Abstract
Objective
Surgical site infections (SSIs) are among the most common healthcare-associated infections occurring following 1%–3% of all surgical procedures. Their rates are the highest following abdominal surgery. They are still associated with increased morbidity and healthcare costs despite the advancement in the medical field. Many risk factors for SSIs following abdominal surgery have been identified. The aim of this study is to comprehensively assess these risk factors as published in peer-reviewed journals.
Design
A systematic review was conducted with accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Setting
The databases for search were PubMed and Cochrane Library, in addition to reference lists. Studies were retrieved and assessed for their quality. Data were extracted in a designed form, and a stratified synthesis of data was conducted to report the significant risk factors.
Participants
Patients undergoing general abdominal surgery.
Intervention
The intervention of general abdominal surgery.
Main outcome measures
To identify and assess the risk factors for SSI following abdominal surgery.
Results
Literature search yielded 813 articles, and the final screening process identified 11 eligible studies. The total number of patients is 11 996. The rates of SSI ranged from 4.09% to 26.7%. Nine studies were assessed to be of high quality, the remaining two studies have moderate quality. Stratified synthesis of data was performed for risk factors using summary measures (OR/risk ratio, 95% CI, and p value). Male sex and increased body mass index (BMI) were identified as significant demographic risk factors, and long operative time was among the major significant procedure-related risk factors.
Conclusions
Male sex, increased BMI, diabetes, smoking, American Society of Anesthesiologists classification of >2, low albumin level, low haemoglobin level, preoperative hospital stay, long operative time, emergency procedure, open surgical approach, increased wound class, intraoperative blood loss, perioperative infection, perioperative blood transfusion, and use of drains are potential independent risk factors for SSI following abdominal surgery.
Keywords: Outcome Assessment (Health Care), Patient Outcome Assessment, Process Assessment (Health Care)
WHAT IS ALREADY KNOWN ON THIS TOPIC
The risk factors associated with the development of a surgical site infection (SSI) varies according to specific patient factors and clinical characteristics, in addition to the nature of the surgical procedure. Despite the considerable number of the clinical studies that have reported on these risk factors for SSIs following abdominal surgeries, it can be challenging to control the level of details available and to comprehensively adjust for all the variables in the estimation of particular risk factors. A comprehensive and detailed assessment of the multifactorial nature of these risk factors could help in improving the quality of surgical care.
WHAT THIS STUDY ADDS
To our knowledge, there are no previous systematic reviews in the literature that comprehensively studied the risk factors of SSIs following the different types of general abdominal surgeries in particular. Moreover, as a high level of evidence is greatly needed in the surgical literature, this systematic review comes to comprehensively identify and assess the current evidence of these predictive factors of postoperative wound infections after abdominal surgery.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We believe that the results of our study will add recent evidence that will help in improving surgical management by forming evidence-based strategies to decrease the rates of preventable SSIs by targeting their potential risk factors; one can improve patient safety.
Introduction
Surgical site infection (SSI) is defined by the Centers for Disease Control and Prevention (CDC) as the infection occurs within 30 days of the surgical operation at the site of the surgery, or within a year if an implant is left in place, and the infection is thought to be secondary to surgery.1 SSIs are classified by the CDC into three categories: superficial incisional if limited to the skin and subcutaneous tissue at the site of the incision, deep incisional when involving fascial and muscle layers, and organ space when involving a body cavity or visceral organs.1 2
These SSIs are among the most common healthcare associated infections, occurring following 1%–3% of all surgical procedures,3 and their rates are much higher following abdominal surgeries than with other types of surgeries, with many studies indicating wide variations in the reported incidences based on the operating conditions.3–6
Although regulations of antisepsis were described very early by Joseph Lister in 1867, SSI is still a common problem in surgical patients despite of the advancements in the medical field.2 It is associated with increased morbidity, readmissions, length of hospital stay, mortality, and healthcare costs.7–10
With the rising rates and morbidities associated with these infections, various studies have made several efforts to find better ways to optimize patients prior to surgery or improve perioperative conditions and management of patients during the recovery period in order to prevent SSIs,11 12 including the recommendations published by the WHO.13
The risk factors associated with the development of an SSI vary according to specific patient factors and clinical characteristics, in addition to the nature of the surgical procedure.14 Despite the considerable number of clinical studies that have reported on these risk factors for SSIs following abdominal surgeries, it can be challenging to control the level of details available and to comprehensively adjust for all the variables in the estimation of particular risk factors.15 A comprehensive and detailed assessment of the multifactorial nature of these risk factors could help in improving the quality of surgical care.
To our knowledge, there are no previous systematic reviews in the literature that studied the risk factors of SSIs following the different types of general abdominal surgeries in particular. Moreover, as a high level of evidence is greatly needed in the surgical literature, this systematic review comes to comprehensively identify and assess the current evidence of these predictive factors of postoperative wound infections after abdominal surgery as they have been published in the medical peer-reviewed literature, taking into consideration the potential confounders. We believe that the results of our study will add recent evidence that will help in improving surgical management by forming evidence-based strategies to decrease the rates of preventable SSIs by targeting their potential risk factors; one can improve patient safety.10
Methods
Reporting
The reporting of this systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.16
Search strategy and study selection
A vigorous, maximum sensitive and online search of the literature was performed. The search strategy was limited to published articles in the last 20 years (January 2002–December 2021) to include the most recent, up-to-date data that reflect the current clinical practice. The databases searched were MEDLINE (via PubMed) and Cochrane Library. The search terms used were “risk factors”, “surgical site infection”, “surgical wound infection”, “abdominal surgical infection”, and “abdominal surgery” combined with “AND” and “OR” operators as appropriate. The reference lists of the included studies were also searched manually to exhaustively retrieve additional relevant articles.
The review and selection of studies were performed based on specific inclusion and exclusion criteria in two screening stages. The first stage of screening was performed based on the titles and abstracts. Eligible studies were screened based on the full text in the second stage. Figure 1 shows the flow of the selection process. The search strategy and study selection were performed by two independent reviewers; any discrepancies were resolved by discussion and consensus.
Figure 1.
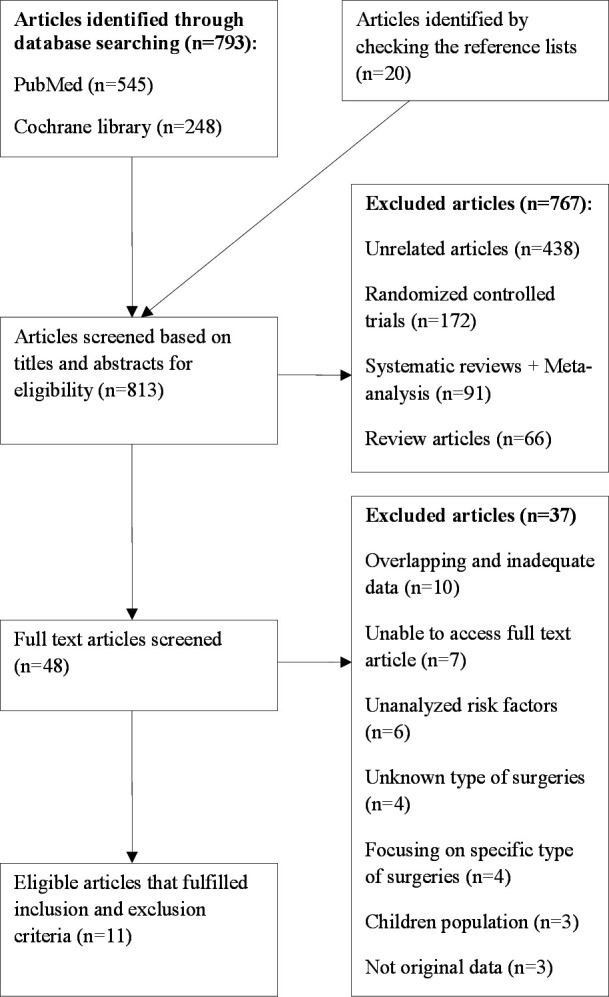
Flow diagram outlining the review process and study selection.
Inclusion and exclusion criteria
Included studies were observational, published in peer-reviewed journal in the last 20 years that assessed risk factors for SSI following abdominal surgery. They should contain original data and adequate analytical summary measures.
Articles were excluded if they were not observational, did not analyze risk factors for SSIs following abdominal surgery, without adequate data if the analysis was general, and did not include the summary measures for the targeted risk factors, making it difficult to interpret the data and to match them with the records in the other studies, or with overlapping data. Articles with patients aged less than 18 years and unknown type of surgeries were also excluded. Reasons for exclusion were documented in figure 1.
Data extraction
Two independent authors extracted the required data in a designed form; the following informations were collected from the included studies: authors, country of the study, year of publication, study design, number of patients, male/female percentage, mean/median age, overall SSI rate, operative procedures, SSI definition, and follow-up period.
Quality assessment
Included articles were scored for their quality by two independent authors using parameters defined by the Agency for Healthcare Research and Quality (AHRQ). These parameters are based on nine criteria, and each on of them is subdivided into further subgroups; these nine major criteria include study population, study question, intervention, comparability of subjects, outcome measures, statistical analysis, conclusion, results, and funding.17–20 A weighted score was provided for each one of the subgroups for a total of 100 points based on AHRQ criteria and with a reference to two previously published systematic reviews that used the same tool,19 21 a score greater than 67 was defined as high quality, a score of 50–67 was considered to be moderate quality, and a score of less than 50 was set to be low quality. Consensus on the final assessment of studies quality was achieved by discussion between the authors. Table 1 shows the quality assessment tool of the included studies.
Table 1.
Quality assessment of the included studies using Agency for Healthcare Research and Quality criteria
| Authors | Study question | Study population | Sample size justification | Inclusion and exclusion criteria | Criteria applied to all groups | Comparability of groups | Comparable with regard to confounding | Use of concurrent controls | Comparability of follow-up | Clear definition of exposure | Measurement methods | Exposure measured equally | Outcomes clearly defined | Outcomes assessed blindly |
| Weighted score | 2 | 5 | 3 | 5 | 3 | 3 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 5 |
| Alkaaki et al22 | 2 | 5 | 1 | 5 | 3 | 0 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 5 |
| Bediako-Bowan et al23 | 2 | 5 | 1 | 5 | 3 | 2 | 3 | 0 | 3 | 5 | 0 | 3 | 5 | 5 |
| Ejaz et al24 | 2 | 5 | 1 | 5 | 3 | 3 | 3 | 5 | 3 | 5 | 0 | 3 | 5 | 0 |
| Giri et al25 | 2 | 5 | 3 | 5 | 3 | 2 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 5 |
| Guzmán-García et al26 | 2 | 5 | 1 | 5 | 3 | 2 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 0 |
| Hernandez et al27 | 2 | 5 | 1 | 5 | 3 | 3 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 5 |
| Isik et al28 | 2 | 5 | 3 | 2.5 | 3 | 2 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 0 |
| Raka et al29 | 2 | 5 | 1 | 5 | 3 | 2 | 0 | 5 | 1 | 5 | 3 | 3 | 5 | 5 |
| Razavi et al30 | 2 | 5 | 1 | 5 | 3 | 2 | 0 | 5 | 1 | 5 | 0 | 3 | 5 | 5 |
| Walz et al31 | 2 | 5 | 1 | 5 | 3 | 3 | 3 | 5 | 3 | 5 | 0 | 3 | 5 | 0 |
| Watanabe et al32 | 2 | 5 | 1 | 5 | 3 | 3 | 3 | 5 | 3 | 5 | 3 | 3 | 5 | 5 |
| CDC definition used | Adequate length of follow-up | Appropriate statistical analysis | Multiple comparisons | Multivariate model used | Power calculation | Confounding assessment | Dose response | Effect measures | Adequate follow-up | Conclusion | Funding | Total | |
| Weighted score | 5 | 5 | 5 | 3 | 2 | 2 | 5 | 2 | 5 | 3 | 5 | 5 | 100 |
| Alkaaki et al22 | 5 | 5 | 5 | 3 | 2 | 0 | 5 | 2 | 5 | 3 | 5 | 0 | 88 |
| Bediako-Bowan et al23 | 0 | 5 | 3 | 3 | 0 | 0 | 5 | 2 | 5 | 3 | 3 | 5 | 79 |
| Ejaz et al24 | 0 | 2 | 5 | 0 | 2 | 0 | 5 | 0 | 5 | 1 | 3 | 0 | 66.5 |
| Giri et al25 | 5 | 5 | 5 | 3 | 2 | 0 | 5 | 2 | 5 | 3 | 3 | 0 | 90 |
| Guzmán-García et al26 | 5 | 5 | 5 | 0 | 2 | 0 | 5 | 0 | 5 | 3 | 3 | 0 | 78 |
| Hernandez et al27 | 5 | 5 | 5 | 3 | 2 | 0 | 5 | 2 | 5 | 3 | 5 | 0 | 91 |
| Isik et al28 | 5 | 5 | 5 | 0 | 2 | 0 | 5 | 0 | 5 | 3 | 5 | 0 | 79.5 |
| Raka et al29 | 5 | 5 | 3 | 3 | 0 | 0 | 0 | 2 | 2.5 | 1 | 3 | 0 | 69.5 |
| Razavi et al30 | 3 | 5 | 3 | 3 | 0 | 0 | 0 | 2 | 2.5 | 3 | 3 | 0 | 66.5 |
| Walz et al31 | 3 | 5 | 5 | 0 | 2 | 0 | 5 | 0 | 5 | 3 | 5 | 0 | 81 |
| Watanabe et al32 | 5 | 5 | 5 | 3 | 2 | 0 | 5 | 2 | 5 | 3 | 5 | 0 | 91 |
CDC, Centers for Disease Control and Prevention.
Outcome measures
The outcome measure in this study was to identify and assess the risk factors for SSI following abdominal surgery.
Data synthesis and analysis
The risk factors identified in this study were variable. The pooled quantitative analysis was precluded due to variable definitions, study characteristics, quality, and types of procedures included. We performed stratified synthesis of data for each one of the included studies to comprehensively assess the risk factors.
Results
Study selection
The online literature search retrieved a total of 793 articles, 545 using PubMed and 248 using Cochrane Library. The reference list checking of the included studies added additional 20 articles. The first stage of screening was performed for 813 articles; 767 articles were excluded in this stage. Forty-eight eligible articles were screened in the second stage based on the full text. A total of 11 articles which fulfilled the inclusion and exclusion criteria were included in this study.22–32 The full screening process is presented in figure 1.
Characteristics of the included studies
All included 11 studies were observational22–32; 7 of them were prospective cohort studies22 23 25 27 29 30 32 and 4 were retrospective cohort studies.24 26 28 31 The studies represent 10 countries; they were published between 2005 and 2020. A total of 11 996 patients were included in these studies, and the rates of SSI in the cohorts ranged from 4.09% to 26.7%. Most of the studies used the CDC definition to identify SSIs. Various general abdominal surgery procedures were identified in the studies. Table 2 summarizes the characteristics of the included studies.
Table 2.
Characteristics of the included studies
| Authors, country | Year of publication | Study design | Patients (n) | Male (%)/female (%) | Median age (IQR)* | Overall SSI rate (%) | Operative procedures | SSI definition | Follow-up period |
| Alkaaki et al,22 Kingdom of Saudi Arabia | 2019 | Prospective cohort | 337 | 42.7/57.3 | Mean 43.6 | 16.3 | Cholecystectomy, ventral hernia repair, gastric bypass, and appendectomy | CDC definition | 30 days |
| Bediako-Bowan et al,23 Ghana | 2020 | Prospective cohort | 358 | 50/50 | 41 (27–56) | 16.2 | Bowel surgeries and ventral hernia repair | No definition | 30 days |
| Ejaz et al,24 USA | 2017 | Retrospective cohort | 1744 | 48.3/51.7 | 58 (47–68) | 7.6 | Hepatobiliary surgery, pancreatectomy, and colectomy | Clinical definition | In patient |
| Giri et al,25 Nepal | 2013 | Prospective cohort | 230 | 57.4/42.6 | Mean 40 | 23.04 | Abdominal gastrointestinal surgeries | CDC definition | 30 days |
| Guzmán-García et al,26 Mexico | 2019 | Retrospective cohort | 755 | 36.3/63.7 | NR | 12.05 | Colorectal surgery-appendectomy-cholecystectomy-ventral hernia repair | CDC definition | 30 days |
| Hernandez et al,27 Peru | 2005 | Prospective cohort | 468 | 59.8/40.2 | Mean 37.2 | 26.7 | Appendectomy, hepatobiliary–gastric–small bowel surgery, colorectal–ventral hernia repair, and laparotomy | CDC definition | 30 days |
| Isik et al,28 Turkey | 2015 | Retrospective cohort | 4690 | 51.9/48.1 | NR | 4.09 | Ventral hernia repair and hepatobiliary–colorectal–abdominal upper gastrointestinal surgery | CDC definition | 30 days |
| Raka et al,29 Kosovo | 2007 | Prospective cohort | 225 | 55.1%/44.9% | 42 (8–88) | 12% | Cholecystectomy, appendectomy, and colonic surgery | CDC definition | 30 days |
| Razavi et al,30 Iran | 2005 | Prospective cohort | 802 | 49.5/50.5 | Mean 46.7 | 17.4% | Cholecystectomy, splenectomy, umbilical hernia–appendectomy, gastric surgery, laparatomy, colectomy, intestinal adhesions–iliostomy | Clinical definition | 30 days |
| Walz et al,31 USA | 2006 | Retrospective cohort | 1446 | 47/53 | 57 (18–96) | 8.7 | Small bowel surgeries and colonic surgeries | Clinical definition | 30 days |
| Watanabe et al,32 Japan | 2008 | Prospective cohort | 941 | 63.7/36.3 | (11–98) | 15.5 | Gastrectomy, small bowel resection, colonic resection, stoma operation, appendectomy, and adhesiolysis | CDC definition | 30 days |
*Data shown represent median (IQR) except otherwise indicated.
CDC, Centers for Disease Control and Prevention; NR, not reported; SSI, surgical site infection.
Quality assessment
AHRQ quality grading tool was used to assess the included studies. Nine of the 11 included studies were found to have high quality (score >67),22 23 25–29 31 32 and 2 articles showed moderate quality in the assessment (score 50–67).24 30
Study question (weighted score of two) was appropriate and focused in all studies. Study population (weighted score of eight) was clearly described in all studies, but only two of them fully justified the sample size.25 28 Comparability of subjects (weighted score of 22) was fully scored for four studies.24 27 31 32 Exposure for intervention (weighted score of 11) was scored fully for seven studies.22 25–29 32 Outcome assessment (weighted score of 20) was fully scored for five studies.22 25 27 29 32 Statistical analysis (weighted score of 19) was not scored fully for any of the included studies due to various characteristics and criteria in subgroup scoring. Results (weighted score of eight) were scored fully in eight studies.22 23 25–28 31 32 All studies’ conclusions (weighted score of 5) were valid, but five of them scored a full mark.22 27 28 31 32 Only one study23 was funded (weighted score of 5).
Risk factors
Various risk factors were analyzed by two or more of the included studies; these factors included age, gender, body mass index (BMI), smoking, diabetes, antibiotics prophylaxis, use of drainage, perioperative blood transfusion, intraoperative blood loss, perioperative infection, intraoperative temperature, preoperative chemotherapy, use of immunosuppressive drugs, haemoglobin level, ASA classification, albumin level, malignancy, preoperative hospital stay, type of admission, operative time, surgical approach, procedure type, wound class, and number of people in the theater. The rates of SSIs ranged from 4.09% to 26.7% with a mean incidence rate of 13.02%. The summary measures used to determine the statistical significance of the included risk factors were p value, OR, and 95% CI; only two studies used risk ratio (RR) instead of OR.23 27 A multivariable analysis model was used in 8 out of 11 studies to obtain the significant independent risk factors. Stratified synthesis of the results of the individual studies is summarized in table 3.
Table 3.
Summary of the significant risk factors identified in the review
| Authors | Risk factors | OR (95% CI)* | P value | Model of analysis | Study quality |
| Alkaaki et al22 | Male sex | 2.6 (1.02 to 6.6) | <0.001 | ||
| Operative time >3 hours | 2.1 (1.23 to 3.6) | <0.001 | Multivariate | High | |
| Emergency procedure | 4.7 (1.58 to 14.4) | <0.001 | |||
| Open approach | 6.5 (2.16 to 19.6) | <0.001 | |||
| Bediako-Bowan et al23 | Overweight | RR 2.1 (1.11 to 3.96) | NR | ||
| Diabetes | RR 1.68 (0.76 to 3.71) | NR | |||
| Chemotherapy in previous 12 weeks | RR 1.57 (0.49 to 5.02) | NR | Univariate | High | |
| Wound class (infected) | RR 3.15 (0.94 to 10.62) | 0.04 | |||
| Operative time >120 min | RR 2.57 (1.19 to 5.52) | 0.015 | |||
| People >10 in the theater | OR 3.12 (0.71 to 13.66) | NR | |||
| Ejaz et al24 | Wound class (dirty-infected) | 2.10 (1.30 to 3.41) | 0.003 | ||
| Perioperative blood transfusion | 3.10 (1.90 to 5.05) | <0.001 | Multivariate | Moderate | |
| EBL >600 mL | 2.04 (1.16 to 3.57) | 0.01 | |||
| Giri et al25 | BMI >25 | 7.6 (2.1 to 27) | 0.002 | ||
| Hemoglobin level <120 g\L | 2.5 (1.1 to 6.1) | 0.03 | Multivariate | High | |
| Operative time >3 hours | 3.6 (1.5 to 8.6) | 0.004 | |||
| Guzmán-García et al26 | Male sex | 2.65 (1.70 to 4.14) | <0.0001 | ||
| BMI >25 | NR | 0.007 | |||
| Diabetes | NR | 0.04 | |||
| Smoking | NR | <0.0001 | |||
| ASA classification (3 and 4) | NR | <0.0001 | Multivariate | High | |
| Low albumin level | NR | <0.001 | |||
| Operative time >1 hour | NR | <0.0001 | |||
| Wound class (dirty-infected) | NR | <0.0001 | |||
| Emergency procedure | 2.94 (1.70 to 5.09) | <0.0001 | |||
| Hernandez et al27 | Wound class (dirty-infected) | RR 3.8 (1.7 to 8.4) | <0.001 | ||
| Operative time >3 hours | RR 2.1 (1.0 to 4.4) | <0.001 | Multivariate | High | |
| Use of drains | RR 6.0 (2.5 to 12.5) | <0.001 | |||
| Isik et al28 | Preoperative hospital stay >8 days | 2.76 (1.46 to 5.28) | <0.001 | ||
| ASA classification (3 and 4) | 2.58 (1.79 to 3.70) | <0.0001 | |||
| Operative time >4 hours | 3.48 (1.54 to 8.21) | 0.0026 | |||
| Emergency procedure | 2.41 (1.57 to 3.67) | <0.0001 | Multivariate | High | |
| Wound class (dirty-infected) | 3.16 (1.47 to 7.19) | 0.003 | |||
| Perioperative blood transfusion | 2.45 (1.52 to 3.90) | 0.0003 | |||
| Use of drains | 1.78 (1.22 to 2.62) | 0.003 | |||
| Raka et al29 | Preoperative hospital stay ≥7 days | OR 6.62 | <0.001 | Univariate | High |
| ASA classification >2 | OR 6.37 | <0.001 | |||
| Operative time >1 hour | OR 8.0 | <0.001 | |||
| Wound class (dirty infected) | OR 5.4 | <0.001 | |||
| Use of drains | OR 6.5 | <0.001 | |||
| Razavi et al30 | Age >65 years | NR | <0.001 | Univariate | Moderate |
| Preoperative hospital stay >15 days | NR | <0.018 | |||
| Operative time >4 hours | NR | <0.001 | |||
| Emergency procedure | NR | <0.001 | |||
| Wound class (dirty-infected) | NR | <0.001 | |||
| Walz et al31 | Perioperative blood transfusion | 1.64 (1.03 to 2.63) | 0.04 | Multivariate | High |
| High intraoperative temperature | 1.33 (1.002 to 1.76) | NR | |||
| Presence of current infection | 2.46 (1.00 to 6.04) | NR | |||
| Watanabe et al32 | Wound class (dirty) | 3.3 (1.63 to 6.67) | 0.0021 | Multivariate | High |
| Intraoperative blood loss | 1 (1.0 to 1.0) | 0.0007 | |||
| Emergency procedure | 3.38 (1.39 to 8.18) | 0.0122 |
*Data shown represent OR (95% CI) until otherwise indicated.
ASA, American Society of Anesthesiologists; BMI, body mass index; EBL, estimated blood loss; NR, not reported; RR, risk ratio.
Demographic risk factors
Age greater than 65 years was a significant risk factor in the univariate model of one study (p<0.001).30 Two studies identified male sex as an independent risk factor for development of SSI in the multivariate model of two high-quality studies,22 26 with summary measures as follows: OR 2.6, 95% CI 1.02 to 6.6, p<0.001, and OR 2.65, 95% CI 1.70 to 4.14, respectively. Overweight is additionally identified as a risk factor in the univariate model of one study (RR 2.1, 95% CI 1.11 to 3.98)23 and in the multivariate model of other two studies that defined BMI of >25 as an independent risk factor,25 26 with summary measures of OR 7.6, 95% CI 2.1 to 27, p=0.002 and p=0.007, respectively.
Preoperative risk factors
Diabetes was identified as a risk factor in two studies, the first study through a univariate model (RR 1.68, 95% CI 0.76 to 3.71)23 and the other one through a multivariate model (p=0.04).26 Two studies identified ASA classifications (3 and 4) as an independent risk factors,26 28 with measures of p<0.0001 and OR 2.58, 95% CI 1.79 to 3.70, p<0.0001, respectively, and another one study defined ASA classification (>2) as a risk factor in the univariate model (OR 6.37, p<0.001).29 Only one study identified previous chemotherapy as a risk factor using univariate model.23 Hemoglobin level of <12 g/dL was identified as an independent risk factor in one study (OR 2.5, 95% CI 1.1 to 6.1, p=0.03).25 Similarly, low albumin level was an independent risk factor in only one study (p<0.001).26 Preoperative hospital stay of >8 days was an independent risk factor in one multivariate model (OR 2.76, 95% CI 1.46 to 5.28, p<0.001)28; additionally, two other studies29 30 defined preoperative hospital stay of ≥7 and >15 days as a risk factor in their univariate models (OR 6.62, p<0.001 and p<0.018), respectively. Smoking was an identified independent risk factor in one high quality study (p<0.0001).26
Procedure-related risk factors
Eight studies identified long operative time as a risk factor using various definitions.22 23 25–30 Emergency admission was a significant risk factor in five studies.22 26 28 30 32 Only one study reported open surgical approach as an independent risk factor (OR 6.5, 95% CI 2.15 to 19.6, p<0.001).22 Dirty-infected wound class was reported as a significant risk factor in eight studies compared with other wound classes.23 24 26–30 32
Intraoperative and postoperative risk factors
Two studies identified intraoperative blood loss as an independent risk factor; the first study defined it as an estimated blood loss of >600 mL (OR 2.04, 95% CI 1.16 to 3.57, p=0.01),24 and the other study didn't give an estimation (p=0.0007).32 High intraoperative temperature nadir was an independent risk factor in only one study (OR 1.33, 95% CI 1.002 to 1.76); the same study reported the presence of perioperative infection as an independent risk factor (OR 2.46, 95% CI 1.00 to 6.04).31 Only one study reported the number of people in the operative theater as a significant risk factor in the univariate model, the study demonstrated that people of >10 in the operative theater increase the risk of wound infection (OR 3.12, 95% CI 0.71 to 13.66).23 Perioperative blood transfusion was found to be an independent risk factor in three studies.24 28 31 Use of drains was identified as a risk factor in three studies.27–29 The summary of reported risk factors is presented in table 3.
Discussion
Despite the widespread adoption of advanced preventive strategies, surgical wound infections continue to occur. Recent evidence in the surgical literature is a base for recommendations and additional options to further reduce the incidence of wound infection. This comprehensive systematic review identified 11 eligible articles that studied risk factors for SSI following abdominal surgery. Nine studies were assessed to be of high quality, and the remaining two studies were of moderate quality.24 30 No restrictions were placed on the geographical region where the study was conducted, but all included studies were conducted with regard to the regular perioperative care. All the included studies were observational cohort studies. This allowed us to report a comprehensive review because observational studies allow a wider breadth of reporting of risk factors for SSI in abdominal surgery; this can be achieved in the routine clinical practice for a larger range of patients, unlike the narrow focus on specific risk factors.
The rates of SSI ranged from 4.09% to 26.7%. These rates in abdominal surgery are higher than other types of surgeries, as compared with similar systematic reviews in spinal surgery,33 dermatological surgery,34 and orthopaedic surgery.35
A number of significant risk factors were identified in the included studies; these include demographic, perioperative, and procedure-related factors. Age was assessed by all studies; only one moderate-quality study identified advanced age as a risk factor for SSI in the univariate model. This is consistent with other reports that associate SSI with people older than 60 years36; however, this result should be interpreted with caution as it is unlikely that older age has a direct and independent relationship due to the number of confounding factors associated with the ageing process that might lead to poorer wound healing. Two studies identified male gender as an independent risk factor22 26; this relationship has been identified in many specific studies,37 and can be supported by the fact that in men, androgens have a proinflammatory effect on wounds, which impairs re-epithelialization process, whereas in women, estrogens have been shown to have an anti-inflammatory effect, which could account for the difference.38 Moreover, the health behaviours and practices of men regarding wound care might contribute to the difference. As expected, well known and reported comorbidities that affect the development of SSI such as increased BMI and diabetes were identified as significant risk factors in our review.39 One study23 defined overweight as a risk factor in the univariate model, and another two studies25 26 defined BMI of >25 as an independent risk factor. This is consistent with the fact that adipose tissue has poor vascularization; as a result, it creates a suitable environment for proliferation of micro-organisms. However, BMI does not accurately reflect the body’s fat composition; we suggest using of parameters such as visceral fat area, subcutaneous fat thickness, and abdomen depth for more reliable SSI prediction in abdominal surgery.40 41 Diabetes was demonstrated as a significant risk factor in two studies23 26; the immunological and vascular complications of diabetes might be associated with wound infection.42
Smoking is generally believed to contribute to infections due to various effects on capillary oxygen transfer, tissue perfusion, and coagulation.43 It is identified as an independent risk factor in one high quality study.26
ASA classification system is a known indicator that reflects combined co-morbidities and physical conditions, the scoring system ranges from (I) for an otherwise healthy patient to (V) for those patients not expected to survive the next 24 hours.44 Three studies in this review found that ASA classification 3 and 4 is a significant risk factor for development of SSI,26 28 29 possibly the higher the classification, the higher risk.
Low albumin level was identified as an independent risk factor in one high quality study.26 In hypoalbuminemia there is no adequate preoperative nutritional support which is essential to optimize surgical outcomes, this result in wound infection.45 Similarly, low hemoglobin concentration associated with an increased risk of wound infection due to tissue hypoxia as reported in past studies,12 this was confirmed in our review as one high quality study defined hemoglobin level <12 g/dL as an independent risk factor.25 One high quality study demonstrated that patients undergoing chemotherapy are at risk of developing SSI in the univariate model23; however, we suggest that further specific studies will help in describing these risks for developing an SSI. Two high quality studies28 29 and one moderate quality study30 identified the length of pre-operative hospital stay as a risk factor using various definitions; colonization of infectious micro-organisms during the prolonged preoperative hospital stay may be the responsible cause for the increased infection risk.
Two studies assessed the significance of the number of people in the operative theater as risk factors23 26; one of them reported a statistical significance of more than 10 people in the univariate model23; however, it is difficult to comprehensively conclude, and these results should be interpreted with caution, so we recommend further specific studies for conclusive outcomes. Not surprisingly, eight studies identified operative time as a significant risk factor using various definitions.22 23 25–30 In fact, this relationship is complex and can be explained by the prolonged exposure to environmental factors, complexity of the case, and occurrence of intraoperative complications, which have been reported in other specific studies.46 Emergency abdominal surgery was reported to have a significant greater risk of postoperative wound infection than elective surgery in five studies22 26 28 30 32; it is regarded as one of the non-modifiable risk factors that could result from lack of readiness for operative procedures. One high-quality study determined open surgical approach as an independent risk factor22; this is consistent with specific reports in abdominal surgery, which conclude that open surgical approach results in larger incision which act as a breeding place for infectious agents.47–49 Dirty-infected wound class was reported as a significant risk factor in eight studies23 24 26–30 32 as compared with other wound classes; this relationship is significantly direct, the wound class; the higher risk of infection (dirty, contaminated, clean-contaminated, or clean), this is not surprising, as the probability of developing SSI is greater due to increased bacterial load. Intraoperative blood loss is another significant independent risk factor which was identified in two studies24 32; as a consequence, it will increase the need for blood transfusion, which is also a risk for SSI. Perioperative infection and high intraoperative temperature nadir were demonstrated as independent risk factors in one study.31 Presence of current infection could complicate surgical outcomes; we suggest that the enhanced perioperative care in patients with concomitant infections would highly decrease the burden of SSIs. To further evaluate intraoperative temperature as a risk factor, we considered a number of specific trials; it was surprising that some reports demonstrated that avoidance of intraoperative hypothermia reduces the incidence of postoperative wound infection50; moreover; our included study reported that despite the statistical significance, difference in temperatures between the both groups is so narrow and clinically negligible, so we recommend further specific assessments regarding this risk factor for SSI. Various reports demonstrated the use of allogeneic blood as a strong factor that increases the risk of infection in patients following surgery.51 Similarly, perioperative blood transfusion is reported as an independent risk factor in three studies24 28 31 due to the known immunosuppressive impact of intraoperative blood transfusion that might give rise to the risk of wound infection. The use of drains postoperatively was identified as a significant risk factor in three studies using various definitions27–29; this is due to colonization and micro-organisms.
It is important to mention that many modifiable risk factors in abdominal surgery such as wound care, operating room environment, preoperative hair removal, and bowel preparation are common risk factors for SSI; we believe that the preoperative prevention interventions for these factors are carried out routinely in the surgical institutions.
Limitations and strengths
As we noted previously, generating pooled estimates across studies is challenging due to many variations in the included studies, we assessed the risk factors focusing on the achievement of statistical significance.
The strengths of this systematic review include the comprehensive nature of risk factor considerations and study eligibilities. However; the lack of sufficient data on some risk factors and the various definitions used were some limitations to this review. We recommend that future studies should aim to specifically and clearly define risk factors; this would improve the impact of the clinical research.
Conclusions
This comprehensive systematic review indicated that male sex, increased BMI, diabetes, smoking, ASA classification of >2, low albumin level, low hemoglobin level, preoperative hospital stay, long operative time, emergency procedure, open surgical approach, increased wound class, intraoperative blood loss, perioperative infection, perioperative blood transfusion, and use of drains are potential independent risk factors for SSI following abdominal surgery. The study also concluded that there is a need for an institutional definition when reporting SSIs; it can be used during prospective, comparative, or even randomized studies targeting the risk factors they have identified.
Footnotes
Contributors: OAM designed this systematic review; performed the study review, data synthesis, and quality assessment; and drafted the final manuscript. AA performed the study review, data synthesis, and quality assessment. EMM was involved in the design, data synthesis and revision of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of Nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–8. [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection. Am J Infect Control 1999;27:97–134. 10.1016/S0196-6553(99)70088-X [DOI] [PubMed] [Google Scholar]
- 3.Eckhauser F, Azoury S, Farrow N, et al. Postoperative abdominal wound infection — epidemiology, risk factors, Identication, and management. CWCMR 2015;2:137. 10.2147/CWCMR.S62514 [DOI] [Google Scholar]
- 4.Aga E, Keinan-Boker L, Eithan A, et al. Surgical site infections after abdominal surgery: incidence and risk factors. A prospective cohort study. Infectious Diseases 2015;47:761–7. 10.3109/23744235.2015.1055587 [DOI] [PubMed] [Google Scholar]
- 5.Legesse Laloto T, Hiko Gemeda D, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis 2017;17:119. 10.1186/s12879-016-2167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mawalla B, Mshana SE, Chalya PL, et al. Predictors of surgical site infections among patients undergoing major surgery at Bugando medical centre in northwestern Tanzania. BMC Surg 2011;11:21. 10.1186/1471-2482-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigelt JA, Lipsky BA, Tabak YP, et al. Surgical site infections: causative pathogens and associated outcomes. Am J Infect Control 2010;38:112–20. 10.1016/j.ajic.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 8.Kaya E, Yetim I, Dervisoglu A, et al. Risk factors for and effect of a one-year surveillance program on surgical site infection at a University hospital in Turkey. Surgical Infections 2006;7:519–26. 10.1089/sur.2006.7.519 [DOI] [PubMed] [Google Scholar]
- 9.Kusachi S, Kashimura N, Konishi T, et al. Length of stay and cost for surgical site infection after abdominal and cardiac surgery in Japanese hospitals: multi-center surveillance. Surgical Infections 2012;13:257–65. 10.1089/sur.2011.007 [DOI] [PubMed] [Google Scholar]
- 10.de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387–97. 10.1016/j.ajic.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Drapeau CMJ, Pan A, Bellacosa C, et al. Surgical site infections in HIV-infected patients: results from an Italian prospective multicenter observational study. Infection 2009;37:455–60. 10.1007/s15010-009-8225-1 [DOI] [PubMed] [Google Scholar]
- 12.Neumayer L, Hosokawa P, Itani K, et al. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1178–87. 10.1016/j.jamcollsurg.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health . Standard treatment guidelines, 6th ed. Accra: Ghana National Drugs Programme (GNDP) Ministry of Health; 2010. Available: https://www.moh.gov.gh/wp-content/uploads/2016/02/Standard-Treatment-Guideline-2010.pdf [Accessed 06 Jun 2018]. [Google Scholar]
- 14.Kirby JP, Mazuski JE. Prevention of surgical site infection. Surg Clin North Am 2009;89:365–89, 10.1016/j.suc.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Concato J. When to randomize, or 'evidence-based medicine needs medicine-based evidence Pharmacoepidemiol Drug Saf 2012;21 Suppl 2:6–12. 10.1002/pds.3245 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 17.West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) 2002;47:1–11. [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 19.Colson J. A systematic review of observational studies on the effectiveness of opioid therapy for cancer pain. Pain Phys 2011;3;14:E85–102. 10.36076/ppj.2011/14/E85 [DOI] [PubMed] [Google Scholar]
- 20.Reisch JS, Tyson JE, Mize SG. Aid to the evaluation of therapeutic studies. Pediatrics 1989;84:815–27. [PubMed] [Google Scholar]
- 21.Subramanyam R, Schaffzin J, Cudilo EM, et al. Systematic review of risk factors for surgical site infection in pediatric scoliosis surgery. Spine J 2015;15:1422–31. 10.1016/j.spinee.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 22.Alkaaki A, Al-Radi OO, Khoja A, et al. Surgical site infection following abdominal surgery: a prospective cohort study. Can J Surg 2019;62:111–7. 10.1503/cjs.004818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bediako-Bowan AAA, Mølbak K, Kurtzhals JAL, et al. Risk factors for surgical site infections in abdominal surgeries in Ghana: emphasis on the impact of operating rooms door openings. Epidemiol Infect 2020;148:e147. 10.1017/S0950268820001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejaz A, Schmidt C, Johnston FM, et al. Risk factors and prediction model for inpatient surgical site infection after major abdominal surgery. J Surg Res 2017;217:153–9. 10.1016/j.jss.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 25.Giri S, Kandel BP, Pant S, et al. Risk factors for surgical site infections in abdominal surgery: a study in Nepal. Surg Infect (Larchmt) 2013;14:313–8. 10.1089/sur.2012.108 [DOI] [PubMed] [Google Scholar]
- 26.Guzmán-García C, Flores-Barrientos OI, Juárez-Rojop IE, et al. Abdominal surgical site infection incidence and risk factors in a Mexican population. Adv Skin Wound Care 2019;32:1–6. 10.1097/01.ASW.0000557833.80431.00 [DOI] [PubMed] [Google Scholar]
- 27.Hernandez K, Ramos E, Seas C, et al. Incidence of and risk factors for surgical-site infections in a Peruvian hospital. Infect Control Hosp Epidemiol 2005;26:473–7. 10.1086/502570 [DOI] [PubMed] [Google Scholar]
- 28.Isik O, Kaya E, Dundar HZ, et al. Surgical site infection: re-assessment of the risk factors. Chirurgia (Bucur) 2015;110:457–61. [PubMed] [Google Scholar]
- 29.Raka L, Krasniqi A, Hoxha F, et al. Surgical site infections in a surgical ward at Kosovo. J Infect Developing Countries 2007;1:337–41. 10.3855/jidc.375 [DOI] [PubMed] [Google Scholar]
- 30.Razavi SM, Ibrahimpoor M, Sabouri Kashani A, et al. Abdominal surgical site infections: incidence and risk factors at an Iranian teaching hospital. BMC Surg 2005;5:2. 10.1186/1471-2482-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walz JM, Paterson CA, Seligowski JM, et al. Surgical site infection following bowel surgery. ARCH SURG 2006;141:1014. 10.1001/archsurg.141.10.1014 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe A, Kohnoe S, Shimabukuro R, et al. Risk factors associated with surgical site infection in upper and lower gastrointestinal surgery. Surg Today 2008;38:404–12. 10.1007/s00595-007-3637-y [DOI] [PubMed] [Google Scholar]
- 33.Fei Q, Li J, Lin J, et al. Risk factors for surgical site infection following spinal surgery: a meta-analysis. World Neurosurgery 2016;95:507–15. 10.1016/j.wneu.2015.05.059 [DOI] [PubMed] [Google Scholar]
- 34.Delpachitra MR, Heal C, Banks J, et al. Risk factors for surgical site infection in minor dermatological surgery: a systematic review. Advances in Skin and Surgical Wounds 2018;0:1–10. 10.1097/01.asw.0000546118.25057.1a [DOI] [PubMed] [Google Scholar]
- 35.Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty 2010;25:1216–22. 10.1016/j.arth.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 36.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection 1999. Infect Control Hosp Epidemiol 1999;20:247–80. 10.1086/501620 [DOI] [PubMed] [Google Scholar]
- 37.Cohen B, Choi YJ, Hyman S, et al. Gender differences in risk of blood stream and surgical site infections. J Gen Intern Med 2013;28:1318–25. 10.1007/s11606-013-2421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langelotz C, Mueller-Rau C, Terziyski S, et al. Gender-specific differences in surgical site infections: an analysis of 438,050 surgical procedures from the German national Nosocomial infections surveillance system. Viszeralmedizin 2014;30:114–7. 10.1159/000362100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser TG, Forrester JD, Forrester JA. Tactics to prevent intra-abdominal infections in general surgery. Surg Infect (Larchmt) 2019;20:139–45. 10.1089/sur.2018.282 [DOI] [PubMed] [Google Scholar]
- 40.Tsujinaka S, Konishi F, Kawamura YJ, et al. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum 2008;51:1757–65; 10.1007/s10350-008-9395-0 [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Wang M, Lu X, et al. Abdomen depth and reclusive abdominis thickness predict surgical site infection in patients receiving elective radical resections of colon cancer. Front Oncol 2019;9:637. 10.3389/fonc.2019.00637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgson K, Morris J, Bridson T, et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015;144:171–85. 10.1111/imm.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverstein P. Smoking and wound healing. Am J Med 1992;93:22S–24S. 10.1016/0002-9343(92)90623-j [DOI] [PubMed] [Google Scholar]
- 44.Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology 1978;49:233–6. 10.1097/00000542-197810000-00001 [DOI] [PubMed] [Google Scholar]
- 45.Haridas M, Malangoni MA. Predictive factors for surgical site infection in general surgery. Surgery 2008;144:496–501. 10.1016/j.surg.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 46.Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs deep/organ-space surgical site infections: implications for quality improvement initiatives. JAMA Surg 2013;148:849–58. 10.1001/jamasurg.2013.2925 [DOI] [PubMed] [Google Scholar]
- 47.Segal CG, Waller DK, Tilley B, et al. An evaluation of differences in risk factors for individual types of surgical site infections after colon surgery. Surgery 2014;156:1253–60. 10.1016/j.surg.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 48.Imai E, Ueda M, Kanao K, et al. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control 2008;36:727–31. 10.1016/j.ajic.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 49.Marzoug OA. Laparoscopic versus open surgical approach of Cholecystectomy in patients with symptomatic cholelithiasis: a systematic review of comparative trials. Int J Sci Rep 2021;7:167. 10.18203/issn.2454-2156.IntJSciRep20210545 [DOI] [Google Scholar]
- 50.Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001;358:876–80. 10.1016/S0140-6736(01)06071-8 [DOI] [PubMed] [Google Scholar]
- 51.Chang H, Hall GA, Geerts WH, et al. Allogeneic red blood cell transfusion is an independent risk factor for the development of postoperative bacterial infection. Vox Sang 2000;78:13–8. 10.1159/000031143 [DOI] [PubMed] [Google Scholar]


