Abstract
Objective
We sought to compare the incidence of early-onset sepsis (EOS) in infants ≥34 weeks’ gestation identified >24 hours after birth, in hospitals using the Kaiser Permanente Sepsis Risk Calculator (SRC) with hospitals using the National Institute for Health and Care Excellence (NICE) guidance.
Design and setting
Prospective observational population-wide cohort study involving all 26 hospitals with neonatal units colocated with maternity services across London (10 using SRC, 16 using NICE).
Participants
All live births ≥34 weeks’ gestation between September 2020 and August 2021.
Outcome measures
EOS was defined as isolation of a bacterial pathogen in the blood or cerebrospinal fluid (CSF) culture from birth to 7 days of age. We evaluated the incidence of EOS identified by culture obtained >24 hours to 7 days after birth. We also evaluated the rate empiric antibiotics were commenced >24 hours to 7 days after birth, for a duration of ≥5 days, with negative blood or CSF cultures.
Results
Of 99 683 live births, 42 952 (43%) were born in SRC hospitals and 56 731 (57%) in NICE hospitals. The overall incidence of EOS (<72 hours) was 0.64/1000 live births. The incidence of EOS identified >24 hours was 2.3/100 000 (n=1) for SRC vs 7.1/100 000 (n=4) for NICE (OR 0.5, 95% CI (0.1 to 2.7)). This corresponded to (1/20) 5% (SRC) vs (4/45) 8.9% (NICE) of EOS cases (χ=0.3, p=0.59). Empiric antibiotics were commenced >24 hours to 7 days after birth in 4.4/1000 (n=187) for SRC vs 2.9/1000 (n=158) for NICE (OR 1.5, 95% CI (1.2 to 1.9)). 3111 (7%) infants received antibiotics in the first 24 hours in SRC hospitals vs 8428 (15%) in NICE hospitals.
Conclusion
There was no significant difference in the incidence of EOS identified >24 hours after birth between SRC and NICE hospitals. SRC use was associated with 50% fewer infants receiving antibiotics in the first 24 hours of life.
Keywords: Neonatal intensive & critical care, Paediatric infectious disease & immunisation, Epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Largest UK study with 99 683 live births comparing neonatal outcomes following the Kaiser Permanante Sepsis Risk Calculator (SRC) versus National Institute for Health and Care Excellence guidance.
Prospective 1-year observational population-wide cohort study using a network approach to ensure capture of all readmissions following discharge due to early-onset neonatal sepsis.
Data were only obtained for infants who had a blood culture received in a laboratory, and therefore it is possible to have missed a few infants who received antibiotics without a blood culture.
Introduction
Early-onset sepsis (EOS) can be defined as bacteraemia occurring within 72 hours of birth. EOS occurs in around 0.7/1000 live births in high-income settings1 and remains a major cause of morbidity in neonates, particularly those born preterm.2 As infants can initially be asymptomatic or present with non-specific symptoms, determining who should receive antibiotics can be a challenge, and is a balance between unnecessary use of antibiotics and avoiding harm from delayed antibiotic therapy. In the UK, most hospitals follow the National Institute for Health and Care Excellence (NICE) guidance CG149, which uses maternal risk factors, clinical indicators and ‘red flags’3 to guide decisions on investigations and antibiotics. However, concerns of associated antibiotic overuse4 have prompted an increasing number of hospitals to adopt the Sepsis Risk Calculator (SRC)5 6 for infants ≥34 weeks’ gestation and within 12 hours of birth.7
The SRC was developed in the USA and estimates the risk of EOS based on background incidence, gestational age, highest maternal antepartum temperature, duration of membrane rupture, maternal group B Streptococcus (GBS) status and type and timing of intrapartum antibiotics. The infant’s evolving clinical presentation is factored into the second part of the model, which adjusts the prior risk of EOS. Depending on the estimated final risk, the SRC provides recommendations for clinical management (routine care/blood culture/empiric antibiotics) and monitoring of vital signs.7 8 The SRC was endorsed by the American Academy of Pediatrics in 2018.9 While the SRC reduces antibiotic usage,10–12 there have been concerns of the potential for missed or delayed identification of EOS compared with NICE.13 14 Despite this, the SARS-CoV-2 pandemic accelerated its uptake in the UK; 10 out of 26 hospitals in London adopted the SRC to ration resources and facilitate earlier discharges. In this 1-year prospective regional study, we aimed to report the incidence of EOS cases and compare the incidence at which it was identified >24 hours after birth in hospitals using SRC with hospitals using NICE guidance.
Methods
Design
We applied a pragmatic study design, developed by a multiprofessional project team (comprising doctors, nurses, midwives and network managers), supported by the London Neonatal Operational Delivery Network. A common minimum data set was collected by a network of trainee and consultant paediatricians in the Neonatal Trainee Research and Improvement Projects (NeoTRIPS). The protocol is published on the NeoTRIPS website.15
Setting
All 26 National Health Service (NHS) hospitals within Greater London providing newborn care and colocated with a maternity service participated in this study. These included 9 tertiary neonatal intensive care units, 13 local neonatal units and 4 special care baby units. In total, 10 hospitals followed SRC and 16 followed NICE guidance. The decision regarding which approach to follow (SRC/NICE) was made by individual hospitals and was not influenced by participation in this study.
The background incidence of EOS used by the SRC hospitals during the study period ranged from 0.6/1000 to 1/1000. There was variation in the application of SRC; in 9/10 units, it was applied only to subsets of infants meeting specified risk thresholds, and there were differences in the management of infants deemed to be at intermediate risk (online supplemental table 1).
bmjopen-2023-072708supp001.pdf (132.7KB, pdf)
Participants
The eligible population was all live births ≥34 weeks’ gestation during a 12-month period from 1 September 2020 to 31 August 2021.
Main outcomes
The primary outcome was the number of cases of EOS identified >24 hours to 7 days of age, as a proportion of live births. EOS was defined as isolation of a bacterial pathogen in the blood or cerebrospinal fluid (CSF) culture of an infant from 24 hours of age (up to 7 days of age). Bacterial pathogens were categorised as per the Vermont Oxford Network Manual of Operations.16 The number of infants commenced empiric antibiotics in the first 24 hours and the number of infants with EOS in the first 72 hours were also assessed. We also evaluated the rate at which empiric antibiotics were commenced >24 hours up to 7 days of age, for a duration of ≥5 days, with negative blood or CSF cultures.
Data collection
The number of all live births ≥34 weeks’ gestation per calendar month at each hospital site was obtained for the duration of the study. Patient-level data were collected for all infants who had a blood culture obtained during the first 7 postnatal days (figure 1). These infants were identified by reviewing weekly lists of blood cultures from all microbiology laboratories serving these hospitals to ensure all screens for suspected EOS were captured from all settings (postnatal ward, neonatal unit, accident and emergency department). If an infant had more than one blood culture, the timing of the first sample was used.
Figure 1.
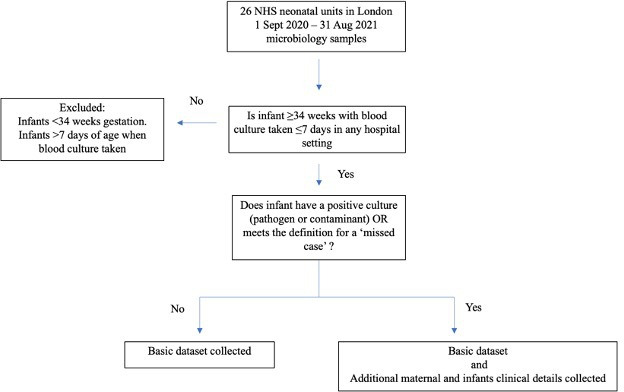
Flowchart of methods. NHS, National Health Service.
For each infant who had a blood culture taken, a basic data set was obtained: time of blood culture (hours of age), receipt of antibiotics and time of administration, admission to a neonatal unit, duration of antibiotics, length of initial hospital stay.
For all EOS cases, additional maternal and infant clinical details were collected (figure 1): gestational age, birth weight, sex, mode of delivery, maternal risk factors (length of rupture of membrane, highest maternal antepartum temperature, GBS status in the current pregnancy, class and timing of intrapartum antibiotics), organisms isolated (blood culture, CSF or both), CSF white cell count, infant’s clinical signs during initial hospital stay, whether the infant presented after discharge home, infant’s symptoms on readmission from home, duration of antibiotics and final clinical outcome. In addition, for SRC hospitals, we collected EOS scores at birth and after clinical examination. We did not collect detailed data for infants with culture-negative sepsis who were treated with antibiotics in the first 24 hours after birth.
Data for readmissions to hospitals other than the birth hospital were obtained through nhs.net correspondence. The NeoTRIPs network covered all London hospitals and frequent communications between members ensured that missing data were minimised.
Anonymised data were collated using Excel through nhs.net, stored on NHS computers and analysed using a centralised Excel spreadsheet through a secure nhs.net server. Monthly data were verified with contributors by three of the authors. Missing data were resolved as far as possible. Cases meeting the definition of EOS were agreed by consensus. Compliance with data submission was supported through feedback at regular meetings throughout the study period. See figure 1, flowchart of methods.
Expected incidence of EOS identified >24 hours after birth
The objective of this pragmatic study was to report the incidence of EOS identified >24 hours after birth to 7 days of age from all London hospitals over a 12-month period. Based on NHS Maternity Statistics,17 estimated ~95 000 live births at ≥34 weeks’ gestation would be born during the study period. With a background EOS incidence of 0.8/1000 live births for Greater London,18 we anticipated ~80 cases of EOS and, based on the estimate defined in the original Kaiser Permanente study,10 we anticipated 5–6 EOS cases identified >24 hours after birth to 7 days.
Statistical analysis
Summary descriptive statistics are presented as medians with their corresponding IQRs for continuous variables and as percentages for categorical variables. All incidence rates are expressed as cases per 1000 or 100 000 live births ≥34 weeks’ gestation, where appropriate, with denominator values based on available data.
Chi-squared tests were used for proportions, independent samples t-test for comparison of means and Mann-Whitney U test for comparisons of medians. Non-parametric data were log transformed to preferentially conduct parametric testing where possible. Shapiro-Wilk test was used for assessing normality of original and log-transformed data. GraphPad Prism was used for analyses. P values <0.05 were considered statistically significant. OR was chosen for events where the incidence was <10%.19
Patient and public involvement
None.
Results
Blood culture data were not available for all months from all hospitals over the study period. Data were missing for 5 months from one SRC hospital and for 32 months from seven NICE hospitals. The live birth denominator corresponding with available data was 42 952 for SRC hospitals and 56 731 for NICE hospitals (table 1). Online supplemental tables 2 and 3 present the live birth denominator data by month for SRC and NICE hospitals.
Table 1.
Outcomes of the participating hospitals
| SRC 10 hospitals |
NICE 16 hospitals |
|
| Live births denominator corresponding to available data | 42 952 | 56 731 |
| Infants screened with blood culture ≤24 hours of age, n (%) | 3297 (7.7) | 8437 (15) |
| Infants who started antibiotics ≤24 hours of age, n (%)* | 3111 (7.2) | 8428 (15) |
| Infants who started antibiotics >24 hours and ≤72 hours of age, n (%)* | 510 (1.3) | 620 (1.3) |
| Infants who started antibiotics >72 hours and ≤7 days of age, n (%)* | 135 (0.3) | 176 (0.4) |
| EOS ≤7 days of age, n, incidence/1000 live births, (95% CI) | 20 (0.47/1000, (0.3 to 0.72)) | 45 (0.79/1000, (0.6 to 1.1)) |
| EOS identified >24 hours and ≤7 days, n (incidence/100 000 live births (95% CI)) | 1 (2.3/100 000, (0.3 to 16)) | 4 (7.1/100 000, (2.7 to 19)) |
| Negative blood culture and started antibiotics >24 hours and ≤7 days for at least 5 days duration, n (incidence/1000 live births, (95% CI)) | 187 (4.4/1000, (3.8 to 5)) | 158 (2.8/1000, (2.4 to 3.3)) |
All live births denote ≥34 weeks’ gestation.
*Timing of antibiotic administration was unavailable for 15 infants (SRC) and 2 infants (NICE).
EOS, early-onset sepsis; NICE, National Institute for Health and Care Excellence; SRC, Sepsis Risk Calculator.
Blood culture screening and intravenous antibiotic use
Overall, 11 734 (12%) infants had a blood culture taken within 24 hours of birth; however, SRC hospitals obtained 50% fewer blood cultures than NICE hospitals (relative risk 0.5, 95% CI (0.47 to 0.51)) (table 1). In both SRC and NICE hospitals, the majority of infants having a blood culture were treated with antibiotics (table 1). Hospital-specific antibiotic use is presented in online supplemental tables 1 and 2. The proportions of infants receiving antibiotics at >24 to ≤72 hours and >72 hours to ≤7 days were similar in both hospital types (OR: 1.1, 95% CI (0.97 to 1.2) vs 1.0, 95% CI (0.81 to 1.3)) with no shift towards later therapy in hospitals using SRC (table 1).
Incidence and characteristics of cases of EOS
Across the entire study population, there were 65 infants with EOS within the first 7 days, 64 within 72 hours (0.64/1000, 95% CI (0.5 to 0.82)) and 1 infant from >72 hours to 7 days. The most common pathogen was GBS (0.44/1000). The incidence of Escherichia coli was 0.07/1000, and the incidence of other pathogens combined was 0.16/1000 (online supplemental table 4).
There was a higher number of EOS cases within the first 7 days in NICE hospitals (n=45; 0·0.79/1000) compared with SRC hospitals (n=20; 0.47/1000) (OR 1.7, 95% CI (1.0 to 2.8)) (table 1). Table 2 shows the clinical characteristics for infants with EOS. Cases in the SRC hospitals were more likely to be symptomatic at time of treatment (10 (53%) vs 11 (26%)). However, the timings of blood culture and initiation of antibiotics across the two groups were similar.
Table 2.
Characteristics of 65 cases of EOS ≤7 days
| SRC (n=20) | NICE (n=45) | P value | |
| Gestational age, weeks, mean (SD) | 38.9 (1.7) | 40.1 (7.4) | 0.43 |
| Birth weight, g, mean (SD) | 3156 (562) | 3255 (436) | 0.45 |
| Male, n (%) | 8 (40) | 24 (53) | 0.33 |
| Vaginal delivery, n (%) | 4 (20) | 25 (56) | 0.008 |
| Highest maternal antepartum temperature, median (IQR)* | 37.6 (36.9–38.3) | 37.3 (36.8–37.6) | 0.32 |
| Maternal GBS status, n (%)† | |||
| Unknown | 9 (47) | 15 (35) | 0.38 |
| Positive | 3 (16) | 17 (40) | 0.07 |
| Negative | 7 (37) | 11 (26) | 0.39 |
| Rupture of membranes, hours, median (IQR)‡ | 12 (8.3–24) | 16 (2.8–32) | 0.28 |
| Maternal antibiotics, n (%)§ | |||
| No antibiotics or any <2 hours prior to birth | 16 (84) | 30 (75) | 0.44 |
| GBS-specific antibiotics >2 hours prior to birth | 0 | 3 (7.5) | 0.22 |
| Broad spectrum antibiotics 2–3.9 hours prior to birth | 3 (16) | 4 (10) | 0.51 |
| Broad spectrum antibiotics >4 hours prior to birth | 0 | 3 (7.5) | 0.22 |
| Initial hospital stay | |||
| Assigned postnatal care and never admitted to neonatal unit, n (%) | 7 (35) | 24 (53) | 0.18 |
| Assigned postnatal care and later admitted to neonatal unit, n (%) | 5 (25) | 11 (24) | 0.93 |
| Admitted to neonatal unit from birth centre, n (%) | 8 (40) | 11 (24) | 0.19 |
| Age at blood culture, hours, median (IQR) | 3.7 (2.1–9.2) | 2.6 (1.5–8.9) | 0.45 |
| Age at antibiotics, hours, median (IQR) | 3.7 (2.5–9.2) | 2.6 (1.5–8.7) | 0.76 |
| Clinical signs at birth, n (%)¶ | 10 (53) | 11 (26) | 0.04 |
| Developed signs before discharge, n (%)¶ | 6 (32) | 14 (33) | 0.94 |
| Never had clinical signs, n (%) | 3 (16) | 17 (40) | 0.07 |
| CSF culture positive, n (%)** | 1 (5) | 2 (4.7) | 0.96 |
| CSF white cell count >20, n (%)** | 1 (5) | 3 (7) | 0.76 |
| Death, n (%) | 0 | 1 (2.2) | 0.50 |
| EOS Score at birth, median (IQR) | 2.0 (0.14–7.8) | – | – |
*Highest maternal antepartum temperature missing for SRC 4, NICE 25 infants.
†Maternal GBS status missing for SRC 1, NICE 2 infants.
‡Rupture of membrane timing missing for SRC 4, NICE 16 infants.
§Maternal antibiotics missing for SRC 1, NICE 5 infants.
¶Timing of clinical signs missing for SRC 1, NICE 3 infants.
**CSF not obtained for SRC 1, NICE 3 infants.
CSF, cerebrospinal fluid; EOS, early-onset sepsis; GBS, group B Streptococcus; IQR, Interquartile range; NICE, National Institute for Health and Care Excellence; SD, Standard deviation; SRC, Sepsis Risk Calculator.
Incidence of EOS identified >24 hours from birth
There were 5 cases of EOS identified by culture >24 hours to 7 days (n=1, 2.3/100 000 for SRC vs n=4, 7.1/100 000 for NICE) (table 1). Owing to the difference in background incidence of EOS, the proportions of cases were compared; (1/20) 5% (SRC) versus (4/45) 8.9% (NICE) (χ=0.3, p=0.59). The maternal and infant characteristics are reported in online supplemental table 5. One infant was born at a NICE hospital, had congenital hydronephrosis and was admitted to the neonatal unit directly. Three infants were readmissions from home following an initial asymptomatic course in hospital (all NICE). One infant developed symptoms while being observed on the postnatal ward (SRC). Detailed case histories are provided in online supplemental file 1. Two infants were excluded because of congenital anomalies predisposing to reduced skin integrity and the pathogenesis of invasive infection was probably postnatal rather than that of EOS. These were Bacillus cereus and Acinetobacter baumannii isolated at 28 hours in an infant with harlequin ichthyosis (SRC) and Staphylococcus aureus isolated at 91 hours in a collodion infant (NICE).
Rate of commencing empiric antibiotics >24 hours after birth for ≥5 days, with negative cultures
There were 345 infants who were commenced empiric antibiotics >24 hours after birth for ≥5 days with negative cultures (187, 440/100 000 for SRC vs 158, 290/100 000 for NICE (OR 1.5, 95% CI (1.2 to 1.9)) (table 1). The maternal and infant characteristics are presented in online supplemental table 6. There were differences in maternal characteristics: length of rupture of membranes (limited interpretation due to missing data), GBS status and antibiotic therapy. Despite more cases in the SRC hospitals, there was no greater proportion of infants admitted to the neonatal unit from the postnatal ward or readmitted from home. Timing and duration of antibiotics were similar. There were no deaths in either group.
Discussion
This large observational, pragmatic study was undertaken to assess and compare the outcomes of the routine use of two widely adopted neonatal sepsis management strategies, the SRC and NICE neonatal infection guideline. Decisions regarding which strategy to use were undertaken locally and therefore reflect a range of local factors, including perceived benefits and risks, caseloads and risk factors.
We found a high proportion of infants born at ≥34 weeks’ gestation who received antibiotics within 24 hours of birth—15% in NICE hospitals vs 7% in the SRC hospitals. This implies that 50% fewer infants received empiric antibiotics in the SRC hospitals. Despite this, there was no evidence of a resultant increase in identification of EOS beyond 24 hours after birth. Indeed, the absolute number of infants meeting this definition of later identification was small. Of the five such cases, only three were readmissions in the first 7 days following an asymptomatic course during the initial hospital stay. These three infants had been cared for in hospitals following NICE. Readmission with bacteraemia, even across a population representing almost 100 000 live births, is therefore a rare event. The rarity is also reflected in other large studies following implementation of SRC: 3 cases across 56 261 live births (5.3/100 000) in Northern California10 and 2 cases across 24 749 live births (8.1/100 000) in Wales.6 All infants in these two studies were also asymptomatic during the initial postnatal stay and without clinical indicators for empiric antibiotics.6 10 This indicates that neither approach will prevent all such cases.
The proportion of infants receiving antibiotics ≤24 hours of age in SRC hospitals in our study is still higher than that reported at Kaiser Permanente hospitals (2.6%)10 and other SRC centres in the USA (3.7%).20 These centres reported on cohorts of infants born ≥35 and ≥36 weeks’ gestation, respectively, where our cohort included ≥34 weeks’ gestation with overall higher incidence of infection. Nevertheless, contributions to higher antibiotic use may be explained by the more conservative SRC approach generally adopted by UK hospitals, in which antibiotics are always started when obtaining a blood culture (online supplemental table 1). Withholding antibiotics is one of the possible SRC recommendations for infants at intermediate risk. A Welsh study showed a similar reduction in antibiotic use to our study (45.5%), with SRC use resulting in 7.7% receiving antibiotics.6 Another reason for the higher proportion treated with antibiotics in our study may be that the SRC was applied only to infants cared for on the postnatal ward, as opposed to those admitted to the neonatal unit. Almost all hospitals implemented a variation of the SRC with differences across hospitals (online supplemental table 1). The high use of antibiotics in the hospitals in our study is highlighted further by an international study in high-income settings (with centres following a variety of approaches in managing risk of EOS) which reported that only 3% of infants were treated.21 It is therefore clear that in our setting large numbers of infants are being exposed to antibiotics relative to the low incidence of EOS.
Although the overall incidence of EOS (0.64/1000 live births ≥34 weeks gestation) is similar to that identified in other UK studies,22 as an observational pragmatic study there are inherent limitations in our ability to interpret the differences we found in outcomes between different hospitals. For example, differences in socioeconomic and ethnic backgrounds of the populations served and of obstetric practice regarding caesarean section rates and intrapartum antibiotic prophylaxis use may have a significant impact on the background risk of EOS.23 24 The difference in the number of EOS identified by culture >24 hours after birth in the groups (SRC=1, NICE=4) is small but could reflect the fact that fewer blood cultures were taken in the SRC hospitals meaning that some infants with transient bacteraemia25 and minimal clinical signs were not captured; this has also been reported by the Kaiser Permanente group where the practice of taking a blood culture and awaiting the result is more common.10
The SRC was developed and validated using EOS confirmed by positive blood cultures.7 8 Because infants can present with signs of sepsis with sterile blood or CSF cultures, we reported an additional 345 infants who commenced ≥5 days of intravenous antibiotics after 24 hours of age with negative cultures. The rate at which this occurred was significantly higher in SRC units than in NICE units. Caution must be exercised when considering a definition of presumed sepsis that includes duration of antibiotic therapy, as this may be influenced by a clinician decision to extend treatment following negative cultures, rather than by clinical indicators. Despite its limitations, a definition of five or more days of antibiotic therapy is used elsewhere.1 16 In the setting of a non-randomised study design, it is also possible that clinicians in SRC hospitals were more cautious following implementation of the SRC. However, there was no skew towards later antibiotic treatment suggesting delayed recognition or later manifestation of sepsis associated with the tool. Additionally, there were no increased adverse outcomes such as neonatal unit admission, readmissions following discharge home or death. Whether later antibiotic therapy for presumed sepsis is associated with later sequelae, such as neurodevelopmental impairment, is not clear.26
A key strength of the study was the support provided by the network of London hospitals embarking on implementation of new practice, feedback at regular intervals and crucially, the trainee network to capture all readmissions with presumed sepsis. This is the largest study of the outcomes of the SRC in the UK to date, with data representing 90% of the eligible birth population, and all hospitals in the network providing maternity care contributing data. Thus, the results are generalisable to the wider population.
There are a number of potential limitations to consider: (1) This was a non-randomised study, and therefore we cannot exclude differences in populations and clinical practices at hospitals that may explain (for example) the higher rate of empiric antibiotic therapy in the context of negative cultures in SRC hospitals. (2) This was a pragmatic design with the capacity to obtain only a limited data set. Broad coverage to capture rare events (identification >24 hours after birth) was prioritised over depth of clinical detail. We therefore did not collect laboratory data such as C reactive protein levels. Data were only obtained for infants who had a blood culture received in a laboratory, and therefore it is possible to have missed a few infants who received antibiotics without a blood culture. There was also variation in the application of the SRC across hospitals, with a modified approach used commonly (online supplemental table 1). Equally, without data on every eligible live birth, uniformity of application of NICE guidance cannot be assessed. (3) We sought to determine the rate at which infants received ≥5 days of antibiotics commenced >24 hours after birth in the context of negative cultures. Infants that died before the intention to complete ≥5 days would not have been captured. (4) Not all hospitals provided data for the entire study period, therefore we cannot assure all readmissions following initial hospital discharge were captured. The possibility of readmission to a hospital outwith Greater London remains, but this is likely to be rare. (5) The SRC was compared with NICE CG149,3 which has since been replaced in 2021 by NICE CG19527 with the removal of maternal broad spectrum antibiotics as a risk factor for neonatal EOS, and previous GBS colonisation mandating intrapartum antibiotic prophylaxis for the subsequent pregnancy, unless the woman has had a negative test in that subsequent pregnancy.27 These new changes may bring about a reduction in neonatal antibiotic exposure and some of the cases identified later observed in our study may have been avoided.
We propose that there is now a need to conduct a UK-wide randomised controlled trial to compare these two strategies. Findings from our study will help inform the design of such a study.
Conclusion
The use of the SRC was associated with 50% fewer infants receiving empiric antibiotics compared with NICE CG149. EOS identified by culture >24 hours after birth was rare, with no difference between the two groups. These findings can help inform clinical guidelines as well as the design of definitive studies to compare outcomes of the SRC with NICE CG195 introduced in 2021.27
Supplementary Material
Acknowledgments
We thank Zeshan Rawn (London Neonatal Operational Delivery Network) for technical assistance and Katie Nichol (NHS England and NHS Improvement London).
Footnotes
Twitter: @ChinthikaP, @Jo_OSullivan, @KatieE1066, @aliciad3, @DrCBattersby
Collaborators: NeoTRIPs team: Sara Abdulla, Remon Agaibi, Elmunzir Ahmed, Luvena Anthony, Luana Ayres da Silva, Nauman Balghari, Archana Bansal, Sunanda Bhatia, Alexandra Briscoe, Andrew Chapman, Chloe Ann Cheang, Sabina Checketts, Jagadish Chintapalli, Li Yan Chow, Jonathan Cookson, Daniel Crane, Lucy Crossman, Angela de Cunto, Andrew DeSilva, Stacey De Atougia, Catherine Douch, Eleanor Duckworth, Nilmi Ekanayake, Ramyia Elangovan, Mariam Elbakry, Sara Farhat Dominguez, Lauren Ferretti, Joana Freitas, Mariana Gaspar Fonseca, Rebecca Gaunt, Adeoya Gbemiga Olabamiji, Daniel Geer, Kazim Ghafoor, Nicola Glogowski, Andrea Gronska, Jennifer Ho, Nichola Hodges, Lukas Huhn, Sarah May Johnson, Ola Joseph, Keisha Kamalanathan, Jessica Kimpton, Niamh Langasco, George Lawson, Rosalie Lear, Yinru Lim, Natasha Liow, Rita Marciano, Ramnik Mathur, Maria Mendoza, Clare Middleton, Nichola Monks, Amanda Moules, Evangelia Myttaraki, Rajvi Nagrecha, Harshini Naidu, Helen Nightingale, Noor Nusair. Chisaraokwu Nwachukwu, Felicity Ockelford, Chineze Okorowo, Yujing Ooi, Evgenia Panagiotopoulou, Neaha Patel, Ayesha Rahim, Saranya Ravindran, Reshmi Raychaudhuri, Matthew Rubens, Kate Ryan, Keya Sahay, Nadia Saleem, Miriam Sanderson, Shivani Shah, Mashal Shamsuddin, Ana Silva Ferreira, Srikanthy Sivakanthan, Helen Smith, Sarah Sturrock, Justinas Teiserskas, Devika Thakur, Sara Tho-Calvi, Naomi Tobi, Stephanie Tolan, Erica Twum-Barimah, Madduka Umeh, Benjamin Ummat, Rebecca Unwin, Aarti Verma, Amit Verma, Hannah Walker, Rebecca Wesson, Adelene Wong, Zijian (Chris) Zhang. Multi-professional project team: Mohammad Alam, Juliet Banya, Tristan Bate, Eleanor Bond, Clare Cane, Rina Chotai, Hayley Clements, Julia Croft, Shu-ling Chuang, Ambalika Das, Alka Desai, Simon Drysdale, Ramyia Elangovan, Elizabeth Eyre, Lydia Eze, Igor Fierens, Jonathan Filkin, Laura Govender, Sophie Griffiths, Michela Groppo, Cassandra Gyamtso, John Ho, Lukas Huhn, Eleanor Hulse, Gopinathannair Harikumar, Zainab Kassim, Christina Kortsalioudaki, Linda Machakaire, Joselyn Morris, Richard Nicholl, Ozioma Obi, Anne Opute, Siddhartha Paliwal, Nandiran Ratnavel, Divyen Shah, Neha Sharma, Ruth Shephard, Amisha Singh, Cheentan Singh, Sonia Spathis, Maria Symeonaki, Uma Srirambhatla, Caroline Sullivan, Sophia Teoh, Lidia Tyszczuk, Mercy Ughwujabo, Catherine Warrick, Kirsty Watts, Louis Yee, Jenny Ziprin.
Contributors: CP wrote the first draft of the article with contributions from CB. CP, SG and RY carried out the analyses. All authors edited and approved the final version of the article. CP and CB conceived the study. CP, CB, GK, KB-S, JR, CH, JJ, AD, TL, PTH, KLD and SS contributed to the development and conduct of the study. CP, SG, RY, JO, DT, CL and KE were involved in data collection, along with the NeoTRIPs team, and supported by the wider multi-professional project team group. CB as guarantor accepts full responsibility for the conduct of the study and controlled the decision to publish.
Funding: Funding was received from the London Operational Delivery Network to support CP and DT. All authors had the final responsibility to submit for publication.
Competing interests: CB reports grants and personal awards funded by the National Institute for Health Research, personal fees from Chiesi Pharmaceuticals and AbbVie Pharmaceuticals and is deputy chair of the NIHR Health Technology Assessment Prioritisation Committee for hospital-based care. PTH was a member of NICE CG149 guideline committee and deputy chair of NICE CG195 guideline committee. The other authors do not have any conflicts of interests to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Neonatal Trainee-Led Research and Improvement Projects (NeoTRIPs) group:
Sara Abdulla, Remon Agaibi, Elmunzir Ahmed, Luvena Anthony, Luana Ayres da Silva, Nauman Balghari, Archana Bansal, Sunanda Bhatia, Alexandra Briscoe, Andrew Chapman, Chloe Ann Cheang, Sabina Checketts, Jagadish Chintapalli, Li Yan Chow, Jonathan Cookson, Daniel Crane, Lucy Crossman, Angela de Cunto, Andrew DeSilva, Stacey De Atougia, Catherine Douch, Eleanor Duckworth, Nilmi Ekanayake, Ramyia Elangovan, Mariam Elbakry, Sara Farhat Dominguez, Lauren Ferretti, Joana Freitas, Mariana Gaspar Fonseca, Rebecca Gaunt, Adeoya Gbemiga Olabamiji, Daniel Geer, Kazim Ghafoor, Nicola Glogowski, Andrea Gronska, Jennifer Ho, Nichola Hodges, Lukas Huhn, Sarah May Johnson, Ola Joseph, Keisha Kamalanathan, Jessica Kimpton, Niamh Langasco, George Lawson, Rosalie Lear, Yinru Lim, Natasha Liow, Catherine Longley, Rita Marciano, Ramnik Mathur, Maria Mendoza, Clare Middleton, Nichola Monks, Amanda Moules, Evangelia Myttaraki, Rajvi Nagrecha, Harshini Naidu, Helen Nightingale, Noor Nusair, Chisaraokwu Nwachukwu, Felicity Ockelford, Chineze Okorowo, Yujing Ooi, Evgenia Panagiotopoulou, Neaha Patel, Ayesha Rahim, Saranya Ravindran, Reshmi Raychaudhuri, Matthew Rubens, Kate Ryan, Keya Sahay, Nadia Saleem, Miriam Sanderson, Shivani Shah, Mashal Shamsuddin, Ana Silva Ferreira, Srikanthy Sivakanthan, Helen Smith, Justinas Teiserskas, Devika Thakur, Sara Tho-Calvi, Naomi Tobi, Stephanie Tolan, Erica Twum-Barimah, Madduka Umeh, Benjamin Ummat, Rebecca Unwin, Aarti Verma, Amit Verma, Hannah Walker, Rebecca Wesson, Adelene Wong, Zijian (Chris) Zhang, Mohammad Alam, Juliet Banya, Tristan Bate, Eleanor Bond, Clare Cane, Rina Chotai, Hayley Clements, Julia Croft, Shu-ling Chuang, Ambalika Das, Alka Desai, Simon Drysdale, Ramyia Elangovan, Elizabeth Eyre, Lydia Eze, Igor Fierens, Jonathan Filkin, Laura Govender, Sophie Griffiths, Michela Groppo, Cassandra Gyamtso, John Ho, Lukas Huhn, Eleanor Hulse, Gopinathannair Harikumar, Zainab Kassim, Christina Kortsalioudaki, Linda Machakaire, Joselyn Morris, Richard Nicholl, Ozioma Obi, Anne Opute, Siddhartha Paliwal, Nandiran Ratnavel, Divyen Shah, Neha Sharma, Ruth Shephard, Amisha Singh, Cheentan Singh, Sonia Spathis, Maria Symeonaki, Uma Srirambhatla, Caroline Sullivan, Sophia Teoh, Stephanie Tolan, Lidia Tyszczuk, Mercy Ughwujabo, Catherine Warrick, Kirsty Watts, Louis Yee, and Jenny Ziprin
Data availability statement
All data relevant to the study are included in the article or uploaded as an online supplemental information. The data that support the findings of this study are available from the corresponding author CB, upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The present cohort study was based on anonymised data collected as part of a service evaluation. The study was deemed to be a service evaluation by the chair and approvals officer of the London South East REC Committee and did not require ethical approval or participant consent.
References
- 1. Cailes B, Kortsalioudaki C, Buttery J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed 2018;103:F547–53. 10.1136/archdischild-2017-313203 [DOI] [PubMed] [Google Scholar]
- 2. Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127:817–26. 10.1542/peds.2010-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neonatal infection (early onset): antibiotics for prevention and treatment | guidance | NICE. NICE; 2012. Available: https://www.nice.org.uk/guidance/cg149 [Google Scholar]
- 4. Mukherjee A, Davidson L, Anguvaa L, et al. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed 2015;100:F248–9. 10.1136/archdischild-2014-306349 [DOI] [PubMed] [Google Scholar]
- 5. Eason J, Ward H, Danko O, et al. Early-onset sepsis: can we screen fewer babies safely? Arch Dis Child 2021;106:86–8. 10.1136/archdischild-2019-317047 [DOI] [PubMed] [Google Scholar]
- 6. Goel N, Cannell S, Davies G, et al. Implementation of an adapted sepsis risk Calculator algorithm to reduce antibiotic usage in the management of early onset neonatal sepsis: a Multicentre initiative in Wales, UK. Arch Dis Child Fetal Neonatal Ed 2022;107:303–10. 10.1136/archdischild-2020-321489 [DOI] [PubMed] [Google Scholar]
- 7. Escobar GJ, Puopolo KM, Wi S, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks' gestation. Pediatrics 2014;133:30–6. 10.1542/peds.2013-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics 2011;128:e1155–63. 10.1542/peds.2010-3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puopolo KM, Benitz WE, Zaoutis TE, et al. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018;142:e20182896. 10.1542/peds.2018-2896 [DOI] [PubMed] [Google Scholar]
- 10. Kuzniewicz MW, Puopolo KM, Fischer A, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr 2017;171:365–71. 10.1001/jamapediatrics.2016.4678 [DOI] [PubMed] [Google Scholar]
- 11. Achten NB, Klingenberg C, Benitz WE, et al. Association of use of the neonatal early-onset sepsis Calculator with reduction in antibiotic therapy and safety. JAMA Pediatr 2019;173:1032–40. 10.1001/jamapediatrics.2019.2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore HL, Battersby C, Piyasena C, et al. Assessing variation in neonatal sepsis screening across England. Arch Dis Child Fetal Neonatal Ed 2023;108:430–1. 10.1136/archdischild-2022-324380 [DOI] [PubMed] [Google Scholar]
- 13. Pettinger KJ, Mayers K, McKechnie L, et al. Sensitivity of the Kaiser Permanente early-onset sepsis Calculator: A systematic review and meta-analysis. EClinicalMedicine 2020;19:100227. 10.1016/j.eclinm.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Snoek L, van Kassel MN, Krommenhoek JF, et al. Neonatal early-onset infections: comparing the sensitivity of the neonatal early-onset sepsis Calculator to the Dutch and the updated NICE guidelines in an observational cohort of culture-positive cases. EClinicalMedicine 2022;44:101270. 10.1016/j.eclinm.2021.101270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan-London early-onset sepsis observational study. 2020. Available: https://neotrips.org/wp-content/uploads/2022/04/studyprotocol2.pdf [Accessed 6 Oct 2020].
- 16. Vermont Oxford Network . 2021 Manual of Operations: Part 2. 2021. Available: https://vtoxford.zendesk.com/hc/en-us/articles/360056768093-2021-Manual-of-Operations-Part-2-Release-25-0-PDF [accessed 2 Apr 2023]. [Google Scholar]
- 17. NHS maternity statistics, England 2018-19 [PAS] - NHS Digital. Available: https://digital.nhs.uk/data-and-information/publications/statistical/nhs-maternity-statistics/2018-19 [Accessed 31 Oct 2019].
- 18. Kimpton JA, Verma A, Thakkar D, et al. Comparison of NICE guideline Cg149 and the sepsis risk Calculator for the management of early-onset sepsis on the postnatal ward. Neonatology 2021;118:562–8. 10.1159/000518059 [DOI] [PubMed] [Google Scholar]
- 19. Sedgwick P. Relative risks versus odds ratios. BMJ 2014;348:g1407. 10.1136/bmj.g1407 [DOI] [Google Scholar]
- 20. Dhudasia MB, Mukhopadhyay S, Puopolo KM. Implementation of the sepsis risk Calculator at an academic birth hospital. Hosp Pediatr 2018;8:243–50. 10.1542/hpeds.2017-0180 [DOI] [PubMed] [Google Scholar]
- 21. Giannoni E, Dimopoulou V, Klingenberg C, et al. Analysis of antibiotic exposure and early-onset neonatal sepsis in Europe, North America, and Australia. JAMA Netw Open 2022;5:e2243691. 10.1001/jamanetworkopen.2022.43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris R, Jones S, Banerjee S, et al. Comparison of the management recommendations of the Kaiser Permanente neonatal early-onset sepsis risk Calculator (SRC) with NICE guideline Cg149 in infants ≥34 weeks. Arch Dis Child Fetal Neonatal Ed 2020;105:581–6. 10.1136/archdischild-2019-317165 [DOI] [PubMed] [Google Scholar]
- 23. Gopal Rao G, Townsend J, Stevenson D, et al. Early-onset group B Streptococcus (EOGBS) infection subsequent to cessation of screening-based Intrapartum prophylaxis: findings of an observational study in West London, UK. BMJ Open 2017;7:e018795. 10.1136/bmjopen-2017-018795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collin SM, Demirjian A, Swann C, et al. Race and Ethnicity in neonatal group B Streptococcal disease in England: 2016-2020. Pediatrics 2022;150:e2021056080. 10.1542/peds.2021-056080 [DOI] [PubMed] [Google Scholar]
- 25. Jaffe DM. Occult bacteremia in children. Adv Pediatr Infect Dis 1994;9:237–60. [PubMed] [Google Scholar]
- 26. Mukhopadhyay S, Puopolo KM, Hansen NI, et al. Neurodevelopmental outcomes following neonatal late-onset sepsis and blood culture-negative conditions. Arch Dis Child Fetal Neonatal Ed 2021;106:467–73. 10.1136/archdischild-2020-320664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NICE . Neonatal infection: antibiotics for prevention and treatment [NG195]. 2021. Available: https://www.nice.org.uk/guidance/ng195 [Accessed 20 Apr 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072708supp001.pdf (132.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as an online supplemental information. The data that support the findings of this study are available from the corresponding author CB, upon reasonable request.


