Commentary: This paper presents a comprehensive approach to the evaluation and management of Charcot Foot Arthropathy. It is authored by a world authority on this challenging subject who presents a nuanced, thoughtful and up to date analysis of the complexities of treating this problem.
Burden of Disease
Both the burden of disease attributable to diabetes and the incidence continues to rise. According to the National Diabetes Statistical Report 2014, 29.1 million Americans, or 9.3% of the population, are diabetic. The National Institutes of Health estimated that there were more than 73 000 lower extremity amputations in 2010. 1 With projections of increasing rates of diabetes and morbid obesity over the past decade, it is likely that the number of lower extremity amputations, the economic and resource burden, and the overall burden of disease attributable to diabetic foot will increase over time. Diabetic foot morbidity can be assigned to 3 categories: (1) abscess and osteomyelitis, (2) neuropathic deformity, and (3) ischemic disease and gangrene.
Health care systems throughout the world are currently addressing the epidemic of diabetic foot morbidity. The medical community is focused on addressing identification of patients at risk and developing strategies to prevent the pathology that presents to the orthopaedic foot and ankle surgeon. This article will specifically address the current clinical approach to the understanding and treatment of patients with neuropathic (Charcot) foot arthropathy.
The goal of this review is to discuss the etiology and current treatment for patients with the most common presentation of midtarsal Charcot foot arthropathy. Although many of the principles can be applied to deformity at the hindfoot or ankle levels, the treatment of these more complex deformity patterns is beyond the scope of this review.
Demographics and Pathophysiology
It has been long accepted that the typical patient who develops Charcot foot arthropathy is in his or her midfifties, has been diabetic for 10 years, and is likely morbidly obese.7,8,19 It is now appreciated that this patient population is also vitamin D deficient with subsequent osteoporosis. Peripheral neuropathy, osteoporosis, and morbid obesity appear to be very important predisposing risk factors. Trauma appears to initiate the pathologic process. This trauma might be an acute fracture or the repetitive bending stress of dynamic equinus loading of poor-quality bone. One should remember that these individuals also have a motor neuropathy that affects smaller nerves (that innervate the dorsiflexors) before impacting the larger nerves (affecting the plantar flexors). This repetitive loading leads to a situation much like a stress fracture.
Many of these patients present with midfoot pain with swelling and often erythema. Because there is no deformity in the initial phase of the active pathologic process, many patients are incorrectly diagnosed as having cellulitis, gout, or tenosynovitis. 15 They get treated with various forms of immobilization. Many are treated with antibiotics, nonsteroidal medications, or even a short course of oral steroids. Some even undergo surgery to treat an “abscess.” In most cases, the acute condition resolves over time and residual deformity is not noted by the physician or patient until the swelling fully resolves. Historically, the focus has been on the severe deformities as most of the resultant deformities are minor and thus can be accommodated with therapeutic footwear. Through careful follow-up, it has been determined that the true incidence of the Charcot process is approximately 0.3 per thousand diabetes patients per year. 6
Researchers have long argued between the neuro-traumatic and neuro-vascular theories of the pathophysiology of Charcot. The neurotraumatic theory proposes a “stress” fracture in the presence of loss of protective sensation, that is, sensory peripheral neuropathy. The patient continues to weight-bear because of the absence of painful stimuli, leading to a deformed hypertrophic “nonunion.” The neurovascular theory implies bony resorption due to increased vascular inflow to the involved area, leading to bony resorption, bony weakness, and mechanical failure leading to deformity. The best available evidence would suggest that the actual pathophysiologic process is a combination of both mechanisms. Trauma upregulates osteoclasts to destroy poor-quality bone that allows or does not allow the acute or subacute fracture to heal. The acquired deformities are observed in those patients whose initial trauma has not healed. This consensus fits very well with the 3 phases of a “time-line” popularized by Eichenholtz in a 1966 text based on a pathologist’s observation of 56 patients with neuropathic disease.5,19
Accommodative Treatment
The classic treatment of acute Charcot foot arthropathy has been non-weight bearing immobilization with a total-contact cast during the active phase of the disease process, followed by longitudinal accommodative bracing with therapeutic shoes or custom-modified orthosis-shoe combinations.9,11,23 This approach is based mainly on expert opinion with little supporting evidence. Accepting the premise that the acute process is akin to a stress fracture, weight-bearing immobilization with a total-contact cast has been demonstrated to be successful in turning “off” the destructive process.3,16 Surgery has historically only been advised for osteomyelitis, wounds that could not be resolved with accommodative bracing with progression to amputation when all else failed.9,11,23 Many experts now advise correction of the deformity to allow the use of commercially available therapeutic footwear without the need for cumbersome accommodative orthoses, thereby enhancing ambulation and leading to an improved quality of life. 19
The metrics of successful treatment of Charcot foot with the historic accommodative treatments were simply eradication of wounds and limb preservation. However, a multicenter observational investigation revealed that patients with Charcot foot reported a severe negative impact on their quality of life, and that impairment did not improve with successful treatment when success was defined as eradication of infection and limb salvage. 4 Two subsequent investigations using different outcome instruments have also reported similar findings.10,18 This information has influenced the current interest in correcting the deformity associated with Charcot foot with the goal of improving quality of life in affected patients.
Evaluation
Initial Presentation
The typical patient who presents with acute Eichenholtz Stage I Charcot foot arthropathy is in their mid- to late fifties, diabetic for 10 or more years, has peripheral neuropathy (measured by insensitivity to the Semmes-Weinstein 5.07 monofilament, 10 g) and is morbidly obese.5,17 It has recently been observed that these patients are likely vitamin D deficient and have osteoporosis secondary to longstanding diabetes. 19 Many will report having had a single inciting episode of trauma that is often trivial in nature. Some patients will not recall a traumatic event and thus many patients are misdiagnosed with gout, tenosynovitis, or cellulitis.
There are several clinical “pearls” that can help to differentiate acute Charcot foot arthropathy from infection. Patients with infection have a feeling of malaise and report having had either increasing blood sugars or an increased insulin requirement during the prodromal period both of which are not the case in Charcot foot. Moreover, when the foot is elevated, with the patient in the supine position, the erythema in Charcot foot will dissipate whereas it will remain in patients with infection.
Differentiating the Active Phase from Infection
Many patients who present with the acute active phase of the Charcot foot disease are incorrectly diagnosed as having cellulitis or an abscess that requires surgical treatment. A great deal of effort has been made to develop advance imaging techniques to distinguish infection from the active phase of the disease process. Because both processes destroy bone, the overlap is too great to allow distinguishing characteristics. Obviously, an open wound that is in continuity with bony destruction speaks for infection, whereas bony destruction without proximity to a wound is only suggestive of infection. The prudent physician will combine primarily clinical acumen with laboratory investigations and imaging techniques when coming to a thoughtful diagnosis. 19
Patients with infection generally have a feeling of malaise, as opposed to the patient with Charcot foot who has no malaise but presents with a swollen and often painful foot. A valuable clinical pearl is a recent increase in blood sugar, increased insulin requirement, or difficulty controlling blood sugars that often accompanies infection, but is not observed with an acute neuropathic process. Diabetics rarely present with a hematogenous infection of the foot, so a careful examination to identify an entry point for the infection should be made. The entry point for initiation of an abscess is often a small crack between the toes of a patient with dry skin, or an ingrown toenail. Normal blood tests can be deceiving, due to the immune compromise of longstanding diabetes. A valuable clinical test can be simple elevation of the foot as discussed above.
Staging
The Eichenholtz description of 3 stages represents a timeline of the destructive disease process. Stage I is the initiation of the destructive process. The foot is very swollen, warm, and erythematous. Radiographs are normal, suggesting the acute inflammatory nature of this phase. The classic treatment advises immobilization in a total-contact cast with non-weight bearing. It is currently appreciated that the early active phase of this process is akin to a stress fracture, making weight bearing acceptable.3,16 Immobilization appears to be the key determinant in turning off the process. This was demonstrated by Simon, who successfully aborted the process by performing acute arthrodesis at this stage. 22
We now appreciate that preexisting osteoporosis and the osteoclastic bony destruction leads to the development of deformity that heralds the onset of Eichenholtz stage II. This has historically been described as fragmentation and the development of deformity. Although the total-contact cast has been demonstrated to “turn off” the destructive process, there is no evidence that it can prevent the development of the deformity that develops in a small percentage of patients. Eichenholtz stage III is simply the consolidation and bony healing that ultimately leads to the establishment of a stable resultant deformity.
It has been demonstrated that weight bearing anterior-posterior radiographs with a relative varus or valgus relationship between the forefoot and hindfoot is predictive of late tissue ulceration over the underlying bony deformity. 2 (Figures 1 and 2). It is now well accepted that patients with clinical and radiographic deformity are likely to fare poorly, thus leading to our current interest in surgical correction of acquired deformity. 12 The current goals of treatment are as follows:
Figure 1.
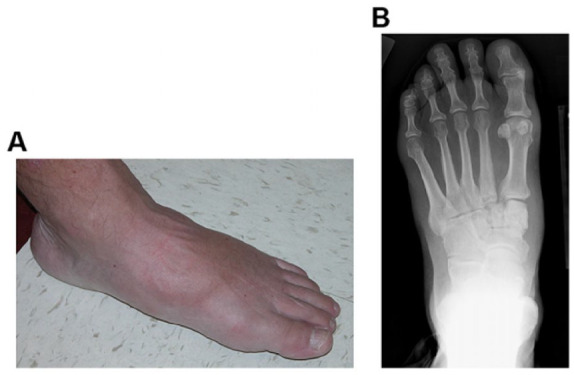
Surgical correction of the acquired deformity is currently advised when patients are clinically or radiographically nonplantigrade. (A) Photograph and (B) weight-bearing anteroposterior radiograph (B) of a patient who is both clinically and radiographically plantigrade. Patients are considered clinically plantigrade when they weight-bear through the plantar tissue of the involved foot. Patients are considered radiographically plantigrade when a line drawn through the axis of the hindfoot (talus) is reasonably colinear with a line drawn through the axis of the forefoot (first metatarsal). Although this patient has evidence of active Charcot foot arthropathy at the tarsal-metatarsal level, he is both clinically and radiographically plantigrade. This patient was treated with a weight-bearing total-contact cast during the active phase of the disease process and managed longitudinally with therapeutic footwear.
Figure 2.
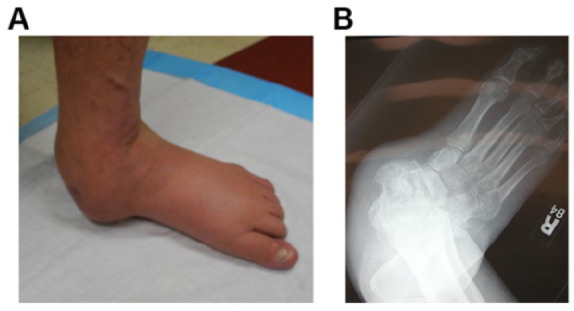
(A) Photograph and (B) weight-bearing anteroposterior radiograph (B) of a patient with a nonplantigrade Charcot foot deformity. Note that he is weight-bearing on nonplantar skin of the medial foot overlying the head of the talus. The best available evidence would suggest that, over time, he will ulcerate the skin overlying the talar head—skin that is not sufficiently durable to accept weight-bearing loads. Surgical correction of this deformity is advised.
A foot that is ulcer and infection free.
A patient who can return to his or her pre-disease activity using commercially available therapeutic footwear.
Avoiding the use of more than a short ankle-foot orthosis.
Treatment
Nonoperative Care
The classic nonoperative treatment of Charcot foot is immobilization in a well-molded total-contact cast during the active phase of the disease process followed by longitudinal accommodative orthotic management. The total-contact cast is simply a carefully applied short leg cast with appropriate padding of bony prominences. It appears that weight bearing does not negatively impact on clinical outcomes.3,16 It also appears that a removable fracture boot that avoids pressure application to bony prominences is similar in effectiveness to the total-contact cast. It has been demonstrated that removeable devices can be as effective if they are not removed.11,23 If our model of Charcot foot as a “stress fracture” phenomenon is correct, then immobilization of the foot during the active phase of the disease process appears to “turn off” the destructive active phase of the disease process, and allow bony healing. In a patient with vitamin D deficiency and osteoporosis, it is simply a question of whether the bone will deform during the healing phase. 19
Surgical Treatment
Surgical Indications
Removal of a bony prominence, that is, exostectomy, to resolve osteomyelitis and allow patients to wear therapeutic footwear with accommodative bracing.
Resection of osteomyelitis and correction of deformity. It remains controversial whether these 2 steps can be combined at a single surgery or should be staged, with correction of deformity being delayed until bony infection is resolved.
Correction of deformity with the goal of allowing walking with commercially-available therapeutic footwear.
Correction of Deformity
Timing of surgery is very controversial. Historically, correction of deformity was delayed until the active pathologic process resolved. This was based on expert opinion. Recently, more experts perform surgery during the active process based on the poor prognosis observed with increased deformity.
Virtually all of the patients who develop deformity have sensory peripheral neuropathy. What is not appreciated is that they also have a motor neuropathy with motor imbalance, as the peripheral neuropathy affects the small nerves, and muscles, earlier than the large nerves, and muscles. All of the patients with midfoot deformity have either a static or dynamic equinus deformity. Thus, the first step in surgery is either a percutaneous Achilles tendon lengthening or a gastrocnemius muscle lengthening.
The deformity is generally corrected by resecting a wedge of bone at the apex of the deformity. In the typical valgus deformity, the incision will be medial and the wedge will be larger medial and plantar. In varus deformities, the incision will be lateral and/or plantar, with the wedge being larger lateral and plantar. Once the deformity is corrected, the correction can be maintained by internal or external fixation (Figure 3).
Figure 3.
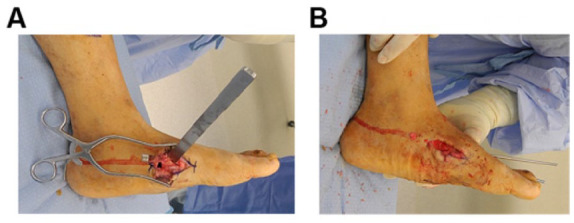
(A) Surgical correction of deformity: A wedge of bone is removed from the apex of the deformity. This wedge is created by making an osteotomy perpendicular to the axis of the proximal segment, and perpendicular to the axis of the distal segment. The resulting wedge will be larger medial and plantar. (B) When removed, the alignment of the forefoot becomes colinear with hindfoot, thus reestablishing a clinically plantigrade foot (B).
Intramedullary Beaming
The most popular method of internal fixation is accomplished with large intramedullary screws or bolts. Long solid or cannulated bolts or screws are typically placed retrograde through the first and other metatarsals, bridging the medial column to the talus with the option of bridging laterally as well. The biomechanics of this construct appear to parallel the comparison of plating as compared with intramedullary nailing for long bone fractures. This technique should not be used in the presence of infection. When infection is present, the infection should be resolved before addressing the internal fixation. Advantages include restoration of sagittal and axial plane alignment, minimally invasive application, intraosseous hardware, compression for arthrodesis, and long-term stability 20 (Figure 4).
Figure 4.
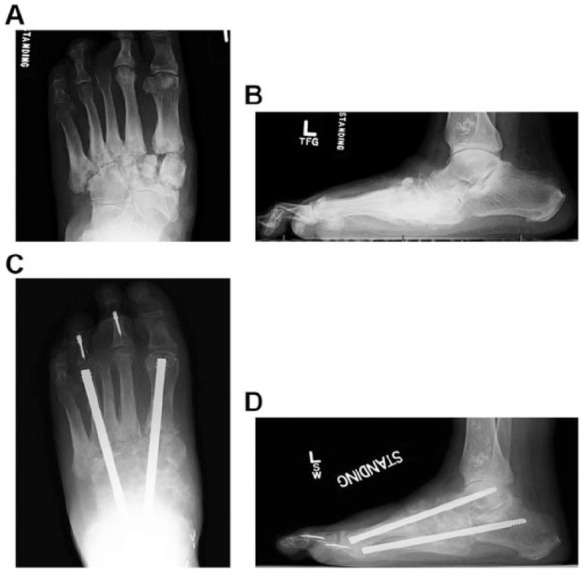
(A, B) Intramedullary beaming: Weight bearing radiographs of a patient with painful midtarsal Charcot foot arthropathy. Correction of the deformity is similar, irrespective of the technique used for maintenance of the correction. (C, D) Follow-up radiographs following correction of the deformity.
Screw-Plate Constructs
Schon initially reported on a plantar plating technique to maintain correction in this complex patient population. 21 Several of the device manufacturers have developed large medial column plates with large-thread “osteoporosis” screws. In spite of the availability of these devices, there is currently a limited role for the screw-plate construct as a primary method of stabilization. This is due to the high risk of infection as a result of the extensive soft tissue stripping required for these devices and the osteoporosis that impairs stability. The most common use of these devices is in “hybrid” fixation to augment intramedullary bolts or external fixation (Figures 5 and 6).
Figure 5.

(A) Medial plating: More invasive than intramedullary beaming, this technique has a high infection and failure rate due to the large medial implants used, necessary soft tissue dissection, and poor-quality bone. This patient underwent successful internal fixation with large threaded screws to accommodate the poor-quality bone. The surgery was complicated by postoperative wound infection that required surgical debridement, antibiotic suppression, and eventual removal of the implants. (B) Image and (C) radiograph following multiple surgical procedures.
Figure 6.
Failure following medial plating: (A) A 36-year-old 360-lb male diabetes patient is seen in photograph and (B) weight-bearing radiograph demonstrating clinical and radiographic nonplantigrade deformity. (C, D) Radiographs following successful correction of deformity. (E, F) In spite of being fully non-weight-bearing, radiographs at 6 weeks demonstrate loss of fixation. We were easily able to obtain correction of the deformity but were unable to maintain the correction because of poor-quality bone.
External Fixation
The most popular method of external fixation is accomplished with acute correction of the acquired deformity and immobilization with a 3-level static external fixator. Gradual correction of deformity is avoided in these patients because of the prolonged time to healing and cumbersome nature of this in patients who are generally morbidly obese. Following correction of the deformity, the frame is applied to maintain the correction.13,14 A construct of all tensioned fine wires is used to avoid the risk for tibial stress fracture that can occur if the tibial ring is secured with half pins (Figure 7).
Figure 7.
External fixation with 3-level static circular external fixation: (A) The frame is prebuilt and sterilized. (B) With the foot centered in the closed foot ring, 2 olive wires are passed from opposite directions through the calcaneus at a 30-degree angle to each other and parallel to the weight-bearing surface of the foot. (C) Two olive wires are then passed from opposite directions through the metatarsals, also at a 30-degree angle to each other and parallel to the weight-bearing surface of the foot. The wires are attached one hole posterior to where they lie, in order to compress the forefoot to the hindfoot (arch wire technique). (D) Olive wires are then passed through the tibia at the level of the proximal and middle rings at a 60-degree angle to each other. The wires are drilled through the bone and then tapped through the soft tissues to avoid neurovascular injury. (E) A modified “frame shoe” is applied to allow the patient to partial weight-bear for transfers.
Fine wire pin breakage can be avoided in larger patients by placing a third forefoot wire and a third proximal wire to decrease the bending moments at the extremes of the fixation construct. Pin tract infection is common. It is treated with relief of the skin tension at the infected pin site and oral first-generation cephalosporin antibiotics. It is extremely rare that an infected wire needs to be removed until it is time for removal of the entire frame. Fine wires are rarely replaced during treatment, even in the presence of infection, as the infection virtually always resolves following removal of the tensioned fine wire and frame.
Postoperative Management
Postoperative management following either internal or external fixation will vary with the method of surgical stabilization and the experience of the operating surgeon. Ability to weight-bear will vary with the size of the patient and the security of the fixation method. Transition to weight bearing with decreasing amounts of immobilization will also depend on the comfort of the surgeon with the technique employed.
Outcomes
Outcomes have generally been reported simply based on eradication of infection and limb salvage. With our current understanding of patient-reported outcomes, the goals for surgery additionally include the ability to ambulate in the community without the encumbrance of heavy accommodative orthotic devices. This goal is a therapeutic depth-inlay shoe with custom accommodative foot orthoses. This can generally be accomplished with correction of the deformity and bony healing. Although favorable clinical outcome appears to be associated with successful arthrodesis, some patients will be clinically successful in the presence of a stable pseudoarthrosis. Successful arthrodesis, however, leads to ambulation with less orthotic encumbrance resulting in more favorable clinical outcomes.
Amputation
Treatment complications and reconstructive failures leading to subsequent amputation are not uncommon. Amputation should also be considered as a primary reconstructive option in patients with severe deformity. The recovery from amputation can be prolonged even if the healing is uneventful in patients affected by morbid obesity, immune deficiency, and renal failure. Morbidly obese patients with renal failure may be difficult to fit with a transtibial prosthesis as a result of stump edema and fluctuating stump volume. Disarticulation at the ankle (Syme’s) or knee should be considered as it may allow an end-bearing amputation stump and the ability to use a volume-adaptable prosthetic socket to accommodate the volume fluctuation
Summary
There is growing interest in the surgical treatment of Charcot foot arthropathy. This review article focused on the most common presentation of Charcot foot at the midtarsal level. Historically, surgery was performed to eradicate infection and allow the use of accommodative bracing. In contrast, contemporary surgery aims to create a clinically and radiographically plantigrade foot, and to eliminate the painful “nonunion” of the Charcot process. Surgical correction consists of soft tissue release, midfoot closing wedge osteotomy, and maintenance of correction with internal and/or external fixation.
Footnotes
This article was originally published as: Pinzur MS. An evidence-based introduction to Charcot foot arthropathy. Foot Ankle Orthop. 2018 Jul 27;3(3):2473011418774269. doi:10.1177/2473011418774269.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Michael S. Pinzur, MD, reports personal fees from Stryker, during the conduct of the study. ICMJE form for author is available online.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. CDC. Facts sheets. https://www.cdc.gov/diabetes/library/factsheets.html. Accessed July 20, 2017.
- 2. Bevan WP, Tomlinson MP. Radiographic measure as a predictor of ulcer formation in diabetic Charcot midfoot. Foot Ankle Int. 2008;29(6):568–573. [DOI] [PubMed] [Google Scholar]
- 3. deSouza L. Charcot arthropathy and immobilization in a weight-bearing total contact cast. J Bone Joint Surg Am. 2008;90(4):754–759. [DOI] [PubMed] [Google Scholar]
- 4. Dhawan V, Spratt KF, Pinzur MS, et al. Reliability of AOFAS diabetic foot questionnaire in Charcot arthropathy: stability, internal consistency, and measurable difference. Foot Ankle Int. 2005;26(9):717–731. [DOI] [PubMed] [Google Scholar]
- 5. Eichenholtz SN. Charcot Joints. Springfield, IL: C.C. Thomas; 1966. [Google Scholar]
- 6. Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23(6):796–800. [DOI] [PubMed] [Google Scholar]
- 7. Game FL, Catlow R, Jones GR, et al. Audit of acute Charcot’s disease in the UK: the CDUK study. Diabetologia. 2012;55(1):32–35. [DOI] [PubMed] [Google Scholar]
- 8. Gouveri E, Papanas N. Charcot osteoarthropathy in diabetes: a brief review with an emphasis on clinical practice. World J Diabetes. 2011;2(5):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson JE. Surgical treatment for neuropathic arthropathy of the foot and ankle. Instr Course Lect. 1999;48:269–277. [PubMed] [Google Scholar]
- 10. Kroin E, Schiff AP, Pinzur MS, Davis ES, Chaharbakhshi E, DiSilvio FA, Jr. Functional impairment of patients undergoing surgical correction for Charcot foot arthropathy. Foot Ankle Int. 2017;38(7):705–709. [DOI] [PubMed] [Google Scholar]
- 11. Pinzur MS. Current concepts review: Charcot arthropathy of the foot and ankle. Foot Ankle Int. 2007;28(8):952–959. [DOI] [PubMed] [Google Scholar]
- 12. Pinzur MS. Surgical vs. accommodative treatment for Charcot arthropathy of the midfoot. Foot Ankle Int. 2004;25(8):545–549. [DOI] [PubMed] [Google Scholar]
- 13. Pinzur MS. Neutral ring fixation for high-risk nonplantigrade Charcot midfoot deformity. Foot Ankle Int. 2007;28(9):961–966. [DOI] [PubMed] [Google Scholar]
- 14. Pinzur MS, Gil J, Belmares J. Treatment of osteomyelitis in Charcot foot with single stage resection of infection, correction of deformity and maintenance with ring fixation. Foot Ankle Int. 2012;33(12):1069–1074. [DOI] [PubMed] [Google Scholar]
- 15. Pinzur MS, Kernan-Schroeder D, Emmanuele NV, Emmanuele MA. Development of a nurse-provided health system strategy for diabetic foot care. Foot Ankle Int. 2001;22(9):744–746. [DOI] [PubMed] [Google Scholar]
- 16. Pinzur MS, Lio T, Posner M. Treatment of Eichenholtz stage I Charcot foot arthropathy with a weight bearing total contact cast. Foot Ankle Int. 2006;27(5):324–329. [DOI] [PubMed] [Google Scholar]
- 17. Pinzur MS, Sage R, Stuck R, Kaminsky S, Zmuda A. A treatment algorithm for neuropathic (Charcot) midfoot deformity. Foot Ankle. 1993;14(4):189–197. [DOI] [PubMed] [Google Scholar]
- 18. Raspovic KR, Wukich DK. Self-reported quality of life in patients with diabetes: a comparison of patients with and without Charcot neuroarthropathy. Foot Ankle Int. 2014;35(3):195–200. [DOI] [PubMed] [Google Scholar]
- 19. Rogers LC, Frykberg RG, Armstrong DG, et al. The diabetic Charcot foot syndrome: a report of the joint task force on the Charcot foot by the American Diabetes Association and the American Podiatric Medical Association. Diabetes Care. 2011;34:2123–2129.21868781 [Google Scholar]
- 20. Sammarco VJ, Sammarco GJ, Walker EW, Guiao RP. Midtarsal arthrodesis in treatment of Charcot midfoot arthropathy. J Bone Joint Surg Am. 2009;91(1):80–91. [DOI] [PubMed] [Google Scholar]
- 21. Schon LC, Easley ME, Weinfeld SB. Charcot neuroarthropathy of the foot and ankle. Clin Orthop. 1998;349:116–131. [DOI] [PubMed] [Google Scholar]
- 22. Simon SR, Tejwani SG, Wilson DL, Santner TJ, Denniston NL. Arthrodesis as an early alternative to nonoperative management of Charcot arthropathy of the diabetic foot. J. Bone Joint Surg Am. 2000;82(7):939–950. [DOI] [PubMed] [Google Scholar]
- 23. Strotman P Reif TJ, Pinzur MS. Current concepts: Charcot arthropathy of the foot and ankle. Foot Ankle Int. 2016;37(11):1255–1263. [DOI] [PubMed] [Google Scholar]




