Abstract
Background
Multiple sclerosis (MS) is a progressively debilitating neurologic disease that poses significant costs to the healthcare system and workforce.
Objective
To evaluate the impact of MS disease progression on societal costs and quality of life (QoL) using data from the German NeuroTransData (NTD) MS registry.
Methods
Cross-sectional cohort study. The cost cohort included patients with MS disability assessed using Expanded Disability Status Scale (EDSS) in 2019 while the QoL cohort included patients assessed using EDSS and EuroQol-5 Dimension 5-Levels between 2009 and 2019. Direct and indirect medical, and non-medical resource use was quantified and costs derived from public sources.
Results
Within the QoL cohort (n = 9821), QoL worsened with increasing EDSS. Within the cost cohort (n = 7286), increasing resource use with increasing EDSS was observed. Societal costs per patient, excluding or including disease-modifying therapies, increased from €5694 or €19,315 at EDSS 0 to 3.5 to €25,419 or €36,499 at EDSS 4 to 6.5, and €52,883 or €58,576 at EDSS 7 to 9.5. In multivariate modeling, each 0.5-step increase in EDSS was significantly associated with increasing costs, and worsening QoL.
Conclusion
This study confirms the major socioeconomic burden associated with MS disability progression. From a socioeconomic perspective, delaying disability progression may benefit patients and society.
Keywords: Multiple sclerosis, disease progression, economic burden of disease, quality of life
Introduction
Multiple sclerosis (MS) is a lifelong demyelinating disease of the central nervous system affecting approximately 2.8 million people worldwide in 2020, with a prevalence of 36 per 100,000 people globally, and 303 per 100,000 in Germany. 1 MS presents heterogeneously, and may include upper/lower extremity disabilities, emotional and cognitive disturbances, balance and coordination disruption, spasticity, abnormal speech, bladder and bowel problems, and fatigue.2,3 As MS progresses, myelin degradation and axonal loss lead to deterioration in nervous communication with the brain.2,4 Subsequently, patients experience progressive loss of motor and sensory functions.2,4 Although there is no cure for MS, symptoms may be managed with physiotherapy, social support, symptomatic medications, and disease-modifying therapies (DMTs).
The socioeconomic burden of MS extends beyond healthcare costs. MS poses not only a significant burden to patients and their families but also to wider society.5,6 MS disability severity is typically measured using the Expanded Disability Status Scale (EDSS), which is commonly used in MS randomized clinical trials.7,8 With disability progression, as reflected in higher EDSS, quality of life (QoL) decreases while socioeconomic costs increase. 9 In patients with MS (PwMS), increased disability correlates with decreased workforce participation.9,10 Costs associated with short- to long-term work absences increase with MS progression.11,12 Productivity costs, especially due to reduced employment, are enhanced by the early age of diagnosis. 13 Direct non-medical costs including care provided by family and professionals play a large role with increasing disability.9,10,14,15
Several survey-based studies have previously highlighted increasing socioeconomic costs and decreasing QoL associated with increased MS disability. 10 These studies have relied on patient recruitment and self-completion of questionnaires. In this cross-sectional, registry-based cohort study of PwMS in Germany, we evaluated the broad impact of disease progression on societal costs and patient QoL, using information routinely collected as part of the patient's standard of care within a registry.
Methods
Study design and population
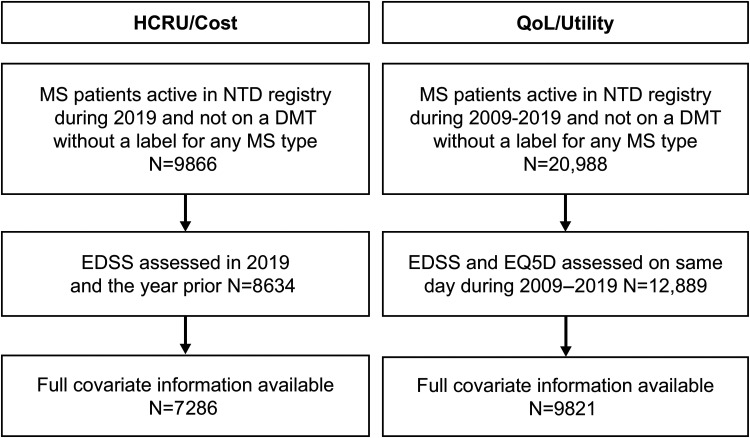
This cross-sectional cohort study of PwMS used routinely collected data from the NeuroTransData (NTD) MS registry. 16 All PwMS were eligible for inclusion. Two cohorts were evaluated. (1) The QoL cohort included data from PwMS who visited NTD clinics between 2009 and 2019. Eligible visits included EDSS and EuroQol-5 Dimension 5-Levels (EQ-5D-5L) assessments on the same day (defined as the index date). (2) The socioeconomic costs cohort (cost cohort) included data from PwMS with ≥1 visit to NTD clinics in 2019 during which EDSS was assessed (index date). This was the most recent year of full data availability at time of analysis and excluded the impact of COVID-19 restrictions on patient healthcare visits. Another visit 365 days prior to the index date with EDSS assessment was required to ensure adequate capture of patient characteristics and socioeconomic costs in that year.
For both populations, patients may be eligible for the study at multiple time points, and for each patient, only the most recent eligible time point was included.
Data source and setting
Data were retrieved from the NTD MS registry database provided by the NTD network, a Germany-wide network of neurologists and psychiatrists specialists founded in 2008. 16 We included patients from each of the 66 neurology and psychiatry clinics comprising the NTD network. The NTD MS registry database currently includes >22,000 PwMS with an average observation period of 5 years. The NTD MS registry routinely captures demographic, medical history, socioeconomic information, patient-reported outcomes, and clinical data during visits to NTD outpatient offices, which are performed on average of approximately 3.5 times per year. 17 A standardized dataset is collected, with the minimal dataset collected at each visit, while additional data are assessed depending on medical need. All data are pseudonymized and pooled to form the NTD MS registry database. 18
Outcomes
The following key variables were retrieved from the NTD registry: EDSS, EQ-5D-5L, healthcare resource utilization (HCRU), sociodemographics (age, sex, educational attainment, living status), and clinical history (disease duration, subtype, relapse history, progression event history, DMT history).
To evaluate QoL, utilities were estimated from the EQ-5D-5L values using the German value set published in Ludwig et al., 2018. 19 The descriptive system comprises five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with each having five levels: no problems, slight problems, moderate problems, severe problems, and extreme problems.
Societal costs were estimated from direct and indirect medical and non-medical resource use. HCRU categories were derived and compared based on prior studies 10 (Table 1). Records of healthcare visits, investigations, treatments, need for equipment, care, etc., from the index date visit and ≥1 other visit in the prior 12 months were used to describe HCRU and generate an annualized cost. If a record for a particular HCRU item was absent, the patient was considered not to use this resource. Costs associated with these variables were derived from prior studies that leveraged public sources (e.g., public information on average costs for inpatient stays, consultations, etc.),9,10 which were adjusted to 2019 values using the consumer price index. Where necessary, this information was supplemented with additional searches of public information to obtain sample costs, for example, pricing of assistive equipment from up to 10 product websites. The human-capital method using national German cost of labor was used to value productivity losses.
Table 1.
Healthcare resource utilization categories.
| Categories | Subcategories | Relevant NTD items |
|---|---|---|
| Direct medical | Inpatient care | Hospital visits, rehab visits |
| Day admissions | Hospital visits, rehab visits | |
| Consultations | Occupational/speech/physio/psycho/acupuncture therapy/neurologist | |
| Tests | Cerebral MRI, spinal MRI, blood samples | |
| Medications | Wide range of medications, which are not DMTs including fampridine, cannabidiol/delta-9-tetrahydrocannabinol spray | |
| DMTs | DMTs | |
| Direct non-medical | Investments | Wheelchair, walker, stairlift, house modification, crutches, incontinence pads, diapers, bed pads, tub lift |
| Community services | Community care, care during day, domestic support | |
| Informal care | Care by family | |
| Indirect | Short-term absence | Sick days |
| Long-term absence, invalidity, early retirement | Disability pensions, other payouts for extended periods including “Uebergangsgeld” (interim pay) and “Krankengeld” (sick pay) |
We aligned our HCRU categories with those applied in Flachenecker et al. 10
DMT, disease-modifying therapy; HCRU, healthcare resource utilization; MRI, magnetic resonance imaging; NTD, NeuroTransData.
Costs of care were estimated based on binary (yes/no) information indicating whether a certain kind of care is needed: daycare (care only provided during the day), care by family, short-term care (temporally restricted stay in an inpatient facility), care in an outpatient setting, and domestic aid (support by community services).
As the number of hours of care is not captured, the average cost of informal care (care by family) per EDSS was imputed, based on prior studies. 10 Investment costs were assigned based on product lifetime with occasional maintenance; these costs and those based on product information were collected. For some products, costs and lifetime value are ill-defined, for example, “house modification” costs vary from <€1000 (ramps) to >€20,000 (complete adaption of a flat). We used the average costs spent per flat in Germany to make a flat barrier fee (excluding costs for tub lifts and stair escalators, as these are separate NTD items).
Statistical analysis
Patient characteristics for both cohorts were described and compared with the overall NTD population to ensure generalizability of findings.
For QoL, average utilities per EDSS are reported. For this descriptive analysis, due to the small sample size at severe EDSS (EDSS 7–9), these patients were categorized to a single EDSS 7+ group. Subsequently, multivariable linear mixed regression modeling was performed to evaluate the association between EDSS and utility adjusted for confounders, including sociodemographics and MS disease characteristics.
For HCRU, the proportion of PwMS with average usage of each HCRU item was reported per EDSS level. Similar to QoL analysis, severe MS patients were categorized to a single EDSS 7+ group. HCRU usage was then mapped to costs, and costs per category were reported according to three levels of disease severity (mild MS, EDSS 0–3.5; moderate MS, EDSS 4–6.5; severe MS, EDSS 7–9), and similar to prior studies.9,10 The prior study 9 relied on a patient-reported EDSS whereby EDSS category 0 to 3 would correspond to EDSS 0 to 3.5 in the official EDSS. Multivariate linear mixed regression modeling was performed to evaluate associations between cost and EDSS, adjusted for confounding. The NTD center was included as a random effect in both models. All analyses were performed using R Statistical Software (v4.1.2; R Core Team 2021) 20 and figures were produced using the package RStudio. 21
Ethical approval and patient consent
The data acquisition protocol was approved by the ethical committee of the Bavarian Medical Board (Bayerische Landesärztekammer; 14 June 2012, No. 11144) and reapproved by the ethical committee of the Medical Board North-Rhine (Ärztekammer Nordrhein, 25 April 2017, ID 2017071). Patient inclusion with informed consent is completed in the respective NTD practice as part of routine clinical care. Patients included in this analysis provided their informed consent (via tablets in NTD practices, electronic questionnaires, or via a patient portal) to the NTD registry. Patients explicitly agreed to any secondary use of their data.
Results
Sample description
Study population and patient demographics
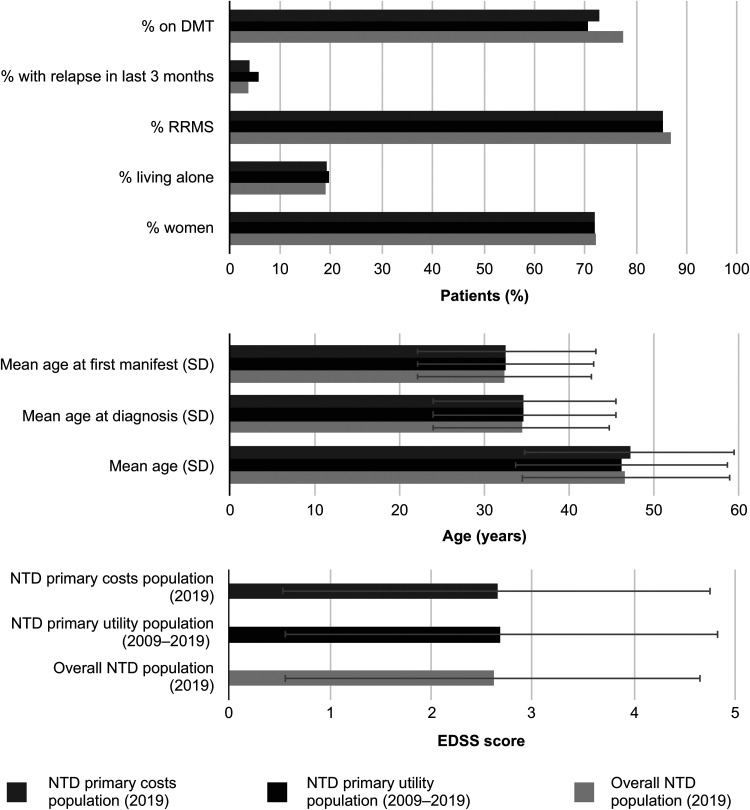
The final sample included 9821 and 7286 PwMS, for the QoL and cost cohorts, respectively (Figure 1). Patients were predominantly female (72%/72%), relapsing-remitting MS (RRMS) patients (85%/87%), treated with DMTs (71%/77%), with a mean EDSS of 2.7/2.6, and age of 46/47 years. Patient characteristics were comparable between the cohorts, and both were representative of the overall NTD population during 2019 (Figure 2), although some differences were noted. The cost cohort had a higher proportion of patients treated with DMTs (77%) than the QoL cohort (71%), which is likely reflective of the changing treatment landscape for the different eras from which patients were included in these cohorts (i.e., 2019 only versus 2009–2019).
Figure 1.
Population flowchart for the QoL and cost populations. Full covariate list: age, sex, living status, educational attainment, time since diagnosis, time since manifestation, MS subtype, time since last relapse, number of relapses in previous year, time since last confirmed disability progression, current DMT, and time since last DMT change. DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EQ-5D-5L, EuroQol-5 Dimension 5-Llevels; HCRU, healthcare resource utilization; MS, multiple sclerosis; NTD, NeuroTransData; QoL, quality of life.
Figure 2.
Study population demographics and MS disease characteristics. DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; NTD, NeuroTransData; RRMS, relapsing-remitting multiple sclerosis; SD, standard deviation.
Quality of life
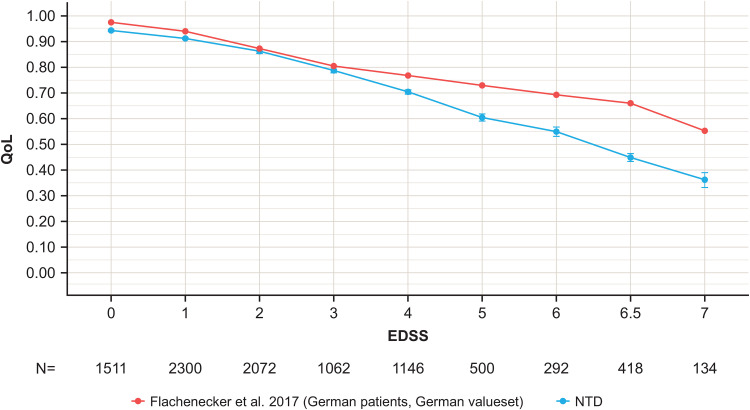
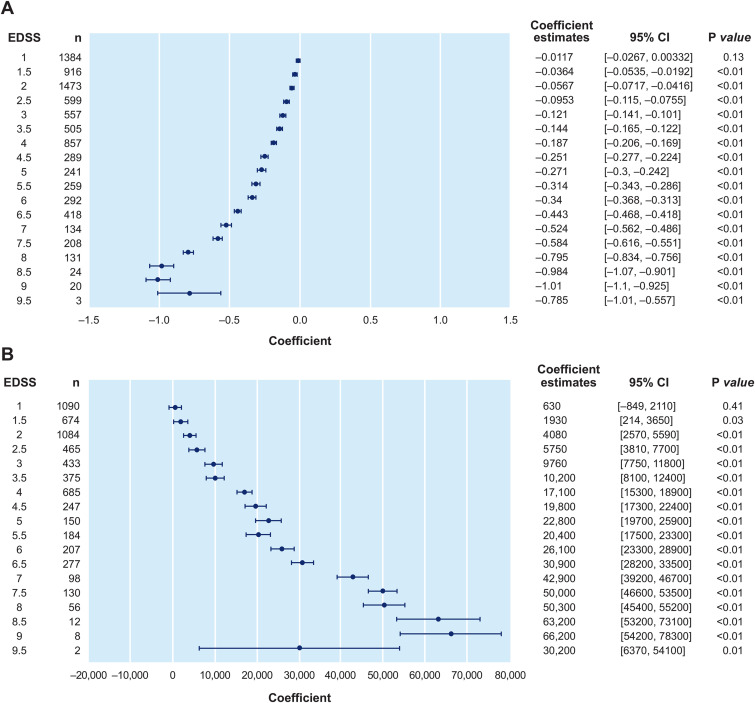
With MS disability progression, QoL was observed to worsen with average utilities decreasing from 0.94 at EDSS 0 to 0.36 at EDSS 7+ (Figure 3). Compared with prior studies of PwMS in Germany, 10 we observed a sharper decrease in utility from moderate to severe EDSS levels (Figure 3). The multivariate linear regression model assessing the association of EDSS with utility identified statistically significant worsening of QoL with each 0.5-step increase in EDSS, adjusting for confounders (Figure 4A).
Figure 3.
Mean utility estimated with the EQ-5D-5L by EDSS level in the NTD registry QoL population (2009–2019) compared with Flachenecker et al. 10 NTD utility population (2009–2019) N = 9435, Flachenecker et al. 10 Patients with EDSS >7 are not included in this chart due to low numbers and wide error bars. Note the previous study relied on patient-reported EDSS, which has minor scoring differences at low EDSS compared with the official EDSS. EDSS, Expanded Disability Status Scale; EQ-5D-5L, EuroQol-5 Dimension 5-Levels; NTD, NeuroTransData; QoL, quality of life.
Figure 4.
Multivariate regression models of the association between EDSS and separately: (A) QoL (n = 9821) and (B) costs (n = 7286). QoL (measured using German value set); multivariate regression model was adjusted for age, living status, sex, time since last relapse, time since MS diagnosis, time since manifestation, time since last DMT change, number of relapses in previous year, time since last confirmed progression event, current DMT use, educational attainment, MS subtype, and included NTD center as a random effect. CI, confidence interval; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; NTD, NeuroTransData; QoL, quality of life.
Direct non-medical healthcare resource utilization
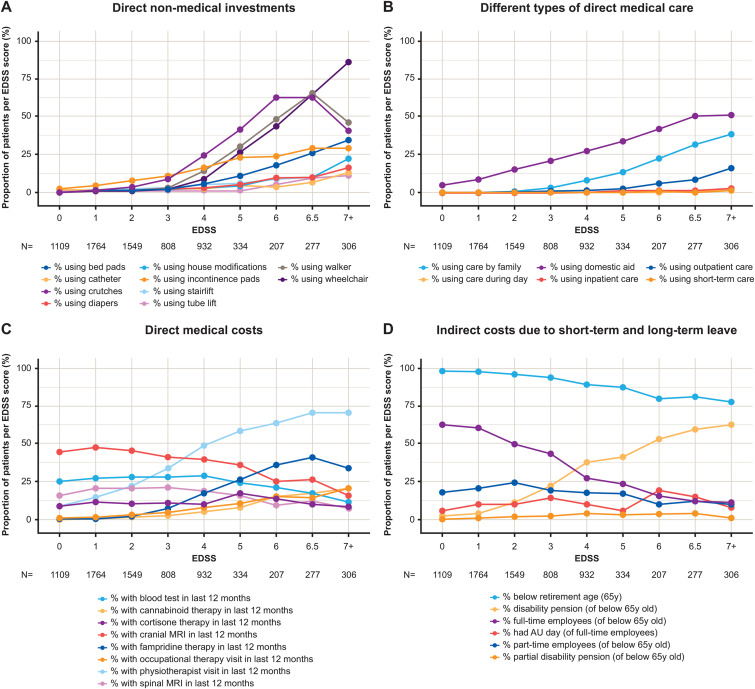
Investments were observed to increase with increasing EDSS score in the cost cohort, with a notable increase in use of aids and equipment from EDSS 4 onwards (Figure 5A). Overall, walking aids were adopted by 15.8% of the NTD cost cohort, 9.6% had wheelchairs, 4.8% had house modifications, and 3.3% had house lifts (Table 2). Community services and informal care were observed at lower EDSS scores, led by domestic support and family care, which became increasingly relevant at EDSS >5 (Figure 5B). Overall, 6.0% of patients required family care, and 18.7% of patients required domestic aid (Table 2).
Figure 5.
Percentage of patients per EDSS score within the NTD registry cost population (2019): (A) requiring direct non-medical investments; (B) requiring direct medical care (including inpatient, outpatient, and short-term care) and direct non-medical care (including family, domestic, and community daycare); (C) incurring direct medical costs (including consultations, tests, and non-DMT medications [fampridine and cannabinoids]); (D) with indirect costs due to short-term and long-term leave. NTD cost population (2019) N = 7286. Note: for those of working age less than 65 years, n = 6838, and for those of working age and working full time, n = 3195. AU, sick day; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; NTD, NeuroTransData; y, year.
Table 2.
Percentage of patients per EDSS category within the NTD registry cost population (2019).
| Overall
a
n = 7286 |
EDSS
0–3.5 n = 5230 |
EDSS
4–6.5 n = 1750 |
EDSS 7+ n = 306 |
|
|---|---|---|---|---|
| Direct medical | ||||
| MS patients using outpatient nursing care | 126 (1.7) | 17 (0.3) | 59 (3.4) | 50 (16.3) |
| MS patients using care during day | 17 (0.2) | 3 (0.1) | 8 (0.5) | 6 (2.0) |
| MS patients using short-term care | 13 (0.2) | 2 (0.0) | 6 (0.3) | 5 (1.6) |
| MS patients using inpatient care | 26 (0.4) | 5 (0.1) | 13 (0.7) | 8 (2.6) |
| MS patients with spinal MRI in last 12 months | 1335 (18.3) | 1029 (19.7) | 284 (16.2) | 22 (7.2) |
| MS patients with cranial MRI in last 12 months | 3055 (41.9) | 2391 (45.7) | 614 (35.1) | 50 (16.3) |
| MS patients with blood test in last 12 months | 1908 (26.2) | 1426 (27.3) | 446 (25.5) | 36 (11.8) |
| MS patients with speech therapist visit in last 12 months | 68 (0.9) | 24 (0.5) | 27 (1.5) | 17 (5.6) |
| MS patients with occupational therapist visit in last 12 months | 350 (4.8) | 108 (2.1) | 179 (10.2) | 63 (20.6) |
| MS patients with psychotherapist visit in last 12 months | 258 (3.5) | 178 (3.4) | 70 (4.0) | 10 (3.3) |
| MS patients with physiotherapist visit in last 12 months | 2182 (30.0) | 978 (18.7) | 986 (56.3) | 218 (71.2) |
| MS patients with acupuncturist visit in last 12 months | 35 (0.5) | 18 (0.3) | 13 (0.7) | 4 (1.3) |
| MS patients with cortisone therapy in last 12 months | 797 (10.9) | 562 (10.8) | 210 (12.0) | 25 (8.2) |
| MS patients with fampridine therapy in last 12 months | 637 (8.7) | 92 (1.8) | 440 (25.1) | 105 (34.3) |
| Direct non-medical | ||||
| MS patients using crutches | 906 (12.4) | 123 (2.4) | 660 (37.7) | 123 (40.2) |
| MS patients using wheelchair | 635 (9.6) | 18 (0.4) | 381 (24.9) | 236 (86.5) |
| MS patients using walker | 707 (9.7) | 56 (1.1) | 509 (29.1) | 142 (46.4) |
| MS patients using stairlift | 237 (3.3) | 83 (1.6) | 105 (6.0) | 49 (16.0) |
| MS patients using house modifications | 353 (4.8) | 52 (1.0) | 195 (11.1) | 106 (34.6) |
| MS patients using catheter | 105 (1.4) | 12 (0.2) | 53 (3.0) | 40 (13.1) |
| MS patients using incontinence pads | 726 (10.0) | 284 (5.4) | 354 (20.2) | 88 (28.8) |
| MS patients using diapers | 152 (2.1) | 20 (0.4) | 85 (4.9) | 47 (15.4) |
| MS patients using bed pads | 190 (2.6) | 39 (0.8) | 83 (4.7) | 68 (22.2) |
| MS patients using tube lift | 87 (1.2) | 5 (0.1) | 48 (2.7) | 34 (11.1) |
| MS patients using walking aids (crutches or ambulators) | 1150 (15.8) | 128 (2.5) | 774 (44.2) | 248 (81.1) |
| MS patients using domestic aid | 1364 (18.7) | 614 (11.7) | 594 (33.9) | 156 (51.0) |
| MS patients using care by family | 439 (6.0) | 62 (1.2) | 258 (14.7) | 119 (38.9) |
| Indirect | ||||
| % of working age (below 65 years) b | 6838 (93.9) | 5086 (97.3) | 1514 (86.5) | 238 (77.8) |
| MS patients on full disability pension (of below 65 years) | 1261 (18.4) | 447 (8.8) | 665 (43.9) | 149 (62.6) |
| MS patients working full time (of below 65 years) | 3195 (46.7) | 2821 (55.5) | 348 (23.0) | 26 (10.9) |
| MS patients on disability pension (partial or full) of below 65 years | 1383 (20.2) | 508 (10.0) | 723 (47.8) | 152 (63.9) |
| MS patients with sick days last 12 months (of full-time working population) | 333 (10.4) | 288 (10.2) | 43 (12.4) | 2 (7.7) |
EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; MS, multiple sclerosis; NTD, NeuroTransData.
% calculated on n of those of working age.
% calculated on n of those working full-time.
Direct medical resource use
During the 12-month period, we observed an overall rate of 0.36% of patients requiring inpatient care, while overall outpatient nursing care was 1.73%, which increased with EDSS (Figure 5B; Table 2). All patients had ≥1 neurologist consultation in the 12 months prior to index date, whereas <5% of patients had speech therapist, occupational therapist, and psychotherapist consultations. For physiotherapy, 30% had at least one consultation, which increased with EDSS (Figure 5C). Usage of medical investigations in the 12 months prior to index date was low; however, magnetic resonance imaging (MRI) and blood tests are not fully captured within the NTD database (Figure 5C).
Indirect resource use and productivity losses
Overall, 93.9% (n = 6838) of the cost cohort were of working age (i.e., <65 years). Full-time employment amongst those of working age in the cost cohort was 46.7% (n = 3195), which decreased with increasing EDSS score (Figure 5D). Of the full-time employed population, 10.4% (n = 333) reported ≥1 day of sick leave in the 12 months preceding the index date. However, sick days are likely underreported in the NTD database, as non-MS sick days are not reported. Amongst those of working age, 20.2% (n = 1383) were receiving full or partial invalidity pensions, which increased from 10.0% at EDSS 0 to 3.5 to 63.9% at EDSS 7+ (Figure 5D).
Socioeconomic costs
Societal costs per patient per year in 2019, excluding or including DMTs, increased from €5694 or €19,315 at mild EDSS, to €25,419 or €36,499 and €52,883 or €58,576 at moderate and severe EDSS levels, respectively (Table 3). The cost of DMTs per EDSS category were €13,621, €11,080, and €5693 for mild, moderate, and severe EDSS levels, respectively (Table 3). The multivariate linear regression model assessing the association of EDSS with overall costs, excluding DMTs, identified statistically significant increases in costs with each 0.5-step increase in EDSS, adjusting for confounders (Figure 4B). Similar results were observed in sensitivity analyses using overall costs including DMTs (Supplemental Figure S1).
Table 3.
Mean healthcare resource utilization costs per EDSS category using the NTD registry cost population (2019).
| NTD (unit costs 2019). Average per patient/year (€) N = 7286 | ||||
|---|---|---|---|---|
| Categories | Subcategories | EDSS
0–3.5 n = 4855 |
EDSS
4–6.5 n = 2125 |
EDSS
7–9.5 n = 306 |
| Direct medical | Inpatient care/day admissions | 250 | 750 | 1418 |
| Consultations | 484 | 1185 | 1595 | |
| Test | 91 | 71 | 31 | |
| Medications | 229 | 1596 | 3043 | |
| Direct non-medical | Investments | 54 | 460 | 1349 |
| Community services | 305 | 2010 | 15,087 | |
| Informal care | 50 | 1214 | 7824 | |
| Indirect | Short-term absence | 212 | 297 | 63 |
| Long-term absence, invalidity, early retirement | 4019 | 17,836 | 22,474 | |
| DMT costs | 13,621 | 11,080 | 5693 | |
| Total excluding DMT costs | 5694 | 25,419 | 52,883 | |
| Total including DMT costs | 19,315 | 36,499 | 58,576 | |
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; NTD, NeuroTransData.
Discussion
This retrospective real-world study of PwMS from the German NTD MS registry confirms EDSS-related trends associated with decreasing QoL and increasing socioeconomic costs associated with MS disease progression reported previously.9,10,22–26 Specifically, with each 0.5-step increase in EDSS, we observed a significant decrease in utility (6% on average based on EQ-5D-5L), and significant increase in annual socioeconomic costs (€3643 on average), becoming more pronounced at higher EDSS levels. Compared with current literature, 10 we observed a steeper decrease in QoL at moderate to severe EDSS levels, while for HCRU, at mild EDSS, absolute direct and indirect medical costs, and non-medical costs observed were lower, but increased at a steeper rate with disease progression.
The greatest cost drivers evolved from DMTs for mild MS (70.5% of total costs) to productivity losses in moderate and severe disease (48.9% and 38.4%, respectively; DMT costs fell to 30.4% and 9.7% of total costs), findings in line with previous studies. 10 We observed how the EDSS-related increasing use of crutches (EDSS >2), walkers (EDSS >3), and wheelchairs (EDSS >3) reflect the motor-driven EDSS step definitions (in rare cases at low EDSS levels, the usage of such aids was observed due to temporary worsening of walking disability due to relapse activity). The use of incontinence pads increases from EDSS 0 linearly throughout all stages, as do diapers from EDSS >3. Modifications at home, stairlifts, and tub lifts become relevant at EDSS >5.
Care needs increase linearly, with support within the family starting at EDSS 0, and care of patients within their families at EDSS >2. Comparing with direct medical care needs, outpatient nursing care becomes relevant at EDSS >4. Very few patients are in full inpatient care. This demonstrates that PwMS typically remain at home being supported and cared for by their families. Furthermore, the EDSS-related increasing use of physiotherapy (EDSS >0) and occupational therapy (EDSS >4) reflected the EDSS step definitions, while acupuncturist visits did not increase throughout all stages.
Indirect costs associated with long-term workplace absences were also a large driver of EDSS-related cost increases. Full-time employment amongst those less than 65 years of age, decreased from 55% at low EDSS to 23% and 11% at moderate and severe EDSS levels, respectively. Similarly, those receiving full disability pensions increased from 10% at low EDSS, to 48% and 64% at moderate and severe EDSS levels, respectively.
Compared with prior studies of PwMS in Germany, 10 we observed similar overall costs and trends with increasing EDSS (Supplemental Figure S2). Overall our findings mirror prior studies from Germany27–29 and other countries9,14,15 whereby the predominant societal cost at higher EDSS levels are the broader impacts resulting in indirect costs due to disability pensions and non-medical costs arising from community services and informal care. These findings highlight the potential value of halting or delaying disease progression through the early use of high-efficacy therapies.30,31
There are strengths and limitations to our study. This study is the first to our knowledge to utilize routinely collected real-world data from outpatient neurology clinics to quantify MS disability progression-related trends in QoL and socioeconomic costs. The similarities with previous studies validate the use of routinely collected registry data within the NTD to quantify QoL and HCRU.9,10 However, a lower number of MS-related hospitalizations were observed, in part explained by a decline in the annualized relapse rate (ARR) during this study observation period. 17 In addition, the NTD has a relapse treatment protocol to prevent inpatient treatment and favors ambulatory treatment of relapses. Compared with other settings, this results in lower hospitalization rates. Since 2009, only 18% of NTD-managed PwMS had intravenous cortisone treatments administered in an inpatient setting. This may have resulted in the observation of lower MS-related hospitalization rates, although similar ARRs to prior studies. Further research would be required to quantify the impact on relapse outcome and cost of relapses resulting from this treatment protocol in an outpatient setting.
Additional strengths in the current study include that EDSS was assessed by the patient's treating neurologist (a certified EDSS rater), while many prior studies relied on patients’ self-reported EDSS.9,10,32–34 Additionally, QoL was measured using the EQ-5D-5L rather than the 3L version relied on previously.9,10,24,35 Furthermore, the NTD population may be more representative of the RRMS distribution in PwMS in Germany, 36 when compared with a recent large-scale survey of PwMS in Germany 10 and prospective observational studies.28,29 We observed that the distribution of MS characteristics, particularly subtype, was more representative of MS population characteristics in comparison to prior survey-based studies, leading to further sample characteristic differences such as lower mean EDSS.10,28,29 Survey-based recruitment requiring self-participation may have excluded healthier PwMS at lower EDSS levels (e.g., recently diagnosed patients not currently enrolled into MS patient society groups), as well as sicker patients at higher EDSS who may decline to participate due to the severity of their disability. These sampling differences may have led to observations of lower costs at lower EDSS, and lower QoL at higher EDSS in the current study compared with the prior study. 10 However, overall, comparable trends of HCRU per EDSS were seen in both the current and prior study. 10 Similarly, prior prospective observational studies were restricted to RRMS patients treated with certain DMTs,28,29 while the current study included all MS patients regardless of treatment and subtype. Some limitations exist regarding the reporting of HCRU in the NTD registry, for example, visits for blood samples not involving a concurrent visit to the neurologist are not recorded in the database, which otherwise may be captured via surveys. MRI appointments at the early stage of MS diagnosis are potentially not fully captured which would have limited impact on the overall costs, however this may have a greater impact on costs at lower EDSS. Sick days are likely underreported in the NTD database. Furthermore, the recording of informal care usage was collected as “yes” or “no,” and costs that could not be accurately assigned as hours were not recorded. Similarly for investments, only the use of a particular item was known, hence the cost had to be assumed. Despite this, the NTD routinely captures a wide range of HCRU as part of standard practice, which are relevant and may be replicable over time. Future studies may consider further augmenting registry data with patient survey data to improve granularity of certain data elements. Bearing in mind the study limitations potentially leading to underestimate some costs, the burden of MS is likely higher than what is reported in our study.
Within a clinically representative MS population, we identified worsening QoL and increasing societal costs as EDSS increases, in line with prior studies, but with a modestly greater decrease in QoL. Significant socioeconomic impacts including informal care requirements and disability pensions were also observed. These findings highlight the socioeconomic burden of MS disease progression and the potential value of halting or delaying disease progression through the early use of high-efficacy therapies. 37 Administering DMTs early in MS could lead to better socioeconomic outcomes related to employment, 38 reduce the need for informal care, and improve patient QoL. 39 From a socioeconomic perspective, delaying disability progression may benefit both patients and society.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173231187810 for The socioeconomic impact of disability progression in multiple sclerosis: A retrospective cohort study of the German NeuroTransData (NTD) registry by Paul Dillon, Yanic Heer, Eleni Karamasioti, Erwan Muros-Le Rouzic, Guiseppe Marcelli, Danilo Di Maio, Stefan Braune, Gisela Kobelt and Jürgen Wasem in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgments
Professional medical writing and editorial assistance were provided by Sonika Singh (Articulate Science, UK) and the manuscript has been submitted by Articulate Science on behalf of the authors; this assistance was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. All authors have authorized submission of the manuscript and approved of all statements included herein.
Footnotes
Data availability: The data used in this study are owned by the NeuroTransData Registry and sharing of the data is subject to their policies. Any reasonable requests for data access can be directed to the NeuroTransData Registry (www.neurotransdata.com).
Paul Dillon was an employee of F. Hoffmann-La Roche Ltd, Basel, Switzerland during completion of the work related to this manuscript and has shares/ownership of F. Hoffmann-La Roche Ltd.
Yanic Heer was an employee of PricewaterhouseCoopers (PwC), Zurich, Switzerland during completion of the work related to this manuscript.
Eleni Karamasioti was an employee of PricewaterhouseCoopers (PwC), Zurich, Switzerland during completion of the work related to this manuscript.
Erwan Muros-Le Rouzic is an employee of and shareholder in F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Giuseppe Marcelli is an employee of F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Danilo Di Maio is an employee of F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Stefan Braune received honoraria from Kassenärztliche Vereinigung Bayerns and health maintenance organizations for patient care, and from Biogen, Merck, NeuroTransData, Novartis, and Roche for consulting, project management, clinical studies, and lectures; he also received honoraria and expense compensation as a board member of NeuroTransData.
Gisela Kobelt is president of EHE International GmbHan and employee of European Health Economics, Mulhouse, France.
Jürgen Wasem is a professor for health services management at University Duisburg-Essen, Germany. He has received an honorarium for consulting study concept and quality assurance of data calculations.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by F. Hoffmann-La Roche Ltd, Basel, Switzerland. F. Hoffmann-La Roche Ltd, Basel, Switzerland was involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
ORCID iDs: Erwan Muros-Le Rouzic https://orcid.org/0000-0002-8925-0481
Stefan Braune https://orcid.org/0000-0002-0541-8196
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Danilo Di Maio, F. Hoffman-La Roche Ltd, Basel, Switzerland.
Gisela Kobelt, EHE International GmbH, St Moritz, Switzerland; European Health Economics, Mulhouse, France.
Jürgen Wasem, Faculty of Economics, University of Duisburg-Essen, Essen, Germany.
References
- 1.The Multiple Sclerosis International Federation. Atlas of MS, atlasofms.org/map/germany/epidemiology/number-of-people-with-ms (2020, accessed 15 September 2022). [DOI] [PMC free article] [PubMed]
- 2.Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 3.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 4.Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun 2014; 48-49: 134–142. [DOI] [PubMed] [Google Scholar]
- 5.Trisolini MA, Wiener J, et al. Global economic impact of multiple sclerosis. https://www.msif.org/wp-content/uploads/2014/09/Global_economic_impact_of_MS.pdf (2010, accessed November 26, 2022).
- 6.De Judicibus MA, McCabe MP. The impact of the financial costs of multiple sclerosis on quality of life. Int J Behav Med 2007; 14: 3–11. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 8.Lavery AM, Verhey LH, Waldman AT. Outcome measures in relapsing-remitting multiple sclerosis: capturing disability and disease progression in clinical trials. Mult Scler Int 2014; 2014: 262350–262350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017; 23: 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flachenecker P, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for Germany. Mult Scler 2017; 23: 78–90. [DOI] [PubMed] [Google Scholar]
- 11.Karampampa K, Gyllensten H, Yang F, et al. Healthcare, sickness absence, and disability pension cost trajectories in the first 5 years after diagnosis with multiple sclerosis: a prospective register-based cohort study in Sweden. Pharmacoecon Open 2020; 4: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyllensten H, Kavaliunas A, Murley C, et al. Costs of illness progression for different multiple sclerosis phenotypes: a population-based study in Sweden. Mult Scler J Exp Transl Clin 2019; 5: 2055217319858383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennum P, Wanscher B, Frederiksen J, et al. The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol 2012; 22: 36–43. [DOI] [PubMed] [Google Scholar]
- 14.Ernstsson O, Gyllensten H, Alexanderson K, et al. Cost of illness of multiple sclerosis—a systematic review. PLoS One 2016; 11: e0159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karampampa K, Gustavsson A, Miltenburger C, et al. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler 2012; 18: 7–15. [DOI] [PubMed] [Google Scholar]
- 16.NeuroTransData GmbH. NTD database. https://www.neurotransdata.com/en/ (2022, accessed April 2022).
- 17.Braune S, Rossnagel F, Dikow Het al. et al. The impact of drug diversity on treatment effectiveness in relapsing-remitting multiple sclerosis (RRMS) in Germany between 2010 and 2018: real-world data from the German NeuroTransData multiple sclerosis registry. BMJ Open 2021; 11: e042480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehrle K, Tozzi V, Braune S, et al. Implementation of a data control framework to ensure confidentiality, integrity, and availability of high-quality real-world data (RWD) in the NeuroTransData (NTD) registry. JAMIA Open 2022; 5: ooac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics 2018; 36: 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021, https://www.R-project.org/ . [Google Scholar]
- 21.RStudio Team. RStudio: Integrated Development for R. Boston, MA, USA: RStudio, PBC, 2020, http://www.rstudio.com/ . [Google Scholar]
- 22.Kavaliunas A, Danylaitė Karrenbauer V, Binzer S, et al. Systematic review of the socioeconomic consequences in patients with multiple sclerosis with different levels of disability and cognitive function. Front Neurol 2021; 12: 737211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones E, Pike J, Marshall T, et al. Quantifying the relationship between increased disability and health care resource utilization, quality of life, work productivity, health care costs in patients with multiple sclerosis in the US. BMC Health Serv Res 2016; 16: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatkowski A, Marissal JP, Pouyfaucon M, et al. Social participation in patients with multiple sclerosis: correlations between disability and economic burden. BMC Neurol 2014; 14: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaksson AK, Ahlström G, Gunnarsson LG. Quality of life and impairment in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2005; 76: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mäurer M, Comi G, Freedman MS, et al. Multiple sclerosis relapses are associated with increased fatigue and reduced health-related quality of life—a post hoc analysis of the TEMSO and TOWER studies. Mult Scler Relat Disord 2016; 7: 33–40. [DOI] [PubMed] [Google Scholar]
- 27.Pakdaman H, Amini Harandi A, Gharagozli K, et al. Health-related quality of life in patients with relapsing-remitting multiple sclerosis treated with subcutaneous interferon β-1a in Iran. Int J Neurosci 2017; 127: 501–507. [DOI] [PubMed] [Google Scholar]
- 28.Visser LA, Louapre C, Uyl-de Groot CA, et al. Health-related quality of life of multiple sclerosis patients: a European multi-country study. Arch Public Health 2021; 79: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller S, Heidler T, Fuchs A, et al. Real-world treatment of patients with multiple sclerosis per MS subtype and associated healthcare resource use: an analysis based on 13,333 patients in Germany. Neurol Ther 2020; 9: 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ness NH, Schriefer D, Haase R, et al. Real-world evidence on the societal economic relapse costs in patients with multiple sclerosis. Pharmacoeconomics 2020; 38: 883–892. [DOI] [PubMed] [Google Scholar]
- 31.Filippi M, Danesi R, Derfuss T, et al. Early and unrestricted access to high-efficacy disease-modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol 2022; 269: 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schriefer D, Ness N-H, Haase R, et al. Gender disparities in health resource utilization in patients with relapsing–remitting multiple sclerosis: a prospective longitudinal real-world study with more than 2000 patients. Ther Adv Neurol Disord 2020; 13: 1756286420960274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziemssen T, Prosser C, Haas JS, et al. Healthcare resource use and costs of multiple sclerosis patients in Germany before and during fampridine treatment. BMC Neurol 2017; 17: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renner A, Baetge SJ, Filser M, et al. Working ability in individuals with different disease courses of multiple sclerosis: factors beyond physical impairment. Mult Scler Relat Disord 2020; 46: 102559. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson J, Kobelt G, Gannedahl M, et al. Association between disability, cognition, fatigue, EQ-5D-3L domains, and utilities estimated with different western European value sets in patients with multiple sclerosis. Value Health 2019; 22: 231–238. [DOI] [PubMed] [Google Scholar]
- 36.Engelhard J, Oleske DM, Schmitting S, et al. Multiple sclerosis by phenotype in Germany. Mult Scler Relat Disord 2022; 57: 103326. [DOI] [PubMed] [Google Scholar]
- 37.Rojas JI, Patrucco L, Alonso R, et al. Effectiveness and safety of early high-efficacy versus escalation therapy in relapsing-remitting multiple sclerosis in Argentina. Clin Neuropharmacol 2022; 45: 45–51. [DOI] [PubMed] [Google Scholar]
- 38.Kavaliunas A, Manouchehrinia A, Gyllensten H, et al. Importance of early treatment decisions on future income of multiple sclerosis patients. Mult Scler J Exp Transl Clin 2020; 6: 2055217320959116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glanz BI, Zurawski J, Casady EC, et al. The impact of ocrelizumab on health-related quality of life in individuals with multiple sclerosis. Mult Scler J Exp Transl Clin 2021; 7: 20552173211007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173231187810 for The socioeconomic impact of disability progression in multiple sclerosis: A retrospective cohort study of the German NeuroTransData (NTD) registry by Paul Dillon, Yanic Heer, Eleni Karamasioti, Erwan Muros-Le Rouzic, Guiseppe Marcelli, Danilo Di Maio, Stefan Braune, Gisela Kobelt and Jürgen Wasem in Multiple Sclerosis Journal – Experimental, Translational and Clinical