Abstract
Sendai virus (SV) infection and replication lead to a strong cytopathic effect with subsequent death of host cells. We now show that SV infection triggers an apoptotic program in target cells. Incubation of infected cells with the peptide inhibitor z-VAD-fmk abrogated SV-induced apoptosis, indicating that proteases of the caspase family were involved. Moreover, proteolytic activation of two distinct caspases, CPP32/caspase-3 and, as shown for the first time in virus-infected cells, FLICE/caspase-8, could be detected. So far, activation of FLICE/caspase-8 has been described in apoptosis triggered by death receptors, including CD95 and tumor necrosis factor (TNF)-R1. In contrast, we could show that SV-induced apoptosis did not require TNF or CD95 ligand. We further found that apoptosis of infected cells did not influence the maturation and budding of SV progeny. In conclusion, SV-induced cell injury is mediated by CD95- and TNF-R1-independent activation of caspases, leading to the death of host cells without impairment of the viral life cycle.
Over the past few years, a growing number of viruses have been found to induce apoptosis in host cells (41, 49). For some of them, mechanisms involved in the initial activation of the apoptotic death cascade have been discovered, such as upregulation of the CD95/Fas receptor by influenza virus (48), upregulation of CD95L/Fas ligand (3, 53), and cleavage of the apoptosis-inhibiting proto-oncogene bcl-2 (46), as well as downregulation of bcl-2 in combination with upregulation of the apoptosis accelerator Bax (39) by human immunodeficiency virus (HIV) and accumulation of p53 in host cells, e.g., during infection by adenovirus, simian virus 40, or human papillomavirus (reviewed in reference 49). However, little is known concerning the effector phase of apoptosis in virus-infected cells. Indirect evidence gained by pharmacological-inhibition experiments or by the cleavage of specific substrates suggested the involvement of caspases without defining how many and which caspases are required to cause suicide of infected host cells (8, 21, 37, 52).
Apoptosis is defined as an active physiological process of cellular self-destruction, with specific morphological and biochemical changes (45). The molecular processes controlling and executing virus-induced apoptosis are still poorly understood. Caspases, a family of cysteine proteases formerly called ICE (interleukin-1β-converting enzyme)-like proteases, play a central role in the execution of the apoptotic process (11, 32). These proteases are synthesized as inactive proenzymes and activated after cleavage at specific aspartate residues. Ten homologs of human origin have been identified, including ICE/caspase-1 (54), CPP32/caspase-3 (16, 31, 50), and FLICE/caspase-8 (29). The last is thought to be the most apical member of the death receptor-mediated pathways, being capable of triggering the processing of other executioner caspases in the apoptotic cascade (30).
As a prototype member of the paramyxovirus family, Sendai virus (SV) is an enveloped negative-strand RNA virus. It is closely related to human parainfluenza viruses and causes acute respiratory tract infections in rodents, such as mice and rats (12). Infection and replication of SV in host cells lead to an extensive cytopathic effect, with subsequent cell death, but the mechanisms of cell injury are poorly understood (12). Previous findings with human peripheral blood mononuclear cells suggested that apoptosis induction might be one mechanism of SV-induced cell death (51). However, peripheral blood mononuclear cells are not typical SV host or propagation cells (12) and apoptosis induction has so far not been linked to SV replication and propagation in other cell types. In addition, there are no data available on the possible mechanisms of SV-induced apoptosis.
Here we show that the strong cytopathic effect after SV infection of target cells can be attributed to apoptotic cell death. Moreover, we show that caspases play a key role in the effector phase of SV-induced cell death and we demonstrate the activation of CPP32/caspase-3 and FLICE/caspase-8. Interestingly, FLICE/caspase-8 activation in infected host cells did not require ligand-induced activation of death receptors, such as CD95 and tumor necrosis factor (TNF)-R1. We further found that apoptosis did not influence the maturation and budding of SV progeny. Thus, our results suggest that the SV-induced cytopathic effect involves CD95- and TNF-R1-independent activation of caspases, which results in apoptosis without affecting the viral life cycle.
MATERIALS AND METHODS
Reagents.
Recombinant human CD95L was expressed in stably transfected 293 cells as soluble Flag-tagged fusion protein and purified by affinity chromatography (unpublished data). TNF-α was purchased from Genzyme, Cambridge, Mass. Chimeric receptor decoy proteins consisting of the extracellular part of CD95 (7) or TNF-R1 (13) fused to immunoglobulin G1 (IgG1)-Fc were kindly provided by Immunex, Seattle, Wash.
Virus and cells.
SV (strain Fushimi) was grown in 9-day-old embryonated chicken eggs as described previously (43). CV-1 (African green monkey kidney) cells were obtained from the American Type Culture Collection (Rockville, Md.), and HepG2 (human hepatoma) cells were obtained from the European Collection of Animal Cell Cultures (Salisbury, United Kingdom). CV-1 cells were maintained in M199 medium, and HepG2 cells were maintained in a HEPES-buffered mixture of minimal essential medium and Dulbecco modified Eagle medium (4:1) containing 4.5 g of glucose/liter, sodium pyruvate, nonessential amino acids, and biotin, all supplemented with 10% fetal calf serum (FCS). Media and supplements were purchased from Life Technologies (Eggenstein, Germany).
Infection of cells.
For infection, cells were used when monolayers had reached 85 to 90% confluence in 35-mm-diameter dishes. As standard inoculation procedure, monolayers were washed twice with medium lacking FCS (washing medium) and overlaid with phosphate-buffered saline (PBS) containing SV at a multiplicity of infection (MOI) of 10. After incubation for 15 min at 37°C, unadsorbed virus was removed by repeated washing of the cells. Medium containing FCS (growth medium) was added, and the cells were incubated for various periods of time at 37°C.
DNA fragmentation assay.
Cells (5 × 107) were collected together with the floating cells in the supernatant at different time intervals postinfection (p.i.), and fragmentation assays were performed as described previously (20). In brief, the cells were washed once in PBS and lysed in 600 μl of DNA lysis buffer (Tris-HCl, pH 7.5, 0.2% Triton X-100, 10 mM EDTA) on ice for 10 min. Cell debris was removed by centrifugation (10 min; 13,000 × g; 4°C), and the supernatants were extracted once with phenol-choloroform–isoamyl alcohol (24:1). Total DNA was precipitated by the addition of 5 M NaCl to a final concentration of 300 mM in the presence of isopropanol, followed by incubation overnight at −20°C. Nucleic acids were pelleted at 12,000 × g (15 min; 0°C), resuspended in 15 μl of Tris-EDTA buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA), and incubated with 1 mg of RNase A (Boehringer Mannheim, Mannheim, Germany)/ml for 30 min. The nucleic acids were electrophoresed through 2% agarose gels (Gibco BRL, Eggenstein, Germany) and stained with ethidium bromide.
In situ-apoptosis assay.
The in situ-cell death detection kit AP (Boehringer Mannheim) was used to detect free 3′ OH ends of fragmented DNA. Terminal deoxynucleotidyltransferase (TDT) catalyzes the polymerization of fluorescein-labeled dUTP in a template-independent manner, labeling ends of fragmented DNA in situ. Subsequently, incorporated fluorescein was detected by alkaline phosphatase-conjugated anti-fluorescein antibody Fab2 fragments, resulting in an intense dark-blue staining of apoptotic cells.
Flow cytometry.
Fragmentation of genomic DNA to hypodiploid DNA was assessed by fluorescence-activated cell sorter (FACS) analysis according to the method described previously (33). In brief, 5 × 106 cells (including floating cells) were collected and washed once in PBS (5 min; 1,000 × g). Pellets were resuspended in 100 μl of PBS, fixed with 1 ml of acetone-methanol (1:1; −20°C), and subsequently washed with PBS. Next, each pellet was resuspended in 400 μl of PBS containing 1 mg of RNase/ml and incubated on ice for 1 h. After the addition of 20 μl of propidium iodide solution (2 mg/ml in PBS; Sigma, Deisenhofen, Germany) and incubation for at least 30 min on ice, flow cytometry was performed (FACS Calibur; Becton Dickinson, Heidelberg, Germany) by using the CellQuest program. Cells to the left of the 2 N peak contained hypodiploid DNA and were therefore considered apoptotic.
Western blot assay.
To detect proteolytic processing of caspases, Western blot assays were done as described previously (25). In brief, 5 × 107 cells were harvested, washed once in PBS, and resuspended in 0.5 ml of lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.5). From each sample 30 μg of cellular protein was electrophoretically separated on sodium dodecyl sulfate–10% polyacrylamide gels under reducing conditions and subsequently transferred to polyvinylidene difluoride membranes (Pall Fluorotrans transfer membrane; Pall Europe, Portsmouth, England). The membranes were blocked in Tris-buffered saline (150 mM NaCl, 13 mM Tris, pH 7.5) containing 5% nonfat dry milk powder for 1 h. Next, the membranes were incubated with the appropriate antibodies (anti-FLICE [Biomedia, Baesweiler, Germany], 1:10; rabbit anti-CPP32 [kindly provided by P. Vandenabeele, University of Ghent, Belgium], 1:1,000; F37720 anti-FasL [Transduction Laboratories, Lexington, Ky.], 1:1,000; and anti-alpha-tubulin [Sigma], 1:2,000) overnight at 20°C, washed three times with TBS-T (TBS containing 0.02% Triton X-100), and incubated with peroxidase-conjugated anti-mouse or anti-rabbit IgG (Amersham-Buchler, Braunschweig, Germany) (1:1,000). Further detection was performed by the ECL Western blotting detection system on Hyperfilm-ECL (Amersham-Buchler). Control cells were incubated with recombinant human CD95L containing supernatant for 12 h and harvested as described above.
Inhibition experiments.
Apoptosis was blocked by addition of the peptide inhibitor z-VAD-fmk [benzoyloxycarbonyl-Val-Ala-Asp (Ome) fluoromethylketone; Enzyme Systems, Dublin, Calif.] (100 μM) every 12 h to the culture supernatant. The supernatants were analyzed by hemagglutination (HA) and 50% tissue culture infectivity dose (TCID50) assays, and the cells were analyzed by flow cytometry.
For inhibition experiments of receptor pathways, 5 × 106 cells were infected with SV (MOI, 10) in 35-mm-diameter dishes. Immediately after infection, chimeric receptor decoy proteins consisting of the extracellular part of either CD95 or TNF-R1 fused to IgG1-Fc were added in a final concentration of 10 or 50 μg/ml. Control experiments in our setting revealed a complete blockage of TNF- and CD95L-induced apoptosis at concentrations of 5 to 20 μg of the decoy constructs/ml (data not shown and reference 7). Forty hours p.i., the cells were harvested and analyzed by FACS as described above.
HA and TCID50 assays.
Virus yield was quantitated by the HA assay, and infectivity of progeny virions was quantitated by the TCID50 assay (TCID50/ml) with supernatants of infected cells, as described previously (5). For the TCID50 assay, FCS was replaced in the growth medium by Nutridoma SR or CS (Boehringer Mannheim) to enable cleavage of the F0 precursor protein by acetylated trypsin prior to the assay, as described previously (5, 24). In our setting, 1 TCID50/ml was equivalent to 5,000 PFU/ml.
RESULTS
SV infection triggers the apoptosis death cascade.
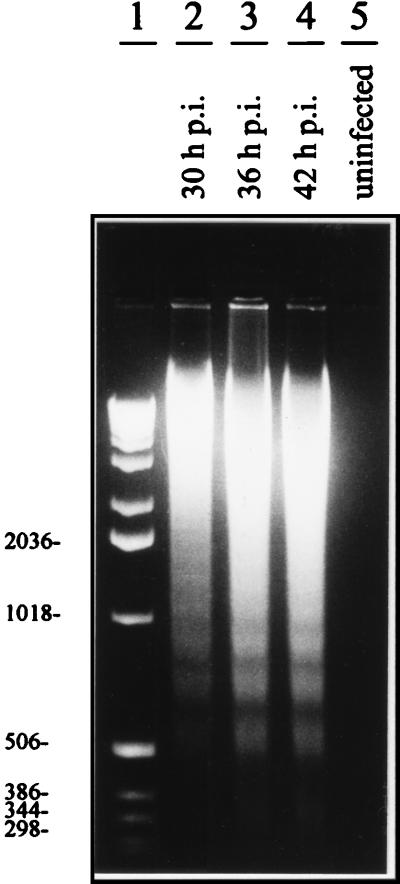
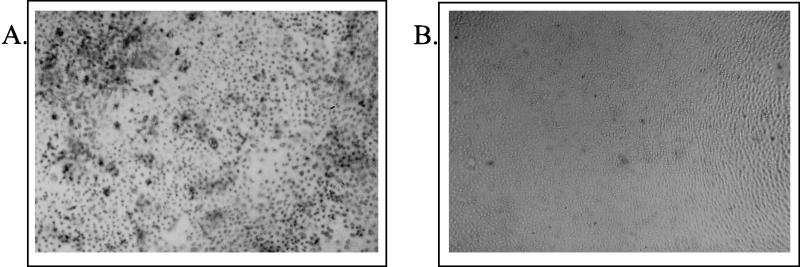
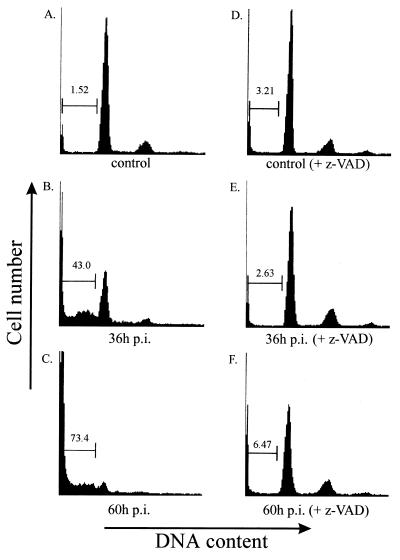
Monkey kidney CV-1 cells infected with SV (MOI, 10) were found to exhibit an extensive cytopathic effect 15 to 24 h p.i., which was morphologically characterized by cell shrinkage, condensation of nuclear chromatin, and cell fragmentation (data not shown). As these alterations were classical signs of apoptotic cell death, we isolated cellular DNA to investigate DNA fragmentation. Starting at 24 to 30 h p.i. (MOI, 10), characteristic DNA fragmentation patterns with oligonucleosomal fragments of 180 to 200 bp and multiples thereof were detected in CV-1 cells (Fig. 1). In contrast, noninfected cells revealed no specific DNA signal, as intact high-molecular-mass DNA was removed during the isolation protocol (Fig. 1, lane 5). To further examine nuclear DNA fragmentation, we used the TDT-mediated dUTP-biotin nick end labeling TUNEL assay (19), in which TDT is employed to label the ends of fragmented DNA in situ with fluorescein-labeled dUTP. Detection of the label by using alkaline phosphatase-conjugated antibodies to the fluorescein-labeled nucleotide generates a dark-blue color whose intensity is proportional to the number of 3′ ends or fragments of nuclear DNA. Intense dark staining was detected in SV-infected CV-1 cells 24 h p.i. (MOI, 10), whereas no staining was seen in uninfected controls (Fig. 2). Figure 3B and C shows rates of apoptosis 36 and 60 h after SV infection of CV-1 cells, determined by the appearance of hypodiploid DNA peaks after propidium iodide staining and flow cytometry analysis. Taken together, these results clearly demonstrate that SV induces apoptotic cell death in CV-1 cells. Corresponding results were obtained for other cell lines, such as HepG2, MDCK, NIH 3T3, HeLa, and 293 cells (data not shown). Thus, apoptotic cell death can be regarded as a general process during SV infection of host cells.
FIG. 1.
DNA fragmentation induced by SV infection of CV-1 cells. Lanes 2 to 4, DNA preparations from productively infected cells at different time points p.i.; lane 1, DNA marker; lane 5, preparation from uninfected control cells.
FIG. 2.
In situ detection of apoptosis in infected CV-1 cells. Apoptotic DNA degradation was visualized by TUNEL staining and subsequent alkaline phosphatase staining as described in Materials and Methods. (A) Intense dark staining of CV-1 cells 24 h p.i.; (B) uninfected CV-1 cells.
FIG. 3.
Detection of hypodiploid DNA in SV-infected cells and inhibition by caspase inhibitor z-VAD. CV-1 cells were infected by SV (MOI, 10) and incubated with 0 (A, B, and C) or 100 (D, E, and F) μM of the caspase inhibitor z-VAD-fmk. Shown are the results of cytometric analysis at 36 and 60 h p.i. as well as the analysis of uninfected CV-1 cells (control) and CV-1 cells incubated with z-VAD-fmk for 60 h [control (+ z-VAD)] to exclude the toxic effects of z-VAD-fmk on these cells. The proportion of sub-2N DNA is indicated in the histograms.
Caspase inhibition blocks SV-induced apoptosis.
It has become clear that the effector phase during apoptotic cell death requires the activation of different caspases (11, 27, 32). We therefore investigated the effect of a broad caspase inhibitor, z-VAD-fmk [benzoyloxycarbonyl-Val-Ala-Asp (Ome) fluoromethylketone] (10, 22, 44), on apoptosis induction in SV-infected host cells. Addition of 100 μM z-VAD-fmk to the supernatant of SV-infected CV-1 cells almost completely inhibited apoptosis, as assessed by a lack of formation of hypodiploid DNA 36 or 60 h p.i. (Fig. 3E and F). These results imply that the activation of caspases is a central mechanism in SV-induced cell death.
Detection of activation of individual caspases.
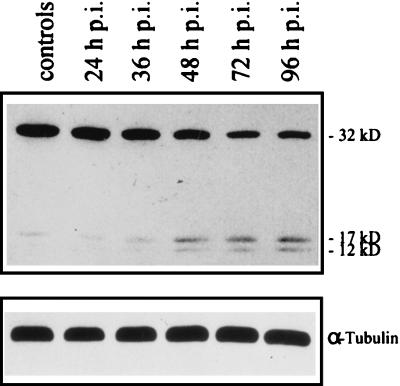
To further define the relevant steps employed in the SV-triggered apoptotic signal transduction pathway, we investigated the activation of individual caspases during SV infection. The cellular protease CPP32/caspase-3 is expressed as a 32-kDa precursor protein, which upon activation is processed into p17 and p12 subunits (31). Earlier work implicated CPP32/caspase-3 as a central executioner protease in mammalian apoptosis. Moreover, sequential activation of CPP32/caspase-3 by other members of the caspase family has been described previously (15, 26, 30, 44). To investigate the involvement of CPP32/caspase-3 in SV-induced apoptosis, cell lysates from infected and noninfected cells were subjected to Western blotting with antibodies directed to CPP32. Proteolytic processing of the 32-kDa precursor in infected cells could be demonstrated by a diminished immunoreactive signal and subsequent detection of the cleavage products p17 and p12 in HepG2 cells (Fig. 4). Corresponding results were obtained for CV-1 cells (data not shown).
FIG. 4.
SV infection triggers CPP32/caspase-3 activation. HepG2 cells were infected with SV (MOI, 10) and were prepared at the indicated time points p.i. Lane 1, preparation of uninfected control cells. The CPP32 proform and subunits p17 and p12 as well as tubulin were detected by Western blot analysis.
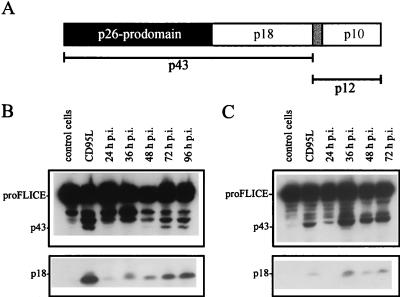
Recently, a novel member of the caspase family, designated FLICE/caspase-8, has been shown to directly cleave CPP32/caspase-3 (30). FLICE/caspase-8 is thought to represent the most upstream protease in the CD95-mediated apoptotic cascade, as it is recruited to the CD95 receptor through the adaptor protein FADD (Fas-associating protein with death domain) (6, 29). Activation of the 55-kDa proFLICE molecule proceeds by a two-step mechanism (Fig. 5A): first, p43 and p12 intermediate cleavage products are generated, which are further processed to the p18 and p10 active subunits (28). We investigated proteolytic activation of FLICE/caspase-8 by using an antibody directed against the p18 subunit. As a positive control, cells treated with recombinant CD95L (10 ng/ml) were included, showing the processing of proFLICE to an intermediate of about 43 kDa and the p18 active subunit (positive control). SV infection resulted in a similar cleavage pattern 24 to 36 h p.i. in both HepG2 and CV-1 cells (Fig. 5B and C). Thus, FLICE/caspase-8 was proteolytically activated during SV infection.
FIG. 5.
FLICE/caspase-8 cleavage in SV-infected host cells. (A) FLICE/caspase-8 cleavage products according to Medema et al. (28); initial cleavage generates p43 and p12 intermediates, followed by further processing to the active p18 and p10 subunits and the FLICE prodomain p26. (B and C) HepG2 (B) and CV-1 (C) cells were infected with SV (MOI, 10), and FLICE and cleavage products p43 and p18 were detected by Western blot analysis at the indicated times p.i. Lanes 1 and 2, preparations from uninfected cells (control cells) and, as a positive control, from uninfected cells incubated with CD95L, respectively.
SV-induced apoptosis does not require activation of TNF-R1 or CD95 by their cognate ligands.
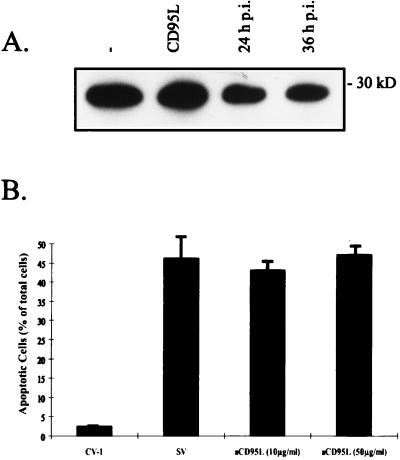
So far, proteolytic activation of FLICE/caspase-8 has been demonstrated only during apoptosis mediated by the death receptors CD95 and TNF-R1 (6, 29). Following CD95 ligation, the adaptor protein FADD and FLICE/caspase-8 are recruited to the receptor and form the so-called death-inducing signaling complex (DISC) (23). Biochemical analysis further revealed that upon receptor ligation the whole cellular amount of FLICE/caspase-8 is processed into the active subunits at the DISC level (26). We therefore investigated whether SV-induced apoptosis involved the CD95 or TNF-R1 pathway, which could be mediated by induction of ligand expression and subsequent receptor ligation. First, using a Western blot assay, we looked at the expression of CD95L in SV-infected CV-1 cells (MOI, 10) in comparison to the expression in uninfected controls. Unexpectedly, we detected a strong signal for CD95L in uninfected CV-1 cells, showing an endogeneous expression of CD95L without signs of apoptosis in these cells (Fig. 6A). By comparing infected cells to uninfected cells at 24 or 36 h p.i., we could detect a slight decrease in CD95L expression. This indicates that FLICE/caspase-8 activation in SV-infected cells was presumably not associated with CD95L upregulation. To further exclude the involvement of receptor-ligand interaction, we incubated CV-1 cells postinfection with neutralizing chimeric receptor decoy proteins consisting of the extracellular part of CD95 fused to IgG1-Fc (7). CD95L-induced apoptosis of CV-1 cells could be blocked in our setting at a concentration of 5 to 10 μg of the decoy proteins/ml (data not shown), as has been shown previously for other cell lines (7). In contrast, as shown in Fig. 6B, SV-induced apoptosis was not affected, even when up to 50 μg of the decoy proteins/ml was employed. Furthermore, as CV-1 cells were not sensitive to TNF-α (10 ng/ml), and corresponding neutralizing TNF-R1 decoy proteins in the supernatant did not influence SV-induced apoptosis in CV-1 cells (data not shown), we therefore conclude that activation of CD95 or TNF-R1 by its cognate ligand does not participate in SV-induced activation of FLICE/caspase-8 leading to apoptotic cell death.
FIG. 6.
CD95L expression in host cells is not responsible for SV-induced apoptosis. (A) CV-1 cells were infected with SV (MOI, 10), and FasL was detected by Western blot analysis at 24 and 36 h p.i. Lanes 1 and 2, preparations from uninfected controls (−) and from cells incubated with CD95L (at 12 h), respectively. (B) After infection with SV (MOI, 10), CV-1 cells were incubated with chimeric receptor decoy proteins consisting of the extracellular part of CD95 (aCD95L) fused to IgG-Fc. At 48 h p.i., the cells were analyzed by flow cytometry (propidium iodide staining). Cells with subgenomic DNA content were considered apoptotic. The bar labeled CV-1 indicates the results of the analysis of uninfected controls, and the bar labeled SV represents the results of the analysis of infected cells without chimeric receptor decoy proteins in the supernatant. In our setting concentrations of 5 to 10 μg of aCD95L/ml in the supernatant blocked CD95L-induced apoptosis completely. The error bars represent standard deviations.
Release and infectivity of virus progeny do not depend on host cell apoptosis.
Two diametrically opposed hypotheses of the role of apoptosis in viral infections have been proposed: apoptosis as a host antiviral defense mechanism versus apoptosis as a pathogen-mediated mechanism to enhance viral replication, induce immune dysregulation, and promote persistent infection. To shed further light on the role of apoptosis in SV infection, we determined virus progeny release in the presence or absence of the caspase inhibitor z-VAD-fmk by a HA assay of culture supernatants (HA units per 5 × 106 cells). These infection studies revealed that apoptosis in host cells was not necessary for efficient virus progeny release (Table 1). In HepG2 cells apoptosis inhibition even seemed to enhance viral replication. The twofold difference observed in HepG2 cells, however, represents only one dilution step and thus may not be significant (Table 1). A mere quantitation of SV progeny virions cannot provide information on the functionality, i.e., infectivity, of such particles. To investigate the functional importance of apoptosis induction for SV particle maturation, the TCID50 of progeny virions was determined. Surprisingly, the calculated ratio of TCID50 per HA unit (TCID50/HA) was found to be independent of apoptosis induction in host cells (Table 1). From these data we conclude that apoptotic death of host cells is not necessary for efficient SV replication or particle maturation.
TABLE 1.
Effect of caspase activation on virus progeny release and infectivitya
| Cell type | z-VADb | HA units/5 × 106 cellsc
|
TCID50d | TCID50/ HA unit | ||
|---|---|---|---|---|---|---|
| 20 h | 24 h | 36 h | ||||
| CV-1 | − | 160 | 640 | 2,560 | 640 | 1 |
| + | 320 | 640 | 1,280 | 640 | 1 | |
| HepG2 | − | 160 | 640 | 5,120 | 320 | 0.5 |
| + | 1,280 | 1,280 | 10,240 | 320 | 0.25 | |
Results are representative of three independent experiments.
The peptide inhibitor z-VAD-fmk (100 μM) was added to the supernatants after infection with SV (MOI, 10) and every 12 h thereafter. −, not present; +, present.
Determined at the indicated times p.i. with SV (MOI, 10).
Determined 24 h p.i. with SV (MOI, 10). Later time points p.i. were not considered due to the short half-life of infective virions in the supernatant of infected cells (11).
DISCUSSION
Mechanisms of virus-induced cell injury play an important role in our understanding of the pathogenesis of viral infections. In this study we show that SV infection leads to apoptotic cell death in all host cell types tested so far. The strong cytopathic effect which can be observed soon after SV infection can thus be attributed to the induction of a SV-triggered apoptotic cell death program. Interestingly, the SV leader region at the 3′ end of the viral RNA, which lies outside the protein coding region, has recently been suggested to influence this process; however, the underlying mechanisms remain to be determined (18).
Caspases play a central role in the effector phase during apoptotic cell death. To date, 10 caspases have been described (11, 32). Still, it is not clear how many different caspases have to be activated for the successful execution of an apoptosis program (27, 47). Furthermore, little is known about which individual caspases are activated during viral infections leading to apoptosis in host cells. The involvement of CPP32/caspase-3 cleavage in virus-infected cells has been shown recently for HIV (4), adenovirus (9), and hepatitis C virus (37). To further understand the role that apoptosis plays in viral infections, it seems to be crucial to define the virus-triggered steps of the apoptosis signal transduction cascade. In parallel, this would potentially open up new possibilities for therapeutic manipulation of these processes. In this context, it has been shown for HIV-1 that apoptosis inhibition by the broad-spectrum caspase inhibitor z-VAD-fmk could result in deleterious consequences for the infected host, such as enhanced viral replication or stimulation of endogenous virus production in cells derived from asymptomatic individuals (8).
In the present study we investigated the molecular mechanisms as well as the consequences of SV-induced apoptosis for virus propagation. Incubation of infected cells with the broad-spectrum caspase inhibitor z-VAD-fmk prevented apoptosis, indicating that caspases were involved in the cytopathic effect of SV. Furthermore, as demonstrated by the processing of its precursor, CPP32/caspase-3 was found to be activated upon SV infection. CPP32/caspase-3 is thought to be a critical executioner protease, because it is activated by a multitude of apoptosis stimuli and is able to cleave various cellular substrates (11, 32).
The most striking finding was the observation that, in addition to CPP32/caspase-3, FLICE/caspase-8 was also proteolytically processed to its active subunits. FLICE/caspase-8 is considered to be an important initiator caspase which is able to activate other caspases, among them caspases 3, 4, 6, and 7 (30). At present, activation of FLICE/caspase-8 has been demonstrated only during death receptor-mediated apoptosis, where it is recruited and activated at the DISC level. It is conceivable that activation of FLICE/caspase-8 during SV infection is mediated by receptor-dependent or -independent mechanisms. Because CD95L-neutralizing decoy proteins failed to prevent SV-induced apoptosis and CV-1 cells were not sensitive to TNF-induced apoptosis, it is unlikely that CD95 or TNF-R1 was involved. However, the possibility cannot be excluded that the TRAIL pathway participates in SV-induced cell death. The cytokine TRAIL (also called Apo2L), which belongs to the TNF family, has previously been reported to activate the caspase cascade by a FADD-independent mechanism (35, 42). We will therefore investigate the possible contribution of TRAIL as soon as the reagents are available.
New insights into the mechanisms of apoptosis were recently provided by the cloning of Apaf-1 (apoptosis-activating factor 1), the mammalian homolog of the ced-4 death gene from Caenorhabditis elegans (55). At its N terminus, Apaf-1 has sequence similarities to the prodomain of certain caspases. This region in Apaf-1 serves as a caspase recruitment domain (CARD) by binding to and activating caspases that have similar CARD motifs. Since FLICE/caspase-8 contains a CARD motif, it is possible that FLICE/caspase-8 is activated upon binding to Apaf-1. In such a scenario, FLICE/caspase-8 activation would not require interaction with the DISC of TNF-R1 or CD95. In support of this assumption, it was recently found that the chemotherapeutic agent betulinic acid triggers FLICE/caspase-8 activation independently of the CD95 pathway and probably of the TNF-R1 and TRAIL pathways (17). Thus, future studies will address the question of whether FLICE/caspase-8 is activated during SV infection by a death receptor-dependent or -independent mechanism. Taking the latter into account, ongoing work has to carefully investigate the role of FLICE/caspase-8 activation in the caspase death cascade of SV-infected host cells.
We further investigated the role of apoptosis in SV replication. Looking at virus progeny release, comparable amounts of virions were released from SV-infected cells incubated either with or without the caspase inhibitor z-VAD-fmk. This demonstrates that efficient SV replication does not depend on apoptosis induction. Therefore, apoptosis inhibition did not lead to dramatically enhanced viral replication, as was demonstrated for HIV (1, 8, 38), or to growth limitation, as shown, e.g., for Semliki Forest virus (40). Our results correspond to recent observations made with reovirus-infected cells, where blocking of apoptosis by bcl-2 did not change the virus yield, suggesting that apoptosis induction is not a major determinant of viral replication efficacy (36). Taken together, these studies show that there is no common role for the apoptosis process during different viral infections; rather it has to be determined individually for different virus species.
Inhibition of apoptosis by the proto-oncogene bcl-2 during influenza virus infection leads to an alteration in the glycosylation pattern of hemagglutinin at the viral surface (34). This hemagglutinin modification impaired infection activity of progeny virions, indicating that the apoptotic death of host cells is necessary for progeny maturation during influenza virus infection. To look at the influence of apoptosis on SV progeny maturation, we determined the infectivity of progeny in relation to the total number of progeny particles (TCID50 per HA unit) from cells undergoing apoptosis or being incubated with the apoptosis inhibitor z-VAD-fmk. As no differences in the infectivity of SV progeny were found, we have to conclude that neither SV replication nor maturation depends on apoptotic death of target cells. Thus, from an evolutionary perspective one could speculate that SV-triggered apoptosis might serve to allow the organism to restrict virus progeny release by eliminating infected cells and preventing the establishment of persistent infections.
ACKNOWLEDGMENTS
Michael Bitzer and Florian Prinz contributed equally to this work.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (BI 669/3-1), the fortüne-program of the Medical Faculty at Tübingen (184) and the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Programm Gesundheitsforschung 2000.
REFERENCES
- 1.Antoni B A, Sabbatini P, Rabson A B, White E. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol. 1995;69:2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragane Y, Kulms D, Metze D, Wilkes G, Pöppelmann B, Luger T A, Schwarz T. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently from its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banki K, Hutter E, Gonchoroff N J, Perl A. Molecular ordering in HIV-induced apoptosis—oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J Biol Chem. 1998;273:11944–11953. doi: 10.1074/jbc.273.19.11944. [DOI] [PubMed] [Google Scholar]
- 5.Bitzer M, Lauer U, Baumann C, Spiegel M, Gregor M, Neubert W J. Sendai virus efficiently infects cells via the asialoglycoprotein receptor and requires the presence of cleaved F0 precursor proteins for this alternative route of cell entry. J Virol. 1997;71:5481–5486. doi: 10.1128/jvi.71.7.5481-5486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 7.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, Green D R. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan A M, Woffendin C, Dixit V M, Nabel G J. The inhibition of pro-apoptotic ICE-like proteases enhances HIV replication. Nat Med. 1997;3:333–337. doi: 10.1038/nm0397-333. [DOI] [PubMed] [Google Scholar]
- 9.Chiou S K, White E. Inhibition of ICE-like proteases inhibits apoptosis and increases virus production during Adenovirus infection. Virology. 1998;244:108–118. doi: 10.1006/viro.1998.9077. [DOI] [PubMed] [Google Scholar]
- 10.Chow S C, Weis M, Kass G E, Holmstrom T H, Eriksson J E, Orrenius S. Involvement of multiple proteases during Fas-mediated apoptosis in T lymphocytes. FEBS Lett. 1995;364:134–138. doi: 10.1016/0014-5793(95)00370-o. [DOI] [PubMed] [Google Scholar]
- 11.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M E, editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 1205–1241. [Google Scholar]
- 13.Crowe P D, VanArsdale T L, Walter B N, Dahms K M, Ware C F. Production of lymphotoxin (LT alpha) and a soluble dimeric form of its receptor using the baculovirus expression system. J Immunol Methods. 1994;168:79–89. doi: 10.1016/0022-1759(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 14.Darlington R W, Portner A, Kingsbury D W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970;9:169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- 15.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes-Alnemri T, Litwack G, Alnemri E S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 17.Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nunez G, Krammer P H, Peter M E, Debatin K M. Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 1997;57:4956–4964. [PubMed] [Google Scholar]
- 18.Garcin D, Taylor G, Tanebayashi K, Compans R, Kolakofsky D. The short Sendai virus leader region controls induction of programmed cell death. Virology. 1998;243:340–353. doi: 10.1006/viro.1998.9063. [DOI] [PubMed] [Google Scholar]
- 19.Gavrieli Y, Sherman Y, Ben Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw V S, Olsen C W, Dybdahl Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoff H S, Donis R O. Induction of apoptosis and cleavage of poly(ADP-ribose) polymerase by cytopathic bovine viral diarrhea virus infection. Virus Res. 1997;49:101–113. doi: 10.1016/s0168-1702(97)01460-3. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen M D, Weil M, Raff M C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyrer S, Bitzer M, Lauer U, Kramer J, Neubert W J, Sedlmeier R. Sendai virus-like particles devoid of HN protein infect cells via the human asialoglycoprotein receptor. J Gen Virol. 1998;79:683–687. doi: 10.1099/0022-1317-79-4-683. [DOI] [PubMed] [Google Scholar]
- 25.Liang X H, Mungal S, Ayscue A, Meissner J D, Wodnicki P, Hockenbery D, Lockett S, Herman B. Bcl-2 protooncogene expression in cervical carcinoma cell lines containing inactive p53. J Cell Biochem. 1995;57:509–521. doi: 10.1002/jcb.240570316. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Kim C N, Pohl J, Wang X. Purification and characterization of an interleukin-1beta-converting enzyme family protease that activates cysteine protease P32 (CPP32) J Biol Chem. 1996;271:13371–13376. [PubMed] [Google Scholar]
- 27.MacFarlane M, Cain K, Sun X M, Alnemri E S, Cohen G M. Processing/activation of at least four interleukin-1beta converting enzyme-like proteases occurs during the execution phase of apoptosis in human monocytic tumor cells. J Cell Biol. 1997;137:469–479. doi: 10.1083/jcb.137.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 30.Muzio M, Salvesen G S, Dixit V M. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–280. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 34.Olsen C W, Kehren J C, Dybdahl-Sissoko N R, Hinshaw V S. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol. 1996;70:663–666. doi: 10.1128/jvi.70.1.663-666.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan G, Ni J, Wei Y-F, Yu G-L, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 36.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggieri A, Harada T, Matsuura Y, Miyamura T. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology. 1997;229:68–76. doi: 10.1006/viro.1996.8420. [DOI] [PubMed] [Google Scholar]
- 38.Sandstrom P A, Pardi D, Goldsmith C S, Chengying D, Diamond A M, Folks T M. bcl-2 expression facilitates human immunodeficiency virus type 1-mediated cytopathic effects during acute spreading infections. J Virol. 1996;70:4617–4622. doi: 10.1128/jvi.70.7.4617-4622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sastry K J, Marin M C, Nehete P N, McConnell K, el Naggar A K, McDonnell T J. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene. 1996;13:487–493. [PubMed] [Google Scholar]
- 40.Scallan M F, Allsopp T E, Fazakerley J K. bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol. 1997;71:1583–1590. doi: 10.1128/jvi.71.2.1583-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, Goddard A D, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 43.Sigmund M, Einberger H, Neubert W J. Simple method for rapid and highly sensitive detection of antiviral-antibodies in serum and cerebrospinal fluid of small laboratory animals. J Virol Methods. 1988;22:231–238. doi: 10.1016/0166-0934(88)90105-x. [DOI] [PubMed] [Google Scholar]
- 44.Slee E A, Zhu H, Chow S C, MacFarlane M, Nicholson D W, Cohen G M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD-FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 46.Strack P R, Frey M W, Rizzo C J, Cordova B, George H J, Meade R, Ho S P, Corman J, Tritch R, Korant B D. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci USA. 1996;93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi A, Earnshaw W C. ICE-related proteases in apoptosis. Curr Opin Genet Dev. 1996;6:50–55. doi: 10.1016/s0959-437x(96)90010-6. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa T, Fukuda R, Miyawaki T, Ohashi K, Nakanishi Y. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology. 1995;209:288–296. doi: 10.1006/viro.1995.1260. [DOI] [PubMed] [Google Scholar]
- 49.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 51.Tropea F, Troiano L, Monti D, Lovato E, Malorni W, Rainaldi G, Mattana P, Viscomi G, Ingletti M C, Portolani M, Ceremelli C, Cossarizza A, Franceschi C. Sendai virus and herpes virus type 1 induce apoptosis in human peripheral blood mononuclear cells. Exp Cell Res. 1995;218:63–70. doi: 10.1006/excr.1995.1131. [DOI] [PubMed] [Google Scholar]
- 52.Ubol S, Park S, Budihardjo I, Desnoyers S, Montrose M H, Poirier G G, Kaufmann S H, Griffin D E. Temporal changes in chromatin, intracellular calcium, and poly(ADP-ribose) polymerase during Sindbis virus-induced apoptosis of neuroblastoma cells. J Virol. 1996;70:2215–2220. doi: 10.1128/jvi.70.4.2215-2220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 54.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 55.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans ced-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]