Abstract
Background:
The bypassing agent, activated prothrombin complex concentrate [aPCC, FEIBA (factor VIII inhibitor bypass activity); Baxalta US Inc, a Takeda company, Lexington, MA, USA], is indicated for the treatment of bleeding episodes, perioperative management, and routine prophylaxis in patients with hemophilia A or B with inhibitors. In certain countries, aPCC is also indicated for the treatment of bleeding episodes and perioperative management in patients with acquired hemophilia A.
Objectives:
To describe long-term, real-world effectiveness, safety, and quality-of-life outcomes for patients with congenital hemophilia A or B and high-responding inhibitors receiving aPCC treatment in routine clinical practice.
Design:
FEIBA Global Outcome (FEIBA GO; EUPAS6691) was a prospective, observational study.
Methods:
Investigators determined the treatment regimen and clinical monitoring frequency. The planned patient observation period was 4 years. Data are from the safety analysis set (patients who received ⩾1 aPCC infusion).
Results:
Overall, 50 patients received either aPCC prophylaxis (n = 37) or on-demand therapy (n = 13) at screening [hemophilia A, n = 49; hemophilia B, n = 1; median (range) age, 16.5 [2–71] years). Mean ± standard deviation overall annualized bleeding rate and annualized joint bleeding rate for patients receiving prophylaxis were 6.82 ± 11.52 and 3.77 ± 5.71, respectively, and for patients receiving on-demand therapy were 10.94 ± 11.27 and 6.94 ± 7.39, respectively. Overall, 177 and 31 adverse events (AEs) were reported in 28 of 40 and 10 of 13 patients receiving prophylaxis or on-demand therapy, respectively. Two serious AEs were considered possibly related to aPCC: acute myocardial infarction due to coronary artery embolism in one patient receiving prophylaxis. No thrombotic microangiopathy was reported. No AEs resulted in death.
Conclusion:
This study demonstrated the long-term, real-world effectiveness and consistent safety profile of aPCC as on-demand therapy and prophylactic treatment in patients with hemophilia and high-responding inhibitors.
Trial registry:
FEIBA Global Outcome Study; EUPAS6691
Keywords: factor VIII inhibitor bypassing activity, hemophilia A, hemophilia B, inhibitors, observational study
Introduction
The main treatment approach for patients with severe hemophilia is replacement therapy with clotting factor concentrates. 1 The development of inhibitory alloantibodies against clotting factor concentrates is a serious complication associated with factor replacement therapy. Inhibitor development occurs in approximately 30% of previously untreated patients with severe hemophilia A and up to 10% of patients with severe hemophilia B.2–5 Inhibitors impede the function of clotting factor concentrates, complicating the treatment of bleeding events, and are associated with higher rates of hospitalization, greater healthcare costs, and higher mortality rates than in patients without inhibitors.1,6–8 Inhibitors are measured by either the Bethesda assay or the Nijmegen-modified Bethesda assay and are classified as either high-responding or low-responding inhibitors. 1 A low-responding inhibitor is an inhibitor <5.0 Bethesda units (BU), whereas high-responding inhibitors are defined as an inhibitor titer ⩾5 BU and are typically persistent. 1
The bypassing agent activated prothrombin complex concentrate [aPCC, FEIBA (factor VIII inhibitor bypass activity); Baxalta US Inc, a Takeda company, Lexington, MA, USA] is indicated for the treatment of bleeding episodes, perioperative management, and routine prophylaxis in patients with hemophilia A or B with inhibitors. In certain countries, aPCC is also indicated for the treatment of bleeding episodes and perioperative management in patients with acquired hemophilia A.9,10 It is important to be aware that indications vary by country. Bypassing agents work by promoting thrombin generation via pathways that do not require activation of factor VIII (FVIII) or factor IX (FIX). 11 The World Federation of Hemophilia (WFH) guidelines for the management of hemophilia, published in 2020, recommend the bypassing agents aPCC or recombinant activated factor VII (rFVIIa, NovoSeven; Novo Nordisk, Bagsvaerd, Denmark) for the management of breakthrough bleeding events in patients with hemophilia A and high-responding inhibitors. 1 Since the publication of the WFH guidelines in 2020, another bypassing agent has been approved for the treatment and control of bleeding events in adults and adolescents (⩾12 years old) with hemophilia A or B with inhibitors.12,13
Another therapeutic option available to patients with hemophilia A and inhibitors is emicizumab (Hemlibra; Genentech Inc., South San Francisco, CA, USA). Emicizumab, a bispecific monoclonal antibody that bridges activated FIX and factor X, was approved in the United States in 2017 and Europe in 2018 and is indicated for routine prophylaxis in patients with hemophilia A with or without FVIII inhibitors (indications vary by country).14,15 The WFH guidelines recommend the use of aPCC or rFVIIa for the management of breakthrough bleeding events experienced by patients with hemophilia A and high-responding inhibitors receiving emicizumab prophylaxis, with rFVIIa preferred over aPCC to avoid the risk of thrombotic microangiopathy. 1 Similarly, rFVIIa over aPCC is recommended for patients with hemophilia A and high-responding inhibitors who undergo major surgery or an invasive procedure while receiving emicizumab. 1
aPCC has been commercially available since 1977 and, during the past 40 years, randomized clinical trials and observational studies have demonstrated the efficacy of aPCC as on-demand therapy for the control of bleeding events, as prophylaxis, and for the perioperative management of patients with hemophilia A or B and inhibitors.16–21 The safety of aPCC administered either as prophylaxis or on-demand therapy was also evaluated in a post-authorization safety surveillance (PASS) study in patients with hemophilia and inhibitors, as well as in a meta-analysis of 39 studies with safety data relating to the use of aPCC.22,23 The FEIBA Global Outcome (FEIBA GO; EUPAS6691) study was designed to describe the long-term, real-world effectiveness, safety, and quality-of-life outcomes for patients with congenital hemophilia A or B and high-responding inhibitors receiving aPCC (FEIBA) treatment in routine clinical practice. The primary objective of the study was to describe the hemostatic effectiveness of aPCC in clinical practice.
Methods
The reporting of this study conforms to the STROBE checklist. 24
Study design
FEIBA GO was a prospective, uncontrolled, observational, non-interventional, open-label, multicenter cohort study. aPCC treatment regimens were prescribed at the discretion of the investigator in accordance with routine clinical practice. Based on prescribing information, a 50- to 100-U/kg body weight aPCC dose was recommended. Investigators were also advised not to exceed a single dose of 100 U/kg and a total maximum daily dose of 200 U/kg unless the severity of bleeding both warranted and justified the use of a higher dose.9,10
Investigators determined the frequency and type of laboratory, radiologic, and clinical monitoring. The study protocol included a screening visit, interval visits, and an end-of-study visit. Interval visits were scheduled at the discretion of the investigator but were anticipated to occur at least once a year. Study visits coincided with routinely scheduled and emergency visits. The protocol did not require any additional testing or monitoring beyond what was deemed necessary by the investigator. The planned overall study duration was approximately 7 years, and the planned observation period for each patient was 4 years from enrollment to the end-of-study visit.
Data from study visits were recorded in electronic case report forms (CRFs). Patients were also provided with a patient diary to complete voluntarily. Information captured in the patient diary (Supplementary Table 1) was entered into the corresponding sections in the CRF for each patient. Where possible, all diary entries were cross-checked against patient case notes.
This study was conducted after ethics committee approval was obtained from each study site. All patients and/or their legally authorized representative provided written informed consent before entering the study. Investigators were required to comply with the protocol, International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. Investigators were responsible for the conduct of all aspects of the study at the study site.
Patients
Male patients of any age diagnosed before study entry with congenital hemophilia A or B with high-responding inhibitors of any titer were eligible for inclusion. Patients were also required to have been prescribed aPCC as part of routine clinical practice, either on demand, as prophylaxis, or during immune tolerance induction.
Patients were excluded from the study if they had a known hypersensitivity to aPCC or any of its components. Patients were also excluded if they had any contraindications to aPCC or any other severe concomitant clinically relevant bleeding disorder. aPCC use was not permitted in patients with either disseminated intravascular coagulation or acute thrombosis or embolism (including myocardial infarction) if therapeutic alternatives were available.
Endpoints
The primary objective to describe the hemostatic effectiveness of aPCC was assessed by annualized bleeding rates (ABRs) and annualized joint bleeding rates (AJBRs), the total number (percentage) of treated bleeds, hemostatic efficacy ratings based on an ‘excellent-to-poor’ 4-point Likert scale, and aPCC administration details.
ABRs were determined by treatment regimen for all patients who had received on-study treatment for ⩾90 days. Patients for whom no bleeding events were recorded must have confirmed zero bleeding events, otherwise they were classified as missing. Bleeding events were counted for the treatment regimen when the event occurred; therefore, patients who switched regimen could appear more than once.
The Likert scale to assess hemostatic efficacy was utilized by the patient or caregiver for treatment administered at home and by the investigator for treatment administered in a hospital or clinic. The overall treatment effectiveness of prophylaxis was evaluated for each infusion log entry under prophylaxis. Patients receiving on-demand therapy, who also ticked prophylaxis-related effectiveness, were excluded. For patients who switched to prophylaxis, the period during which prophylactic treatment was received was evaluated. The following rating scale was used:
Excellent: definitely low bleeding rate with improvement in daily activities and quality of life. Very satisfied with the treatment and worth being continued.
Good: relatively low bleeding rate with some improvement in daily activities and quality of life. Satisfied with the treatment and worth being continued.
Fair: minimal change in breakthrough bleeding episodes with only partial benefit in terms of activity level and quality of life. Partially satisfied with the treatment. Not sure if it is worth continuing treatment.
Poor: frequent breakthrough bleeding episodes interfering with activity level and quality of life. Not satisfied with the treatment.
The effectiveness of aPCC treatment for acute bleeding episodes was documented at interval visits and at the end-of-study visit. Acute bleeding cessations were assessed based on on-demand infusions that were administered within 72 h after a bleeding event. The date and time were used to connect bleeding events with infusions. The following rating scale was used:
Excellent: full relief of pain and cessation of objective signs of bleeding (e.g. swelling, tenderness, and decreased range of motion in the case of musculoskeletal hemorrhage) within approximately 6–12 h and after one or two infusions. No additional infusion required for the control of bleeding. Any additional infusion for treatment of bleeding will preclude this rating. Administration of further infusions to maintain hemostasis would not affect this scoring.
Good: definite pain relief and/or improvement in signs of bleeding within approximately 6–24 h requiring more than two infusions for complete resolution. Administration of further infusions to maintain hemostasis would not affect this scoring.
Fair: probable and/or slight relief of pain and slight improvement in signs of bleeding within approximately 6–24 h. Requires multiple infusions for complete resolution.
Poor: no improvement of signs or symptoms or conditions worsen.
Secondary endpoints included the total number of target joints at screening and the incidence of new target joints. A target joint was defined as a joint in which three or more bleeding events occurred in a 6-month period. The number of patients who underwent invasive surgical procedures was also assessed. The management of surgical procedures was at the discretion of the investigator. Safety outcomes were assessed by the incidence, severity, and relatedness of serious and non-serious adverse events (AEs). The incidence, severity, and relatedness of thromboembolic events and thrombotic microangiopathy events were also assessed. AEs were coded using the Medical Dictionary for Regulatory Activities (version 23.0).
Statistical analysis
The study sample size was not based on statistical considerations because no hypothesis testing or interval estimation was applied. The all-patients analysis set included all patients enrolled in the study. The safety analysis set consisted of data for all patients who were enrolled, met all inclusion and exclusion criteria, and received at least one infusion of aPCC. The safety analysis set was used for the evaluation of study endpoints.
Statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC, USA), version 9.4. Inferential statistical testing procedures could be applied for selected parameter comparison, although they were exploratory in nature. Continuous variables were analyzed using standard descriptive measures. Categorical variables were summarized by counts and the percentage of patients in corresponding categories. Percentages for missing values were not displayed because they were not included in the percentage calculations for other categories.
In general, no imputations were made for missing values. Missing or partial dates of bleeding events were imputed to assign bleeding events to observational periods. Any missing patient weight was imputed using the last-observation-carried-forward approach. Any AE without a relationship categorization was considered as possibly related to the study drug. Dates were imputed for assigning AEs to pre-treatment/treatment emergent and medications into previous and concomitant categories, and for the calculation of the duration of historical hemophilia treatment. For missing dates of treatment history, a missing start date remained missing, and a missing end date was set to the day before the screening date. Missing days and months were imputed as 01 or 01–01 (for start) and 31 or 31–12 (for end), respectively.
Results
Study participants
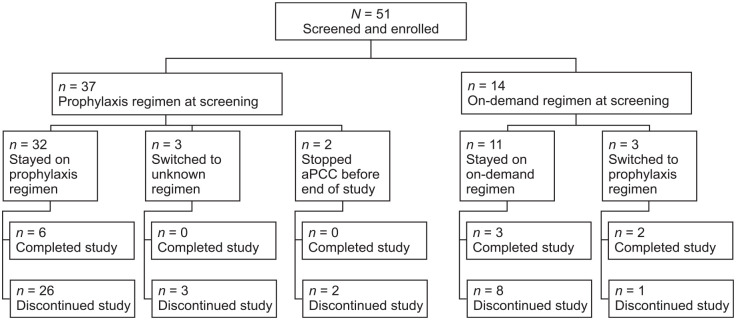
Enrollment commenced on 3 September 2014 and completed on 19 December 2017. The study was terminated on 28 February 2020. Patients were enrolled at 27 sites across 11 countries: Germany, United Kingdom, Poland, France, Russian Federation, Spain, Italy, Norway, Hungary, Portugal, and Belgium. Two patients from one site were excluded from the analysis owing to compliance issues.
The all-patients analysis set consisted of 51 patients: Germany (n = 15), United Kingdom (n = 7), Poland (n = 6), France (n = 5), Russian Federation (n = 4), Spain (n = 3), Italy (n = 3), Norway (n = 3), Hungary (n = 2), Portugal (n = 2), and Belgium (n = 1), of whom 37 were receiving aPCC prophylaxis and 14 were receiving on-demand therapy at screening (see Figure 1). In total, 40 patients (78.4%) withdrew prematurely from the study over time, of whom 27 switched to another clinical trial or product. Of these, 11 patients switched to emicizumab. Eleven patients completed the study and seven patients had >48 months of follow-up (Supplementary Figure 1).
Figure 1.
Patient disposition (all-patients analysis set).
The all-patients analysis set included all patients enrolled in the study. The safety analysis set consisted of data for all patients who were enrolled, met all inclusion and exclusion criteria, and received at least one infusion of aPCC. In total, the all-patients analysis set included 51 patients and the safety analysis set included 50 patients.
aPCC, activated prothrombin complex concentrate.
The safety analysis set included 50 patients enrolled at 25 sites across 11 countries, of whom 37 were receiving aPCC prophylaxis and 13 were receiving on-demand therapy at screening. One of the 51 patients in the all-patients analysis set was lost to follow-up and excluded from the safety analysis set. Demographics and baseline characteristics for patients in the safety analysis set are reported in Table 1. All patients had documented prior therapy with aPCC, with a median (range) treatment duration of 14.09 (0.4–188.3) months. Seventeen patients (34.0%) had also received prior therapy with either rFVIIa or other treatments (Supplementary Table 2). In total, 49 patients had hemophilia A and one patient had hemophilia B. All patients had severe hemophilia (factor activity <1% at screening). Overall, the median (range) patient age was 16.5 (2–71) years. Most patients receiving aPCC prophylaxis at screening were aged 18 years or younger, whereas most patients receiving on-demand therapy were adults aged 30 years or older (see Table 1). Patient medical history by treatment regimen at screening is presented in Supplementary Table 3. Historical FVIII inhibitor titers (titers measured before screening) were documented in 44 patients: 30 patients (68.2%) had high titers (⩾5 BU/ml) and 14 patients (31.8%) had low titers (0.6 to <5 BU/ml). Historical FIX inhibitor titers were documented in one patient who had a high titer (⩾5 BU/ml). During the study period, 21 surgical procedures were reported in 16 patients, of whom 11 were receiving aPCC prophylaxis and 5 were receiving on-demand therapy (see Supplementary Materials).
Table 1.
Demographics and baseline characteristics of patients (safety analysis set).
| Treatment regimen at screening | |||
|---|---|---|---|
| Prophylaxis (n = 37) |
On-demand (n = 13) |
Total (N = 50) |
|
| Age at informed consent, years | |||
| Mean ± SD | 19.3 ± 16.31 | 34.4 ± 21.91 | 23.2 ± 18.91 |
| Median (range) | 15.0 (2–71) | 36.0 (5–65) | 16.5 (2–71) |
| Age category, n (%) | |||
| Pediatric: 0–12 years | 14 (37.8) | 3 (23.1) | 17 (34.0) |
| Adolescent: >12–18 years | 10 (27.0) | 1 (7.7) | 11 (22.0) |
| Young adult: >18–30 years | 6 (16.2) | 1 (7.7) | 7 (14.0) |
| Adult: >30–60 years | 6 (16.2) | 7 (53.8) | 13 (26.0) |
| Elderly: >60 years | 1 (2.7) | 1 (7.7) | 2 (4.0) |
| Ethnicity, n (%) a | |||
| Asian | 1 (2.7) | 0 (0.0) | 1 (2.0) |
| Black or African American | 4 (10.8) | 0 (0.0) | 4 (8.0) |
| White | 23 (62.2) | 13 (100.0) | 36 (72.0) |
| Other | 2 (5.4) | 0 (0.0) | 2 (4.0) |
| Not collected | 7 (18.9) | 0 (0.0) | 7 (14.0) |
| Hemophilia type, n (%) | |||
| Hemophilia A | 36 (97.3) | 13 (100.0) | 49 (98.0) |
| Hemophilia B | 1 (2.7) | 0 (0.0) | 1 (2.0) |
| Time since diagnosis, years b | n = 33 | n = 12 | n = 45 |
| Mean ± SD | 18.73 ± 15.50 | 32.31 ± 21.72 | 22.35 ± 18.15 |
| Median (range) | 14.31 (2.6–62.0) | 35.42 (4.5–63.0) | 15.62 (2.6–63.0) |
SD, standard deviation.
Due to specific country regulations, ethnicity was not collected in France or Portugal.
Time since diagnosis of hemophilia in years was calculated as the time between first diagnosis and informed consent in months, divided by 12.
Annualized bleeding rates
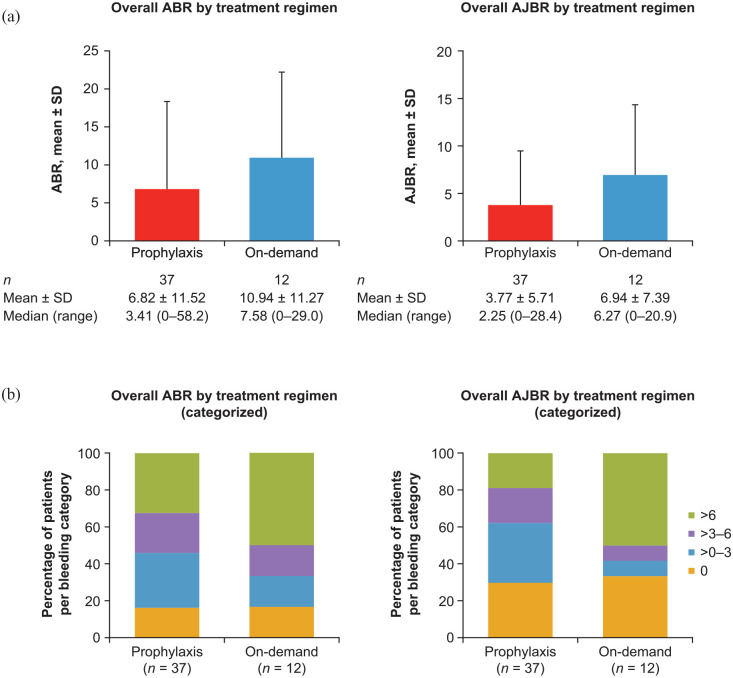
The mean ± standard deviation (SD) overall ABR and AJBR for patients receiving aPCC prophylaxis (n = 37) were 6.82 ± 11.52 and 3.77 ± 5.71, respectively. Six patients receiving aPCC prophylaxis had zero ABRs and 11 patients had zero AJBRs. The mean ± SD overall ABR and AJBR for patients receiving on-demand therapy (n = 12) were 10.94 ± 11.27 and 6.94 ± 7.39, respectively. Two patients receiving on-demand therapy had zero ABRs and four had zero AJBRs (see Figure 2).
Figure 2.
Overall ABR and AJBR by (a) treatment regimen and (b) categorized (safety analysis set).
ABR is the number of all bleeding events standardized to 12 months. AJBR is the number of joint bleeding events standardized to 12 months. ABR and AJBR were only included in the analysis for patients with a regimen duration of ⩾90 days. All patients without confirmed zero bleeding events were set to missing. Bleeding events were counted for the regimen where the event occurred; therefore, patients who switched regimen could appear more than once. Overall AJBR treatment regimen (categorized): one patient in the prophylaxis group was classified as missing.
ABR, annualized bleeding rate; AJBR, annualized joint bleeding rate; SD, standard deviation.
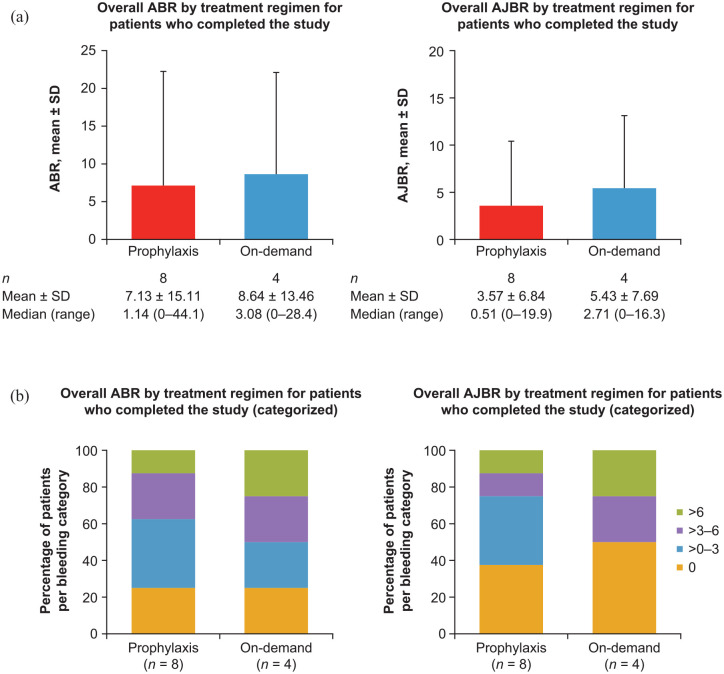
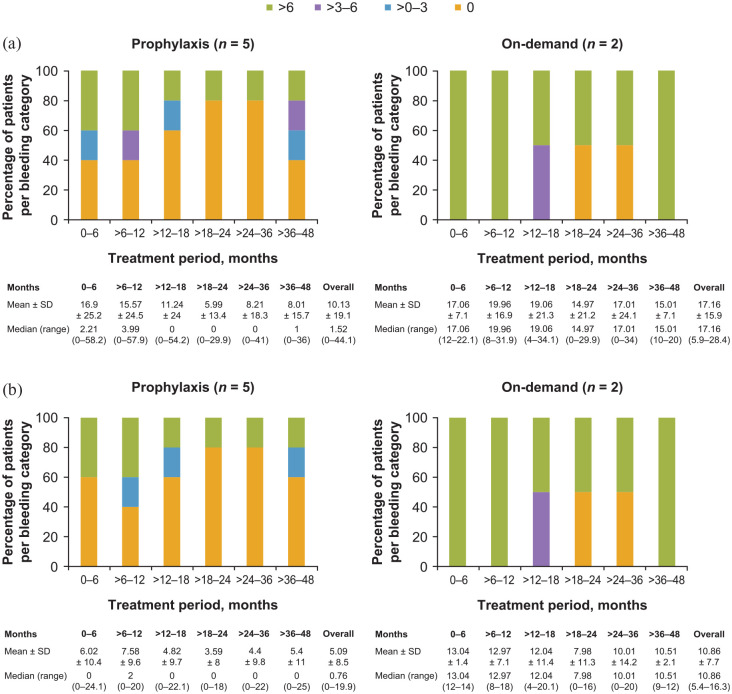
The mean ± SD overall ABR and AJBR for patients receiving aPCC prophylaxis who completed the study (n = 8) were 7.13 ± 15.11 and 3.57 ± 6.84, respectively. Two patients receiving aPCC prophylaxis who completed the study had zero ABRs and three patients had zero AJBRs. The mean ± SD overall ABR and AJBR for patients receiving on-demand therapy who completed the study (n = 4) were 8.64 ± 13.46 and 5.43 ± 7.69, respectively. One patient receiving on-demand therapy had a zero ABR and two had zero AJBRs (see Figure 3). The overall ABR and AJBR for the seven patients who had >48 months of follow-up are shown in Figure 4. ABRs and AJBRs by on-study treatment duration are also shown in Figure 4.
Figure 3.
Overall ABR and AJBR for patients who completed the study by (a) treatment regimen and (b) categorized (safety analysis set).
ABR is the number of all bleeding events standardized to 12 months. AJBR is the number of joint bleeding events standardized to 12 months. ABR and AJBR were only included in the analysis for patients with a regimen duration of ⩾90 days. All patients without confirmed zero bleeding events were set to missing. Bleeding events were counted for the regimen where the event occurred; therefore, patients who switched regimen could appear more than once. One patient switched regimen during the study and an ABR was calculated for both regimens.
ABR, annualized bleeding rate; AJBR, annualized joint bleeding rate; SD, standard deviation.
Figure 4.
(a) ABRs and (b) AJBRs for patients with >48 months of follow-up (safety analysis set).
ABR is the number of all bleeding events standardized to 12 months. AJBR is the number of joint bleeding events standardized to 12 months. ABR and AJBR were only included in the analysis for patients with a regimen duration of ⩾90 days. All patients without confirmed zero bleeding events were set to missing. Bleeding events were counted for the regimen where the event occurred; therefore, patients who switched regimen could appear more than once.
ABR, annualized bleeding rate; AJBR, annualized joint bleeding rate; SD, standard deviation.
Bleeding occurrence
Overall, 31 patients (81.6%) receiving aPCC prophylaxis experienced a bleeding event and 21 patients (55.3%) experienced spontaneous bleeding events. Overall, 10 patients (83.3%) receiving on-demand therapy experienced a bleeding event and 9 patients (75.0%) experienced spontaneous bleeding events (see Table 2).
Table 2.
Occurrence of bleeding events (safety analysis set).
| Number of patients, n (%) | Treatment regimen | |||
|---|---|---|---|---|
| Prophylaxis | On-demand | Unknown | No aPCC | |
| With any bleeding event | 31 (81.6) | 10 (83.3) | 0 (0.0) | 1 (50.0) |
| With any treated bleeding event | 26 (68.4) | 9 (75.0) | 0 (0.0) | 0 (0.0) |
| With any spontaneous bleeding event | 21 (55.3) | 9 (75.0) | 0 (0.0) | 0 (0.0) |
| With any injury/traumatic bleeding event | 23 (60.5) | 8 (66.7) | 0 (0.0) | 1 (50.0) |
| With any undetermined cause of bleeding event | 18 (47.4) | 5 (41.7) | 0 (0.0) | 1 (50.0) |
aPCC, activated prothrombin complex concentrate.
Any bleeding event includes breakthrough bleeding events. A treated bleeding event is defined as an event with a documented on-demand treatment administered within 72 h after the start of the event. The number of bleeds was calculated for each regimen; therefore, patients who switched regimens can appear more than once. Only patients with regimen duration of ⩾90 days are included in this analysis.
Overall, the mean ± SD number of bleeding events per patient was 14.9 ± 31.8 for patients receiving prophylaxis (n = 37). The mean ± SD number of joint bleeding events per patient was 8.4 ± 15.4. For patients receiving prophylaxis, the mean ± SD overall number of bleeding events per patient for target joints and non-target joints was 2.2 ± 6.2 and 6.2 ± 10.9, respectively. The mean ± SD number of bleeding events per patient was 20.8 ± 31.6 for patients receiving on-demand therapy (n = 12). The mean ± SD number of joint bleeding events per patient was 13.2 ± 18.9. For patients receiving on-demand therapy, the mean ± SD overall number of bleeding events per patient for target joints and non-target joints was 1.4 ± 4.0 and 11.8 ± 18.8, respectively.
Treatment effectiveness
The effectiveness of aPCC prophylaxis as measured by patients/caregivers or investigators using the Likert scale was assessed for 12,739 infusions. In total, 77.8% of infusions were assessed as ‘good’ and 18.2% were considered ‘excellent’ (see Table 3). Overall, 539 on-demand infusions that were administered within 72 h of a bleeding event were assessed for effectiveness in terms of breakthrough bleed cessation. For patients receiving prophylaxis who also received on-demand infusions, 51.9% of the on-demand infusions were assessed as ‘good’ and 14.3% were assessed as ‘excellent’. For patients receiving on-demand therapy, 41.3% of infusions were assessed as ‘good’ and 45.3% were assessed as ‘excellent’ (see Table 4).
Table 3.
Overall effectiveness of prophylactic infusions measured by Likert scale (safety analysis set).
| Treatment effectiveness measured by Likert scale | Classification of infusion | |||
|---|---|---|---|---|
| Prophylaxis ,n (%) (n = 11,414) |
On-demand, n (%) (n = 1320) |
Unclassified, n (%) (n = 5) |
Total, n (%) (n = 12,739) |
|
| Excellent | 2123 (18.6) | 194 (14.7) | 1 (20.0) | 2318 (18.2) |
| Good | 8957 (78.5) | 956 (72.4) | 4 (80.0) | 9917 (77.8) |
| Fair | 276 (2.4) | 156 (11.8) | 0 (0.0) | 432 (3.4) |
| Poor | 58 (0.5) | 14 (1.1) | 0 (0.0) | 72 (0.6) |
Data presented by treatment group at screening. Data presented for patients receiving on-demand therapy at screening switched to prophylaxis during the study. The treatment columns are defined as the infusion type specified in the infusion log. In instances where the infusion type was not specified, the infusion was considered as unclassified. Effectiveness of prophylactic treatment was based on the number of infusions in the infusion log. Classification of infusion as documented by the patient or caregiver for treatments given at home or by the investigator for treatments given in the hospital/clinic. For patients receiving on-demand therapy, infusions that were classified as prophylactic were excluded.
Table 4.
Treatment effectiveness for acute bleeding event cessation (safety analysis set).
| Treatment effectiveness measured by Likert scale | Treatment regimen | ||
|---|---|---|---|
| Prophylaxis, n (%) (n = 314) |
On-demand, n (%) (n = 225) |
Total, n
(%) (N = 539) |
|
| Excellent | 45 (14.3) | 102 (45.3) | 147 (27.3) |
| Good | 163 (51.9) | 93 (41.3) | 256 (47.5) |
| Fair | 81 (25.8) | 30 (13.3) | 111 (20.6) |
| Poor | 25 (8.0) | 0 (0.0) | 25 (4.6) |
Classification of infusion as documented by the patient or caregiver for treatments given at home or by the investigator for treatments given in the hospital/clinic.
aPCC therapy regimen and consumption
The total annualized dosage was 13,345.56 U/kg for prophylaxis and 2100.69 U/kg for on-demand therapy (see Table 5). The median (range) dose per infusion was 65.76 U/kg (37–100 U/kg). The median (range) number of weekly infusions was 4.12 (0.5–14).
Table 5.
Overall aPCC consumption (safety analysis set).
| Median (range) | Prophylaxis (n = 35) |
On-demand (n = 9) |
|---|---|---|
| Number of weekly infusions | 4.12 (0.5–14) a | 0.83 (0.2–6) |
| Weekly dose, U/kg | 255.77 (32–900) | 40.26 (14–629) |
| Dose per infusion, U/kg | 65.76 (37–100) | 67.72 (48–104) |
| Total annualized dosage, U/kg | 13,345.56 (1670–46,947) | 2100.69 (712–32,831) |
aPCC, activated prothrombin complex concentrate.
Only patients with a regimen duration of ⩾90 days are included in this analysis.
n = 36.
Target joints
In the safety analysis set, 17 patients had target joints at screening, of whom 12 were receiving aPCC prophylaxis and 5 were receiving on-demand therapy (see Table 6). The overall mean ± SD ABR and AJBR in patients who received aPCC prophylaxis and had target joints at screening (n = 12) were 4.26 ± 3.86 and 3.24 ± 3.40, respectively. For patients who received on-demand therapy and had target joints at screening (n = 4), overall mean ± SD ABR and AJBR were 18.01 ± 12.26 and 11.58 ± 8.98, respectively. Five patients developed new target joints during study participation, of whom four were receiving prophylaxis and one was receiving on-demand therapy (see Table 6).
Table 6.
Target joints a at screening and incidence of new target joints (safety analysis set).
| Treatment regimen | ||||||
|---|---|---|---|---|---|---|
| Prophylaxis | On-demand | Switcher | No aPCC | Total | ||
| Any target joint, at screening, n (%) | Yes | 12 (32.4) | 5 (38.5) | 0 (0.0) | 0 (0.0) | 17 (34.0) |
| No | 25 (67.6) | 8 (61.5) | 0 (0.0) | 0 (0.0) | 33 (66.0) | |
| Any target joint, whole study (overall), n (%) | Yes | 13 (38.2) | 5 (50.0) | 3 (50.0) | 0 (0.0) | 21 (42.0) |
| No | 21 (61.8) | 5 (50.0) | 3 (50.0) | 0 (0.0) | 29 (58.0) | |
| Any new target joint, whole study (overall), n (%) | Yes | 4 (12.5) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 5 (10.6) |
| No | 28 (87.5) | 8 (88.9) | 6 (100.0) | 0 (0.0) | 42 (89.4) | |
aPCC, activated prothrombin complex concentrate.
For any target joint at screening, data are classified by treatment regimen at screening. All other parameters are classified by treatment regimen reported throughout the study duration.
A target joint was defined as a joint in which three or more bleeding events had occurred in a 6-month period.
Safety
For the safety analysis set, 177 AEs were reported in 28 of 40 patients (70.0%) receiving aPCC prophylaxis, and 31 AEs were reported in 10 of 13 patients (76.9%) receiving on-demand therapy (see Table 7). The most frequently reported AEs (⩾10% of patients) were infections and musculoskeletal disorders (see Supplementary Table 4). No thrombotic microangiopathy events were reported, and no patients experienced an AE that resulted in death.
Table 7.
Summary of AEs and study drug–related AEs (safety analysis set).
| Prophylaxis (n = 40) | On-demand (n = 13) | No aPCC (n = 3) | ||||
|---|---|---|---|---|---|---|
| Patients, n (%) |
Events, n |
Patients, n (%) |
Events, n |
Patients, n (%) |
Events, n |
|
| AEs | 28 (70.0) | 177 | 10 (76.9) | 31 | 1 (33.3) | 11 |
| AEs leading to study drug interruption | 3 (7.5) | 3 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Severe AEs | 10 (25.0) | 22 | 3 (23.1) | 5 | 0 (0.0) | 0 |
| Serious AEs | 17 (42.5) | 54 | 5 (38.5) | 9 | 0 (0.0) | 0 |
| Life-threatening | 3 (7.5) | 3 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Leading to drug interruption | 2 (5.0) | 2 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Study drug–related AEs | 4 (10.0) | 10 | 1 (7.7) | 1 | 0 (0.0) | 0 |
| Probably related | 2 (5.0) | 6 | 1 (7.7) | 1 | 0 (0.0) | 0 |
| Hypersensitivity | 1 (2.5) | 1 | 1 (7.7) | 1 | 0 (0.0) | 0 |
| Drug hypersensitivity | 1 (2.5) | 4 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Hemarthrosis | 1 (2.5) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Possibly related | 2 (5.0) | 4 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Acute myocardial infarction | 1 (2.5) | 1 a | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Coronary arterial embolism | 1 (2.5) | 1 a | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Headache | 1 (2.5) | 2 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Leading to study drug interruption | 1 (2.5) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Serious study drug–related AEs | 1 (2.5) | 2 a | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Non-serious study drug–related AEs | 3 (7.5) | 8 | 1 (7.7) | 1 | 0 (0.0) | 0 |
AE, adverse event; aPCC, activated prothrombin complex concentrate.
Based on Medical Dictionary for Regulatory Activities coded terms. A patient with more than one event in a specific category was only counted once. Percentages were based on the total number of patients for each regimen. Patients can appear in more than one regimen group depending on when the AE occurred.
Two serious AEs (acute myocardial infarction due to coronary artery embolism) reported in one patient receiving prophylaxis were considered possibly related to treatment.
Ten AEs considered related to aPCC occurred in 4 of 40 patients (10.0%) receiving aPCC prophylaxis and 1 aPCC-related AE occurred in 1 of 13 patients (7.7%) receiving on-demand therapy. The patient with hemophilia B experienced four drug hypersensitivity events while receiving prophylaxis. Each of these events was considered probably related to aPCC (see Table 7). In total, 54 serious AEs occurred in 17 of 40 patients (42.5%) receiving prophylaxis and 9 events occurred in 5 of 13 patients (38.5%) receiving on-demand therapy. Three patients (7.5%) receiving prophylaxis experienced a serious AE that was classified as life-threatening (klebsiella sepsis, device-related sepsis, n = 1; epistaxis, n = 1; abdominal wall hematoma, n = 1). Life-threatening events were defined as events in which the patient was, in the judgment of the investigator, at risk of death at the time of the event. Two of these events resolved and one event (klebsiella sepsis, device-related sepsis) resolved with sequelae. Two thromboembolic events were reported: acute myocardial infarction due to coronary artery embolism. Both events were reported in a patient who was receiving aPCC prophylaxis for the treatment of a gastro-intestinal bleed and were considered serious AEs possibly related to aPCC. The patient recovered and aPCC treatment was not discontinued, although the dose was reduced after these events. This patient also experienced a duodenal ulcer hemorrhage that was considered a serious AE.
Three (7.5%) of the 40 patients who received prophylaxis experienced an AE leading to study drug interruption: 1 patient experienced hematuria, a serious AE that was not considered related to aPCC; 1 patient experienced a skin injury, a serious AE that was not considered related to aPCC; 1 patient experienced hemarthrosis, which was not a serious AE but was considered probably related to aPCC. No patients who were receiving on-demand therapy experienced an AE that led to study drug interruption. No AEs led to study drug withdrawal. No AEs led to withdrawal or discontinuation from the study.
Pediatric data
Demographics and baseline characteristics for the 17 patients aged 0–12 years old are shown in Supplementary Table 5. At screening, 14 patients were receiving aPCC prophylaxis and 3 were receiving on-demand therapy. The mean ± SD overall ABR and AJBR for patients receiving aPCC prophylaxis were 12.32 ± 16.99 and 6.62 ± 7.98, respectively. The mean ± SD overall ABR and AJBR for patients receiving on-demand therapy were 16.77 ± 10.65 and 11.76 ± 7.93, respectively (see Supplementary Figure 2).
Overall, 111 AEs were reported in 12 of 14 patients (85.7%) aged 0–12 years receiving aPCC prophylaxis, and 11 AEs were reported in 3 patients receiving on-demand therapy. Eight AEs considered related to aPCC (drug hypersensitivity, n = 4; hypersensitivity, n = 1; hemarthrosis, n = 1; headache, n = 2) occurred in 3 of 14 patients (21.4%) receiving aPCC prophylaxis. No AEs considered related to aPCC were reported in patients receiving on-demand therapy. No serious AEs considered related to aPCC were reported in patients aged 0–12 years.
Discussion
The FEIBA GO study demonstrates the effectiveness of aPCC as both an on-demand therapy and as prophylaxis in the treatment of patients with hemophilia A and high-responding inhibitors. The safety profile for aPCC reported in this study is also generally consistent with previous studies.17,20,21,23 Notably, a meta-analysis of 39 studies determined that the incidence rate of thromboembolic events was 5.09 (95% CI: 0.01–1795.60) per 100,000 infusions for patients receiving aPCC on-demand therapy, whereas no thromboembolic events were reported in patients receiving prophylaxis. 23 In this study, two thromboembolic events were reported and no thrombotic microangiopathy events were reported.
aPCC prophylaxis can be challenging to administer as it can require a long time to reconstitute the lyophilized product, a high volume per dose, and a prolonged infusion duration. 25 Despite these challenges, findings from the FEIBA GO study support the viability of aPCC prophylaxis in patients with hemophilia and high-responding inhibitors. Findings from two previous randomized clinical trials also support the use of aPCC prophylaxis in patients with hemophilia A or B and inhibitors.17,20
The FEIBA GO study shows that there is a wide variation in the prophylaxis regimens being administered to patients in real-world clinical practice, with regimens ranging from 0.5 to 14 weekly infusions and a dose per infusion of 37–100 U/kg, with a median dose per infusion of 66 U/kg. The median dose per infusion observed in the FEIBA GO study was below the recommended dosing of 85 U/kg every other day outlined in the US prescribing information for routine prophylaxis. 9 This would suggest that, in clinical practice, physicians typically adjust aPCC prophylaxis to meet the individual needs of the patient.
The 2020 WFH guidelines recommend the use of aPCC or rFVIIa for the management of breakthrough bleeding events experienced by patients with hemophilia A and high-responding inhibitors receiving emicizumab prophylaxis, with rFVIIa preferred over aPCC to avoid the risk of thrombotic microangiopathy. 1 aPCC and rFVIIa have been shown to have a similar effect on the management of joint bleeding events, although the efficacy of the products was rated differently by a substantial proportion of patients.1,26 This interpatient variability further highlights the need to individualize treatment and suggests that some patients with hemophilia may respond better to aPCC treatment.
A key limitation was the number of patients who discontinued from the study and the small number of patients who had >48 months of follow-up. The FEIBA GO study was terminated prematurely on 28 February 2020 because it was not feasible to reach the target patient number or achieve the planned length of clinical observation for the enrolled patients. Emicizumab was approved in the United States in 2017 and in Europe in 2018 for routine prophylaxis in patients with hemophilia A with inhibitors.14,15 The subsequent availability of emicizumab during the course of the FEIBA GO study may have contributed to the high discontinuation rate. Difficulties in maintaining regular follow-up visits, especially if patients had moved from pediatric to adult centers or switched between centers during the study duration, also contributed to the high discontinuation rate, which resulted in a low number of patients completing the planned observation period of 4 years.
Another limitation was the fact that this was an observational study which relied on data collection in real-world clinical practice. It was not always possible to anticipate clinical monitoring; therefore, some types of data (e.g. individual bleeding event information, treatment details, and laboratory measures) were not collected consistently or efficiently. In addition, limited data were available for many secondary endpoints, such as quality of life, pain, and joint assessments. This may reflect difficulties in obtaining data in clinical practice because of patient/physician time constraints as well as the number and complexity of available questionnaires. In addition, we were unable to collect detailed information regarding treatment and outcomes from surgical procedures as their management was at the discretion of the investigator.
Bypassing agents remain an important therapeutic option for the treatment of patients with hemophilia A and high-responding inhibitors, particularly in countries where emicizumab is not available. It is also important to acknowledge that emicizumab is not indicated for the treatment of patients with hemophilia B. 1 Owing to small patient numbers, it was not possible to draw any clinically meaningful conclusions from the FEIBA GO study regarding aPCC treatment in patients with hemophilia B. Further research into the effectiveness and safety of aPCC treatment in patients with hemophilia B would, however, be valuable.
Conclusions
In conclusion, despite encountering challenges inherent to observational real-world studies, FEIBA GO demonstrated the long-term, real-world effectiveness and consistent safety profile of aPCC as an on-demand therapy for the control of bleeding events and as a prophylactic treatment in patients with hemophilia and high-responding inhibitors.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207231184323 for Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study by Carmen Escuriola Ettingshausen, Cedric Hermans, Pål A. Holme, Ana R. Cid, Kate Khair, Johannes Oldenburg, Claude Négrier, Jaco Botha, Aurelia Lelli and Jerzy Windyga in Therapeutic Advances in Hematology
Supplemental material, sj-eps-2-tah-10.1177_20406207231184323 for Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study by Carmen Escuriola Ettingshausen, Cedric Hermans, Pål A. Holme, Ana R. Cid, Kate Khair, Johannes Oldenburg, Claude Négrier, Jaco Botha, Aurelia Lelli and Jerzy Windyga in Therapeutic Advances in Hematology
Supplemental material, sj-eps-3-tah-10.1177_20406207231184323 for Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study by Carmen Escuriola Ettingshausen, Cedric Hermans, Pål A. Holme, Ana R. Cid, Kate Khair, Johannes Oldenburg, Claude Négrier, Jaco Botha, Aurelia Lelli and Jerzy Windyga in Therapeutic Advances in Hematology
Acknowledgments
We would like to thank Nataliya Kemenyash, an employee of Takeda Pharmaceuticals International AG, for her contribution to the management of the study and the preparation of data for analysis. Medical writing support for this article was provided by Sarah Morgan, PhD, employee of Excel Medical Affairs (Fairfield, CT, USA), and was funded by Takeda Development Center Americas Inc., a Takeda company, Lexington, MA, USA. Sarah Morgan, PhD, employee of Excel Medical Affairs (Fairfield, CT, USA) also submitted the article on behalf of the authors, who authorized the submission of their article via a third party.
Footnotes
ORCID iD: Cedric Hermans  https://orcid.org/0000-0001-5429-8437
https://orcid.org/0000-0001-5429-8437
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Carmen Escuriola Ettingshausen, Hämophilie-Zentrum Rhein Main – HZRM GmbH, Mörfelden-Walldorf, Germany.
Cedric Hermans, Hemostasis and Thrombosis Unit, Division of Haematology, Cliniques Universitaires Saint-Luc, UCLouvain, Brussels, Belgium.
Pål A. Holme, Department of Haematology, Oslo University Hospital and Institute for Clinical Medicine, University of Oslo, Oslo, Norway
Ana R. Cid, Unidad de Hemostasia y Trombosis, Hospital Universitario y Politécnico La Fe, València, Spain
Kate Khair, Centre for Outcomes and Experience Research in Children’s Health, Illness and Disability (ORCHID), Great Ormond Street Hospital for Children, London, UK.
Johannes Oldenburg, Institute of Experimental Hematology and Transfusion Medicine, Bonn University Clinic, Bonn, Germany.
Claude Négrier, University Claude Bernard Lyon 1, Lyon, France.
Jaco Botha, Takeda Pharmaceuticals International AG, Zürich, Switzerland.
Aurelia Lelli, Takeda Pharmaceuticals International AG, Zürich, Switzerland; R&D, Plasma Derived Therapy, Takeda Pharmaceuticals International AG, Thurgauerstrasse 130, 8152 Glattpark-Opfikon, Zürich, Switzerland.
Jerzy Windyga, Department of Hemostasis Disorders and Internal Medicine, Institute of Hematology and Transfusion Medicine, Warsaw, Poland.
Declarations
Ethics approval and consent to participate: This study was conducted after ethics committee approval was obtained from each study site. All patients and/or their legally authorized representative provided written informed consent before entering the study. Investigators were required to comply with the protocol, International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. Investigators were responsible for the conduct of all aspects of the study at the study site.
Consent for publication: Not applicable.
Author contributions: Carmen Escuriola Ettingshausen: Formal analysis; Investigation; Methodology; Writing – review & editing.
Cedric Hermans: Formal analysis; Investigation; Methodology; Writing – review & editing.
Pål A. Holme: Formal analysis; Investigation; Methodology; Writing – review & editing.
Ana R. Cid: Formal analysis; Investigation; Methodology; Writing – review & editing.
Kate Khair: Formal analysis; Investigation; Methodology; Writing – review & editing.
Johannes Oldenburg: Formal analysis; Investigation; Methodology; Writing – review & editing.
Claude Négrier: Formal analysis; Investigation; Methodology; Writing – review & editing.
Jaco Botha: Formal analysis; Writing – review & editing.
Aurelia Lelli: Formal analysis; Writing – review & editing.
Jerzy Windyga: Formal analysis; Investigation; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The FEIBA GO study was funded by Baxalta Innovations GmbH, a Takeda company, Vienna, Austria.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Carmen Escuriola Ettingshausen: grant/research support [Biotest, CSL Behring, Octapharma, Shire (a Takeda company), Sobi]; honoraria for lectures/advisory boards [Bayer, Biotest, CSL Behring, Grifols, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Shire (a Takeda company), Sobi]; and consultancy [BioMarin, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Roche/Chugai, Shire (a Takeda company), Sobi]. Cedric Hermans: grant/research support [Bayer, Pfizer, Shire (a Takeda company), Sobi]; honoraria for lectures/advisory boards [Bayer, Biogen, CAF-DCF, CSL Behring, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]; and consultancy [Bayer, Biogen, CAF-DCF, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]. Pål A. Holme: grant/research support [Bayer, Octapharma, Pfizer, Shire (a Takeda company), Sobi]; and honoraria for lectures/advisory boards [Bayer, CSL Behring, Novo Nordisk, Pfizer, Shire (a Takeda company), Sobi]. Ana R. Cid: honoraria for lectures/advisory boards [Novo Nordisk, Roche, Shire (a Takeda company), Sobi]; and consultancy (Roche, Sobi). Kate Khair: grant/research support [Baxalta/Shire (a Takeda company), CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi, uniQure]; honoraria for lectures/advisory boards [Bayer, Novo Nordisk, Pfizer, Roche, Shire (a Takeda company), Sobi]; consultancy [Bayer, Novo Nordisk, Roche, Shire (a Takeda company), Sobi]; support attending meetings and/or travel (Bayer, Novo Nordisk); leadership or fiduciary role in other board, society, committee or advocacy group (Vice President board of Trustees The Haemophilia Society); and stock or stock options (Haemnet Ltd, Medikhair). Johannes Oldenburg: grant/research support [Bayer, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Shire (a Takeda company)]; honoraria for lectures/advisory boards [Bayer, Biogen, Biotest, Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]; and consultancy [Bayer, Biogen, Biotest, Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Shire (a Takeda company), Sobi]. Claude Négrier: grant/research support [CSL Behring, Octapharma, Shire (a Takeda company), Sobi]; and consultancy [Bayer, BioMarin, CSL Behring, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Shire (a Takeda company), Sobi, Spark]. Jaco Botha: employee of Takeda Pharmaceuticals International AG, and Takeda stock owner. Aurelia Lelli: employee of Takeda Pharmaceuticals International AG, and Takeda stock owner. Jerzy Windyga: grant/research support and honoraria for lectures [Amgen, Alnylam, Baxalta/Shire (a Takeda company), Bayer, CSL Behring, LFB, Novartis, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Siemens, Sobi, Swixx Biopharma].
Availability of data and materials: The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection regulations, and requirements for consent and anonymization.
References
- 1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26(Suppl. 6): 1–158. [DOI] [PubMed] [Google Scholar]
- 2. Gouw SC, Van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood 2013; 121: 4046–4055. [DOI] [PubMed] [Google Scholar]
- 3. Van den Berg HM, Fischer K, Carcao M, et al. Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood 2019; 134: 317–320 [DOI] [PubMed] [Google Scholar]
- 4. Male C, Andersson NG, Rafowicz A, et al. Inhibitor incidence in an unselected cohort of previously untreated patients with severe haemophilia B: a PedNet study. Haematologica 2021; 106: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ljung R, Auerswald G, Benson G, et al. Inhibitors in haemophilia A and B: management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients. Eur J Haematol 2019; 102: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckhardt CL, Loomans JI, Van Velzen AS, et al. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost 2015; 13: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 7. Guh S, Grosse SD, McAlister S, et al. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia 2012; 18: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindvall K, Von Mackensen S, Elmståhl S, et al. Increased burden on caregivers of having a child with haemophilia complicated by inhibitors. Pediatr Blood Cancer 2014; 61: 706–711. [DOI] [PubMed] [Google Scholar]
- 9. Baxalta US Inc. FEIBA (anti-inhibitor coagulant complex). Highlights of prescribing information, https://www.shirecontent.com/PI/PDFs/FEIBA_USA_ENG.pdf (2023, accessed 27 June 2023).
- 10. Takeda UK Ltd. FEIBA 50 U/ml powder and solvent for solution for infusion. Summary of product characteristics, https://www.medicines.org.uk/emc/medicine/30168 (2021, accessed 15 March 2023).
- 11. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood 2014; 124: 3365–3372. [DOI] [PubMed] [Google Scholar]
- 12. Laboratoire Français du Fractionnement et des Biotechnologies. CEVENFACTA. Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/cevenfacta-epar-product-information_en.pdf (2022, accessed 15 March 2023).
- 13. HEMA biologics. SEVENFACT [coagulation factor VIIa (recombinant)-jncw]. Highlights of prescribing information, https://sevenfact.com/Sevenfact_PI.pdf (2022, accessed 15 March 2023).
- 14. Roche Registration GmbH. HEMLIBRA. Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf (2023, accessed 15 March 2023).
- 15. Genentech Inc. HEMLIBRA (emicizumab-kxwh). Highlights of prescribing information, https://www.gene.com/download/pdf/hemlibra_prescribing.pdf (2023, accessed 27 June 2023).
- 16. Brackmann HH, Schramm W, Oldenburg J, et al. Origins, development, current challenges and future directions with activated prothrombin complex concentrate for the treatment of patients with congenital haemophilia with inhibitors. Hamostaseologie 2020; 40: 606–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antunes SV, Tangada S, Stasyshyn O, et al. Randomized comparison of prophylaxis and on-demand regimens with FEIBA NF in the treatment of haemophilia A and B with inhibitors. Haemophilia 2014; 20: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hilgartner M, Aledort L, Andes A, et al. Efficacy and safety of vapor-heated anti-inhibitor coagulant complex in hemophilia patients. FEIBA study group. Transfusion 1990; 30: 626–630. [DOI] [PubMed] [Google Scholar]
- 19. Hilgartner MW, Knatterud GL. The use of factor eight inhibitor by-passing activity (FEIBA immuno) product for treatment of bleeding episodes in hemophiliacs with inhibitors. Blood 1983; 61: 36–40. [PubMed] [Google Scholar]
- 20. Leissinger C, Gringeri A, Antmen B, et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N Engl J Med 2011; 365: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 21. Négrier C, Lienhart A, Numerof R, et al. SURgical interventions with FEIBA (SURF): international registry of surgery in haemophilia patients with inhibitory antibodies. Haemophilia 2013; 19: e143–e150. [DOI] [PubMed] [Google Scholar]
- 22. Negrier C, Voisin S, Baghaei F, et al. Global post-authorization safety surveillance study: real-world data on prophylaxis and on-demand treatment using FEIBA (an activated prothrombin complex concentrate). Blood Coagul Fibrinolysis 2016; 27: 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rota M, Cortesi PA, Crea R, et al. Thromboembolic event rate in patients exposed to anti-inhibitor coagulant complex: a meta-analysis of 40-year published data. Blood Adv 2017; 1: 2637–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teitel JM. Treatment and prevention of bleeding in congenital hemophilia A patients with inhibitors. Transfus Apher Sci 2018; 57: 466–471. [DOI] [PubMed] [Google Scholar]
- 26. Astermark J, Donfield SM, DiMichele DM, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood 2007; 109: 546–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207231184323 for Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study by Carmen Escuriola Ettingshausen, Cedric Hermans, Pål A. Holme, Ana R. Cid, Kate Khair, Johannes Oldenburg, Claude Négrier, Jaco Botha, Aurelia Lelli and Jerzy Windyga in Therapeutic Advances in Hematology
Supplemental material, sj-eps-2-tah-10.1177_20406207231184323 for Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study by Carmen Escuriola Ettingshausen, Cedric Hermans, Pål A. Holme, Ana R. Cid, Kate Khair, Johannes Oldenburg, Claude Négrier, Jaco Botha, Aurelia Lelli and Jerzy Windyga in Therapeutic Advances in Hematology
Supplemental material, sj-eps-3-tah-10.1177_20406207231184323 for Real-world data in patients with congenital hemophilia and inhibitors: final data from the FEIBA Global Outcome (FEIBA GO) study by Carmen Escuriola Ettingshausen, Cedric Hermans, Pål A. Holme, Ana R. Cid, Kate Khair, Johannes Oldenburg, Claude Négrier, Jaco Botha, Aurelia Lelli and Jerzy Windyga in Therapeutic Advances in Hematology