Abstract
Introduction
Kidney transplant candidates (KTCs) need to be in optimal physical and psychological condition prior to surgery. However, KTCs often experience compromised functional capacity which can be characterised as frailty. Prehabilitation, the enhancement of a person’s functional capacity, may be an effective intervention to improve the health status of KTCs. The PREhabilitation of CAndidates for REnal Transplantation (PreCareTx) study aims to examine the effectiveness of a multimodal prehabilitation programme on the health status of KTCs, and to explore the potential of implementation of prehabilitation in daily clinical practice.
Methods and analysis
This study uses a single centre, effectiveness-implementation hybrid type I study design, comprised of a randomised controlled trial and a mixed-methods study. Adult patients who are currently on the transplant waiting list or are waitlisted during the study period, at a university medical centre in The Netherlands, will be randomly assigned to either prehabilitation (n=64) or care as usual (n=64) groups. The prehabilitation group will undergo a 12-week home-based, tailored prehabilitation programme consisting of physical and/or nutritional and/or psychosocial interventions depending on the participant’s deficits. This programme will be followed by a 12-week maintenance programme in order to enhance the incorporation of the interventions into daily life. The primary endpoint of this study is a change in frailty status as a proxy for health status. Secondary endpoints include changes in physical fitness, nutritional status, psychological well-being, quality of life and clinical outcomes. Tertiary endpoints include the safety, feasibility and acceptability of the prehabilitation programme, and the barriers and facilitators for further implementation.
Ethics and dissemination
Medical ethical approval was granted by the Medical Ethics Committee Groningen, Netherlands (M22.421). Written informed consent will be obtained from all participants. The results will be disseminated at international conferences and in peer-reviewed journals.
Trial registration number
ClinicalTrials.gov, NCT05489432.
Keywords: Renal transplantation, NUTRITION & DIETETICS, Rehabilitation medicine, MENTAL HEALTH, Quality of Life
Strengths and limitations of this study.
The intervention was developed in co-creation with kidney transplant candidates and recipients, their significant others and healthcare providers involved in kidney transplant care.
A randomised controlled trial will provide a high-quality assessment of the effect of a multimodal, tailor-made prehabilitation programme on frailty and other important patient-centred outcomes regarding physical fitness, nutritional status and psychosocial well-being.
A mixed-methods study will provide insight into the feasibility and acceptability of prehabilitation in a real-world setting by analysing the barriers and facilitators associated with this intervention.
This study is being conducted at a single centre and only includes kidney transplant candidates.
The study is not double-blinded due to the nature of the intervention.
Introduction
Kidney transplant candidates (KTCs) may have a compromised health status due to disease progression, comorbidities and the adverse effects of dialysis. This may lead to impaired physical fitness, lower quality of life and an increased risk of developing psychological problems.1–5 Poor health status is related to a low level of physical activity, eliciting a cycle of deteriorating physical fitness in which multiple factors are involved, including muscle wasting, malnutrition, inflammation and fatigue.4 Data from the TransplantLines Biobank and Cohort study6 at our centre, the University Medical Center Groningen (UMCG), showed that of 424 KTCs, 87% had one or more problems related to physical or psychological fitness prior to transplantation. Regarding physical fitness, 55% of KTCs had problems related to decreased muscle strength and/or walking ability and 45% had a suboptimal nutritional status. Concerning psychological well-being, 36% showed high symptom levels of anxiety and/or depression. In addition, 58% of the KTCs experienced severe fatigue and 19% experienced moderate fatigue. These findings show that KTCs are a vulnerable patient population and exhibit signs of frailty. Frailty is a multidimensional syndrome and captures the multiple domains involved in the health status of KTCs. It is a physiological condition caused by declines across physical, cognitive and physiological reserves.7–9 Among KTCs, frailty is associated with an increased inflammatory state, hospitalisations and waitlist mortality.10–12 It is estimated that one in six kidney transplant (KT) recipients is frail prior to transplantation.13
Studies have shown that physical fitness and psychological well-being can be improved by the means of prehabilitation.14–18 Prehabilitation is an intervention aimed at optimising the patient’s overall fitness before an operation to enhance recovery after the surgery and improve outcomes. Prehabilitation may also be effective in improving the overall health status of KTCs prior to the KT. It focuses on implementing lifestyle changes in order to enable patients to withstand the stress of surgery, reduce the risk of postoperative complications, unplanned readmissions and to enhance recovery.19 Prehabilitation comprises physical training, dietary management and psychosocial interventions.19 The waiting-list period before the KT provides a window of opportunity to improve the overall fitness of KTCs by prehabilitation. In The Netherlands, the duration of the waiting-list period ranges from less than 3 months for those who receive a kidney from a living donor to over 3 years in case of deceased donor kidney transplantation. Especially for the latter, the duration of the waiting-list period is unpredictable. By offering a prehabilitation programme tailored to the needs and possibilities of KTCs prior to transplantation, patients may be more likely to adopt a sustainable, healthy lifestyle.
Studies have shown that prehabilitation during the waiting-list period in transplant candidates is feasible.15 18 20–22 Three studies showed that prehabilitation significantly improved physical activity, fatigue, walking time and grip strength during the waitlist period in KTCs.18 20 21 However, these studies had a small sample size, and the interventions were not provided in a multimodal approach. As KTCs experience deficits across multiple reserves, a multimodal approach is essential. Additionally, complex interactions between the physical and psychological health of a patient are addressed when multimodal interventions are implemented.23 Therefore, the effectiveness of a multimodal tailored prehabilitation programme in KTCs still needs to be determined.
The primary objective of this study is to measure the effect of a 12-week home-based, tailor-made multimodal prehabilitation programme on changes in frailty status between T0 (screening for modifiable problems) and T1 (13 weeks after start of the prehabilitation programme). Furthermore, changes in physical functioning, nutritional status, psychological well-being, quality of life and clinical outcomes between T0 and T1 will be measured.
The secondary objectives are to determine the sustainability of the results regarding frailty status and changes in physical functioning, nutritional status, psychological well-being and quality of life at 6 months after the start of the study.
The tertiary objective of this study is to explore the potential for further implementation of prehabilitation in a daily clinical practice. This will be done by examining the safety, feasibility and acceptability of the prehabilitation programme and barriers and facilitators for further implementation.
Methods and analysis
Trial design
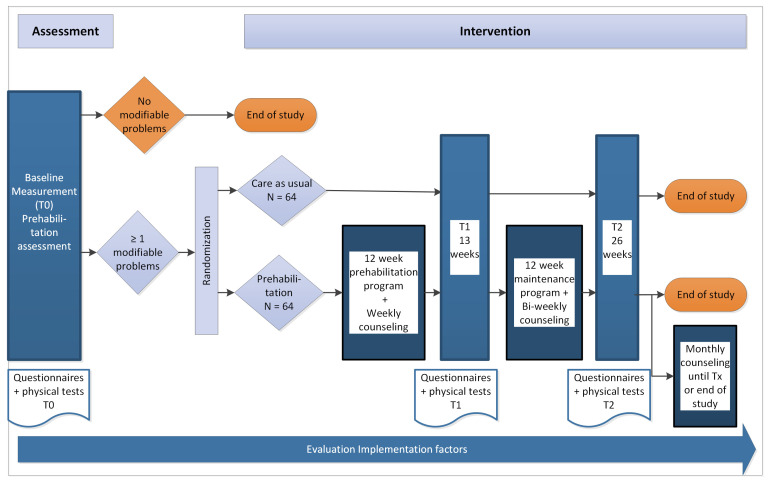
The PreCareTx study uses a single centre, effectiveness-implementation hybrid type I study design, consisting of a randomised controlled trial and a mixed-methods study. An overview of the study is given in figure 1. The duration of the study will be 3 years, starting in January 2023. The study is reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials statement (online supplemental material 1).24 The study has been registered on ClinicalTrials.gov.
Figure 1.
Overview PreCareTx study.
bmjopen-2023-072805supp001.pdf (67.7KB, pdf)
Study setting
The intervention will be conducted in the KTCs’ home environment, depending on their needs and preferences. Study visits will be conducted at the UMCG in The Netherlands at the following time points: baseline (T0), and at week 13 (T1) and week 26 (T2) after randomisation.
Recruitment
Patients on the UMCG waiting list for kidney transplantation or waitlisted during the inclusion period, will be recruited by their treating physician and receive an information letter about the risks and benefits of the study. Written informed consent will be obtained from the patient (online supplemental material 2). Patient recruitment will start in January 2023 and end in June 2025.
bmjopen-2023-072805supp002.pdf (215.2KB, pdf)
Eligibility criteria
In order to be eligible to participate in this study, potential participants must meet all the following inclusion criteria:
Adult KTC (≥18 years).
Listed for kidney transplantation on the UMCG KT waiting list at the start of the study or waitlisted during the inclusion period (January 2023 to June 2025).
The exclusion criteria include:
Inability to read and/or speak the Dutch language.
Combined organ transplantation (eg, kidney+pancreas, kidney+liver).
In case of living donor KT: a transplantation planned within 3 months.
Involved in a lifestyle intervention study.
Participant screening and assessment
After informed consent, participants will be screened for problems regarding physical activity, nutritional status or psychological well-being in an assessment.
To evaluate physical functioning, participants will complete several questionnaires, including the Duke Activity Status Index (DASI), the physical subscale of the Short Form 36 (SF-36) and the Short QUestionnaire to ASsess Health-enhancing physical activity (SQUASH). Additionally, participants will wear an activity tracker for 3 days and their handgrip, biceps and quadriceps strength will be measured. Furthermore, the Short Physical Performance Battery (SPPB) and the steep ramp test (SRT) will be performed.
To assess nutritional status, participants will complete the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) and maintain a food diary for 3 days. In addition, the participant’s hip-waist ratio and their body mass index (BMI) will be measured. Lastly, a bioimpedance analysis (BIA) will be conducted.
For the evaluation of psychological functioning, participants will be asked to complete the following questionnaires: State-Trait Anxiety Inventory (STAI6), Patient Health Questionnaire 9 (PHQ-9) and Checklist Individual Strength: subjective fatigue (CIS8R). Finally, the Montreal Cognitive Assessment (MoCA) will be administered.
To assess frailty status and health-related quality of life (HRQoL), each participant will complete the Tilburg Frailty Indicator (TFI) and the SF-36, respectively.
The details of the assessment are described in table 1 and under outcome measurements. Participants who present with one or more modifiable problem(s) will be eligible to take part in the intervention study. In this study, a modifiable problem is defined as a problem that can be altered by the means of prehabilitation.
Table 1.
Overview of questionnaires and measurements at various measurement points
| Baseline (T0) | Week 13 (T1) | Week 26 (T2) | |
| Measurements at home | |||
| Food diary (3 days) | X | X | X |
| Activity tracker (3 days) | X | X | X |
| Questionnaires (online or paper-and-pencil) | |||
| Physical activity | |||
| Duke Activity Status Index (DASI) | X | X | X |
| Nutritional status | |||
| Patient-Generated Subjective Global assessment | X | X | X |
| Psychological fitness | |||
| State-Trait Anxiety Inventory | X | X | X |
| Patient Health Questionnaire | X | X | X |
| Checklist Individual Strength | X | X | X |
| Outcomes | |||
| HRQoL-SF-36 | X | X | X |
| Questionnaires Com-B model | |||
| Capability | |||
| Psychological Capability | |||
| Health literacy (SBSQ-D) | X | ||
| Physical Capability | |||
| Physical sub scale SF-36 | (X) | ||
| DASI | (X) | ||
| Opportunity | |||
| Social influences | |||
| Social support (SSL-I) | X | ||
| Environmental context and resources | |||
| Barriers and motivators questionnaire | X | ||
| Health-smart behaviour inventory | X | ||
| Motivation | |||
| Beliefs about capabilities | |||
| Self-efficacy (SE-MCDS) | X | ||
| Personal control (Mastery Scale) | X | ||
| Goals and planning | |||
| Action planning and control planning questionnaire | X | ||
| Tests and questionnaires during study visit | |||
| Physical activity | |||
| Handgrip strength | X | X | X |
| Biceps strength | X | X | X |
| Quadriceps strength | X | X | X |
| Short Physical Performance Battery | X | X | X |
| Steep ramp test | X | X | X |
| Short QUestionnaire to ASsess Health-enhancing physical activity | X | X | X |
| Nutritional status | |||
| Bioimpedance analysis | X | X | X |
| BMI (height and weight measurement) | X | X | X |
| Hip-waist ratio | X | X | X |
| Cognitive ability | |||
| Montreal Cognitive Assessment | X | ||
| Outcomes | |||
| Tilburg Frailty Indicator | X | X | X |
BMI, body mass index; HRQoL, health-related quality of life; SBSQ, Set of Brief Screening Questions; SE-MCDS, Self-Efficacy to Manage Chronic Disease Scale; SF-36, Short Form 36; SSL-I, Social Support List-Interactions.
Randomisation and allocation concealment
All participants who present with at least one modifiable problem, as determined during the assessment at the baseline study visit, will be randomised to the intervention or control group on a 1:1 ratio using block randomisation after stratification for sex and pre-emptive/non-pre-emptive transplantation. This study will not be blinded as it is not possible to blind the participant, or healthcare professionals involved in the intervention. Randomisation will take place using ALEA (www.aleaclinical.eu). The randomisation will be performed by an independent researcher who is not involved in screening, recruitment, clinical care or data collection.
Intervention
The home-based, multimodal, tailor-made programme will focus on three domains (physical activity, nutritional advice and psychosocial support) depending on the preferences and needs of the participant. For each domain, interventions have been developed based on the behavioural change wheel method.16 17 A context analysis was performed to gain insight into the problems KTCs face, and the factors (ie, preferences, barriers, limitations and facilitating factors) that are important to them for the creation and implementation of a prehabilitation programme. Two certified lifestyle coaches, a physiotherapist and a dietitian, will be involved in the intervention. The lifestyle coach, together with the participant and their significant other, will compose a personalised, goal-directed prehabilitation programme that can be incorporated into the daily life of the participant. During the intervention, the lifestyle coach will provide (bi)weekly counselling sessions with the participant. In these sessions, the progress of the participant, including their goals, facilitators and barriers, will be discussed. Participants will be offered monthly counselling sessions after completing the maintenance programme. Counselling ends when the participant chooses not to make use of the counselling sessions, when they undergo kidney transplant, or at the end of study (September 2025).
Physical activity
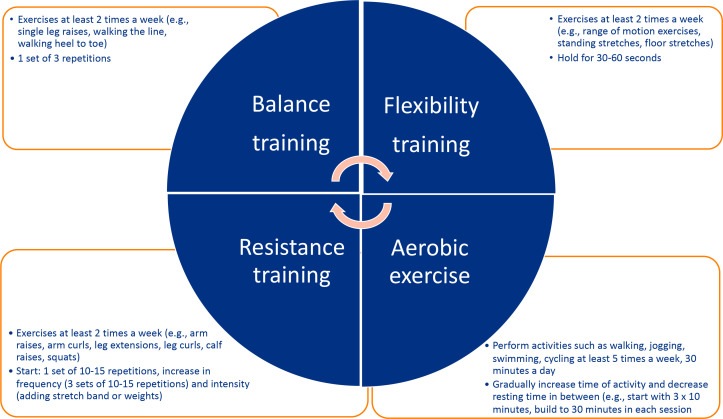
The aim of the physical activity interventions will be to improve the strength and endurance of KTCs. The criteria of The Nederlandse Norm Gezond Bewegen (in English: Dutch Healthy Physical Activity Guidelines), which includes: (1) performing activities that are moderately intense in nature for at least 30 min a day/5 days per week, and (2) performing activities to increase muscle strength for 20 min a day/ 2–3 days a week, will serve as guidance.25 The intervention will differ per participant depending on his/her baseline fitness level, preferences and whether they are on dialysis.26 Participants will receive a bag of weights (1–4 kg) and resistance bands (very light, light, medium, heavy), in order to perform lightweight and bodyweight exercises at home. Additionally, participants will be offered to participate in activities such as swimming, walking and cycling. Figure 2 shows the various components which will be considered while creating the tailor-made intervention for each participant.
Figure 2.
Components of the physical activity intervention and examples of possible activities.
Nutritional advice
Nutritional interventions will focus on improving nutritional status and body composition by supporting participants to engage in healthy and sustainable dietary habits. If participants already receive guidance from a dietician in the context of regular care, the nutritional advice will be coordinated with his/her dietician. The intervention will be tailored to the nutritional problems and/or dietary restrictions of each individual participant and focus on optimising and preventing shortages or imbalances of energy, protein and/or other nutrients for all participants.
Psychosocial support
Psychosocial interventions will consist of individual coaching by a certified lifestyle coach during (bi)weekly counselling sessions. The sessions will focus on the use of effective coping strategies, stress and energy management and promoting social support. Significant others may take part in these sessions if the participant wishes that they do. In addition, interventions aimed at relaxation such as sleep hygiene and relaxation interventions (eg, progressive muscle relaxation techniques, visual and auditory stimulation, breathing techniques) will be offered. Participants with clinically relevant scores regarding anxiety (STAI6 ≥12) or depression (PHQ-9 ≥10) will be referred to a social worker at their local hospital for further evaluation, treatment and/or referral to a psychologist.27 28
Control group
The control group will receive care as usual. Standard medical care for KTCs consists of a consultation with a nephrologist and/or nurse practitioner at their local hospital every 3 months approximately. In addition, a consultation with a dietician is scheduled if laboratory values are not consistent with expected results from dietary restrictions for chronic kidney disease or on demand of the KTC. Depending on the needs of the KTC a social worker can be consulted. Physical therapy consults may be advised by a nephrologist and/or nurse practitioner for those KTCs who experience declines in their fitness levels. The contents of the physical therapy session will depend on the fitness level of the KTC. Data on the use of allied healthcare will be collected. Regarding measurements, the same time intervals will be used in between assessments. A study visit at the UMCG will be planned at week 13 (T1) and week 26 (T2) after randomisation.
Participant withdrawal
Participants may always withdraw from the study, without any consequences. The investigator can decide to withdraw a subject from the study for urgent medical reasons. Participants will be withdrawn if they get transplanted during the study.
If participants withdraw from the study prior to measurement point T1, new participants will be included to ensure sufficient power of the study. Participants who have withdrawn from the study after T1 but indicate that their data may be used in the follow-up studies (eg, on the effect of prehabilitation on outcomes after transplantation) will be followed according to the specifications of the patient.
Outcome measurements
All outcome measurements are summarised in table 1. The primary, secondary and clinical outcomes will be measured at three time points: T0 (baseline assessment), T1 (week 13) and T2 (week 26). If a participant is unable to make it to the study visit at week 13 or week 26, a study visit will be planned within a 1 week time frame of these time points.
Primary outcome
The primary outcome will be change in frailty status between T0 and T1 as measured by the TFI.29 This validated tool has been chosen as it covers multiple components of frailty. In addition, the sustainability of the intervention will be examined by change in frailty status between T1 and T2. The TFI is a multidimensional, validated questionnaire for measuring frailty among community dwelling older adults.29 It consists of 15 items reflecting the different components of frailty: physical frailty (8 items), psychological frailty (4 items) and social frailty (3 items). The total TFI score ranges between 0 and 15. A score ≥5 is used as a cut-off point for frailty.
Secondary outcomes
Secondary endpoints include changes in physical functioning, nutritional status, psychological well-being and quality of life. To measure these changes, a set of questionnaires will be filled out prior to the study visits (T0/T1/T2) in the UMCG using an online survey. Participants who prefer a pen-and-paper survey, will receive one via mail. Physical tests will be done during the study visit at the UMCG.
Physical functioning will be measured by two questionnaires and five performance tests.
The SQUASH will be used to gain insight into engagement in physical activities in one’s daily life.30
The DASI will be used to measure functional capacity.31
An activity tracker will be used to measure the number of steps taken by the participant. Participants will be asked to wear the activity tracker for 3 days and note the steps per day in their food diary (see nutritional assessment).
Handgrip strength will be assessed using the Jamar Hydraulic Hand Dynamometer (Patterson Medical JAMAR 5030J1, Warrenville, Canada).32
Quadriceps and biceps strength will be measured with a hand-held dynamometer CITEC CT 3002/30 handheld dynamometer (Haren, Netherlands).33 34
The SPPB will be used to measure physical performance regarding balance, gait speed and leg muscle strength.35 The SPPB consists of a balance test, a 4-metre walking test and the 5 Times Sit-To-Stand test.
The SRT will be performed on an electronically braked cycle ergometer to measure one’s aerobic capacity. During the SRT, the resistive load is accelerated in a fast schedule (25 W/10 s) until exhaustion of the participant.36
Nutritional status will be assessed by a questionnaire, a food diary and three body measurements.
The PG-SGA SF will be used to assess nutritional status across various domains: changes in body weight, changes in nutritional intake, symptoms which negatively influence intake, absorption and usage of nutrients and level of activities and function.37
Participants will be asked to complete a food diary throughout consecutive 3 days, including 1 weekend day, to gather information on fat, protein and energy intake.
BMI will be calculated as follows: weight (in kg) divided by height (m) squared (kg/m2).
Hip and waist circumference will be measured in centimetres to calculate a waist-hip ratio.
BIA will be conducted to non-invasively measure body composition (eg, lean tissue index, fat tissue index, extracellular and intracellular volume) by using the InBody S10.
Psychosocial well-being will be measured by three questionnaires.
Health-related quality of life
To assess HRQoL, the SF-36 health survey will be used. It is a 36-item, self-reported questionnaire that captures participants’ perceptions of their own health and well-being. Based on the item scores, a Physical Component Score and a Mental Component Score will be calculated.40 41
Clinical outcomes
Clinical outcomes, including waitlist mortality, delisting and the number of hospital admissions, will be assessed by medical record review until time of transplantation and recorded on a case record form.
Other measures
To gain insight into the capability, opportunity and motivation of participants to engage in behaviour change the following questionnaires and test will be administered at T0.
Health literacy will be measured using the Dutch version of the Set of Brief Screening Questions.42 43
Barriers and motivators regarding physical activity will be measured using the Barriers and Motivators Questionnaire.44
Barriers and motivators regarding nutritional intake will be measured using a subset of the Motivators and Barriers to Health-Smart Behaviours Inventory regarding health food and healthy drinks.45
Barriers and motivators regarding social support will be measured using the short version of the Social Support List-Interaction (SSL-I).46
The Self-Efficacy to Manage Chronic Disease Scale will be used to gain insight into the confidence of a person in the ability to successfully perform a specific task or behaviour related to one’s health in various situations.47 48
Personal control will be measured using the Pearlin-Schooler Mastery Scale.41 48
To gain insight into goal directedness and action planning skills of participants, the Action and Coping planning questionnaire developed by Sniehotta et al will be used.49
MoCA will be used a screening tool for cognitive deterioration.50
Tertiary outcomes
Data regarding feasibility and acceptability of the prehabilitation programme will be collected throughout the study period. To assess feasibility the following data will be collected:
Enrolment (number of eligible participants, consent rate, reasons for refusal (if known)).
Attrition (percentage of completion of the programme, reasons for dropout).
Fidelity (adherence to the programme, barriers and facilitators; adjustments to the programme).
Safety (number of adverse events).
Logistical problems.
The acceptability of the prehabilitation programme will be assessed among participants using the Treatment Acceptability and Preference questionnaire and among involved healthcare professionals using the Normalisation MeAsure Development (NoMAD) questionnaire.51–53 In addition, satisfaction, feedback regarding the programme, barriers and facilitators for further implementation will be obtained by six focus group meetings with participants of the intervention group and involved healthcare providers at the end of the study period. The focus group meetings will be led by an experienced senior researcher.
Demographic and patient characteristics will be recorded throughout the study.
Sample size calculation
An a priori sample size calculation was performed based on an effect size of 0.5, which is generally found across outcomes and across populations as indicative of a minimal clinically important difference. To find a statistically significant difference between the control and intervention groups in the change of frailty at the end of the prehabilitation programme (T1) with a medium effect size (0.5), alpha value of 0.05 (two-sided) and a power of 0.80 at least 128 participants are needed in the study, n=64 in each group. Based on a dropout rate of 15%, 148 KTCs will be needed for randomisation. Given the estimated exclusion after the assessment of participants with no problems of 15%, 176 KTCs need to be included for assessment.
Based on a conservative estimation of 50% regarding response rate to the invitation to participate in the study, and an initial exclusion of 10% of the target population (eg, because of a language barrier), a total of 388 KTCs (2×176 needed for assessment+10% exclusion) will be needed as potential eligible participants.
Statistical analysis
All analyses will be performed using Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, V.28.0. IBM, Armonk, New York, USA). The analyses will be based on the intention-to-treat principle. A two-sided p value of <0.05 will be considered to indicate statistical significance for all analyses.
An intention-to-treat analysis will be carried out to study the difference in outcome measures between the intervention and the control group. The primary outcome will be the change in frailty status between T0 and T1. Differences between groups will be performed using the Student’s t-test or Mann-Whitney U test depending on normality of data. Differences within groups will be tested with a paired-samples t-test or Wilcoxon signed-rank test depending on normality of data.
Regarding missing data, imputation by mean or modus will be done if missing at random (MAR) is less than 5%. If MAR>5%, multiple imputation will be used. Imputation will not be performed if missing data are not random.
Explorative analysis will be performed to gain insight into differences between the intervention and the control group regarding changes in frailty status (T1-T2), physical functioning, nutritional status, psychosocial well-being, quality of life and clinical outcomes at the various measurement points. These changes will be analysed using the appropriate tests based on measurement level and distribution. Differences in proportions between groups will be examined using χ2 tests. Differences between groups will be performed using the Student’s t-test or Mann-Whitney U test depending on normality of data. Differences within groups will be tested with a paired-samples t-test or Wilcoxon signed-rank test depending on normality of data. Changes over time between T0 and T2 will be analysed using general linear models analysis with group×time interaction.
Data regarding feasibility, acceptability and barriers and facilitators for further implementation (eg, enrolment, attrition, adherence, safety, logistical problems) will be described using descriptive statistics.
Qualitative data from the focus group meetings will be audio recorded and transcribed verbatim. Transcriptions will be imported into ATLAS.ti 22 (Scientific Software Development GmbH, Berlin, Germany). Data will be iteratively analysed and discussed using six analysis steps: familiarisation with the data, generation of initial codes, searching for themes, reviewing themes, defining and naming themes and writing the report.54 55
Data management
Data will be handled in accordance with the General Data Protection Regulation and the Dutch Act on Implementation of the General Data Protection Regulation. All participant data will be pseudonymised. Data collection forms will be stored in RoQua, a routine outcome measurement system used in the UMCG, and REDCap, a secured web application for building and managing online surveys and databases. A key list (identification list) will be kept to be able to link data of the electronic patient dossier to a pseudonymised patient. This key list will be secured by a password and saved on a locked research drive. Hardcopy research data of the project will be stored in a locked filing cabinet in the office of the principal investigator, which will also be locked. The principal investigator will have access to the final trial data set. After the completion of the research project, as soon as all research data have been analysed and processed, all hardcopy research documents will be sent to the central archive of the UMCG.
Data monitoring
The principal investigator has deemed the implementation of a data monitoring committee unnecessary due to the low-risk nature of this study.
Remuneration
Participants will not receive remuneration for their contribution to this study. However, they will receive reimbursement for the cost of travel and parking costs.
Patient and public involvement
The patient advisory committee (PAC) of the UMCG Transplant Center was involved in the process of development of the study by exchanging ideas and giving feedback on the research proposal. The patient council of the Dutch Kidney Foundation contributed to the acceptance of the grant that helped fund this study. Also, a context analysis was performed to gain insight into the problems that KTCs face and the help that they receive prior to transplantation.
The project’s steering committee consists of patients, including a representative of the PAC of the Transplant Center, and professionals. This group discusses the progress of the study quarterly. Patients will be involved in further development of the prehabilitation programme.
Ethics and dissemination
Medical ethical approval for this study has been granted by the Institutional Review Board of the UMCG (registration no. METc 2022/421). The study will adhere to institutional policies, local laws and the Declaration of Helsinki. Written informed consent will be obtained from all participants by their treating physician. Important protocol modifications will be communicated to relevant parties.
Although the risk of injury during exercise is negligible, this will be monitored weekly by a lifestyle coach. All adverse events will be followed until they have abated, or until a stable situation has been reached.
The results will be disseminated at international conferences and in peer-reviewed journals.
Trial sponsor
University Medical Center Groningen.
P.O. Box 30 001 (FA 12), 9700 RB Groningen.
Supplementary Material
Footnotes
Collaborators: The PreCareTx Investigators are C Annema, E J Finnema, A V Ranchor, M J Schroevers, A J Haanstra, Y van der Veen, S P Berger, S J L Bakker, C F M Franssen, A E de Joode, R Westerhuis, R Dekker, H Maring, E E Quint, R A Pol, J M Klaase and S M L Niamut.
Contributors: The protocol was designed and written by CA and EEQ. It was critically reviewed by AJH, YvdV, HM, SPB, AR, SJLB, EF and RAP. All authors approved the final version of this manuscript.
Funding: This work was supported by The Dutch Kidney Foundation, grant number 20OS008. This funding source had no role in the design of this study, nor will it have any role during its execution.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the PreCareTx Investigators:
C Annema, EJ Finnema, AV Ranchor, MJ Schroevers, AJ Haanstra, Y van der Veen, SP Berger, SJL Bakker, CFM Franssen, AE de Joode, R Westerhuis, R Dekker, H Maring, EE Quint, RA Pol, JM Klaase, and SML Niamut
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Yarlas AS, White MK, Yang M, et al. Measuring the health status burden in hemodialysis patients using the SF-36® health survey. Qual Life Res 2011;20:383–9. 10.1007/s11136-010-9764-8 [DOI] [PubMed] [Google Scholar]
- 2. McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg 2017;266:1084–90. 10.1097/SLA.0000000000002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation 2015;99:805–10. 10.1097/TP.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zelle DM, Klaassen G, van Adrichem E, et al. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol 2017;13:318. 10.1038/nrneph.2017.44 [DOI] [PubMed] [Google Scholar]
- 5. Kittiskulnam P, Sheshadri A, Johansen KL. Consequences of CKD on functioning. Semin Nephrol 2016;36:305–18. 10.1016/j.semnephrol.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eisenga MF, Gomes-Neto AW, van Londen M, et al. Rationale and design of transplantlines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018;8:e024502. 10.1136/bmjopen-2018-024502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gobbens RJJ, van Assen MALM, Luijkx KG, et al. The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc 2010;11:344–55. 10.1016/j.jamda.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 10. McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013;61:896–901. 10.1111/jgs.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAdams-DeMarco MA, Chu NM, Segev DL. Frailty and long-term post-kidney transplant outcomes. Curr Transplant Rep 2019;6:45–51. 10.1007/s40472-019-0231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, inflammatory markers, and Waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation 2018;102:1740–6. 10.1097/TP.0000000000002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quint EE, Zogaj D, Banning LBD, et al. Frailty and kidney transplantation: a systematic review and meta-analysis. Transplant Direct 2021;7:e701. 10.1097/TXD.0000000000001156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luther A, Gabriel J, Watson RP, et al. The impact of total body Prehabilitation on post-operative outcomes after major abdominal surgery: a systematic review. World J Surg 2018;42:2781–91. 10.1007/s00268-018-4569-y [DOI] [PubMed] [Google Scholar]
- 15. Pesce de Souza F, Massierer D, Anand Raje U, et al. Exercise interventions in solid organ transplant candidates: a systematic review. Clin Transplant 2020;34:e13900. 10.1111/ctr.13900 [DOI] [PubMed] [Google Scholar]
- 16. Hernández Morante JJ, Sánchez-Villazala A, Cutillas RC, et al. Effectiveness of a nutrition education program for the prevention and treatment of malnutrition in end-stage renal disease. J Ren Nutr 2014;24:42–9. 10.1053/j.jrn.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 17. Wilkinson TJ, McAdams-DeMarco M, Bennett PN, et al. Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr Opin Nephrol Hypertens 2020;29:471–9. 10.1097/MNH.0000000000000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: results from a pilot study. Clin Transplant 2019;33:e13450. 10.1111/ctr.13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol 2018;44:919–26. 10.1016/j.ejso.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 20. Lorenz EC, Hickson LJ, Weatherly RM, et al. Protocolized exercise improves frailty parameters and lower extremity impairment: a promising prehabilitation strategy for kidney transplant candidates. Clin Transplant 2020;34:e14017. 10.1111/ctr.14017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma X, Zhang Z, Peng M, et al. Face-to-face mentoring, remotely supervised home exercise Prehabilitation to improve physical function in patients awaiting kidney transplantation: a randomized clinical trial. Front Psychol 2022;13:831445. 10.3389/fpsyg.2022.831445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quint EE, Ferreira M, van Munster BC, et al. Prehabilitation in adult solid organ transplant candidates. Curr Transplant Rep 2023;10:70–82. 10.1007/s40472-023-00395-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin 2015;33:17–33. 10.1016/j.anclin.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 24. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kemper HCG, Ooijendijk WTM, Stiggelbout M. Consensus over de Nederlandse norm voor gezond bewegen; 2000.
- 26. Baker LA, March DS, Wilkinson TJ, et al. Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol 2022;23:75. 10.1186/s12882-021-02618-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 28. Spielberger C. Manual for the state-trait anxiety inventory (self-evaluation questionnare). Consulting Psychogyists Press, 1970. [Google Scholar]
- 29. Gobbens RJ, Boersma P, Uchmanowicz I, et al. The Tilburg Frailty Indicator (TFI): new evidence for its validity. Clin Interv Aging 2020;15:265–74. 10.2147/CIA.S243233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wendel-Vos GCW, Schuit AJ, Saris WHM, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–9. 10.1016/s0895-4356(03)00220-8 [DOI] [PubMed] [Google Scholar]
- 31. Ravani P, Kilb B, Bedi H, et al. The duke activity status index in patients with chronic kidney disease: a reliability study. Clin J Am Soc Nephrol 2012;7:573–80. 10.2215/CJN.07990811 [DOI] [PubMed] [Google Scholar]
- 32. Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984;9:222–6. 10.1016/s0363-5023(84)80146-x [DOI] [PubMed] [Google Scholar]
- 33. Merlini L, Mazzone ES, Solari A, et al. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve 2002;26:64–70. 10.1002/mus.10166 [DOI] [PubMed] [Google Scholar]
- 34. Schreuders TAR, Roebroeck ME, Goumans J, et al. Measurement error in grip and pinch force measurements in patients with hand injuries. Phys Ther 2003;83:806–15. [PubMed] [Google Scholar]
- 35. Freiberger E, de Vreede P, Schoene D, et al. Performance-based physical function in older community-dwelling persons: a systematic review of instruments. Age Ageing 2012;41:712–21. 10.1093/ageing/afs099 [DOI] [PubMed] [Google Scholar]
- 36. Rozenberg R, Bussmann JBJ, Lesaffre E, et al. A steep ramp test is valid for estimating maximal power and oxygen uptake during a standard ramp test in type 2 diabetes. Scand J Med Sci Sports 2015;25:595–602. 10.1111/sms.12357 [DOI] [PubMed] [Google Scholar]
- 37. Abbott J, Teleni L, McKavanagh D, et al. Patient-generated subjective global assessment short form (PG-SGA SF) is a valid screening tool in chemotherapy outpatients. Support Care Cancer 2016;24:3883–7. 10.1007/s00520-016-3196-0 [DOI] [PubMed] [Google Scholar]
- 38. van der Bij AK, de Weerd S, Cikot RJLM, et al. Validation of the dutch short form of the state scale of the Spielberger state-trait anxiety inventory: considerations for usage in screening outcomes. Community Genet 2003;6:84–7. 10.1159/000073003 [DOI] [PubMed] [Google Scholar]
- 39. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 41. Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19:2–21. [PubMed] [Google Scholar]
- 42. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 43. Fransen MP, Van Schaik TM, Twickler TB, et al. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun 2011;16 Suppl 3:134–49. 10.1080/10810730.2011.604383 [DOI] [PubMed] [Google Scholar]
- 44. van Adrichem EJ, Krijnen WP, Dekker R, et al. Multidimensional structure of a questionnaire to assess barriers to and motivators of physical activity in recipients of solid organ transplantation. Disabil Rehabil 2017;39:2330–8. 10.1080/09638288.2016.1224274 [DOI] [PubMed] [Google Scholar]
- 45. Tucker CM, Marsiske M, Rice KG, et al. Patient-centered culturally sensitive health care: model testing and refinement. Health Psychol 2011;30:342–50. 10.1037/a0022967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hagedoorn M, Sanderman R, Buunk BP, et al. “Failing in spousal caregiving: the 'identity-relevant stress' hypothesis to explain sex differences in caregiver distress”. Br J Health Psychol 2002;7:481–94. 10.1348/135910702320645435 [DOI] [PubMed] [Google Scholar]
- 47. Lorig KR, Sobel DS, Ritter PL, et al. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256–62. [PubMed] [Google Scholar]
- 48. Ritter PL, Lorig K. The English and Spanish self-efficacy to manage chronic disease scale measures were validated using multiple studies. J Clin Epidemiol 2014;67:1265–73. 10.1016/j.jclinepi.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 49. Sniehotta FF, Scholz U, Schwarzer R. Action plans and coping plans for physical exercise: a longitudinal intervention study in cardiac rehabilitation. Br J Health Psychol 2006;11:23–37. 10.1348/135910705X43804 [DOI] [PubMed] [Google Scholar]
- 50. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 51. Finch TL, Girling M, May CR, et al. Improving the normalization of complex interventions: part 1 - development of the nomad instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18:135. 10.1186/s12874-018-0591-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finch TL, Girling M, May CR, et al. Improving the normalization of complex interventions: part 2 - validation of the Nomad instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18:135. 10.1186/s12874-018-0591-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sidani S, Epstein DR, Bootzin RR, et al. Assessment of preferences for treatment: validation of a measure. Res Nurs Health 2009;32:419–31. 10.1002/nur.20329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Braun V, Clarke V, Hayfield N, et al. Handbook of research methods in health and social sciences. New York: Springer, 2018. [Google Scholar]
- 55. Friese S. Qualitative data analysis with Atlas.ti. Third ed. Los Angeles: SAGE, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072805supp001.pdf (67.7KB, pdf)
bmjopen-2023-072805supp002.pdf (215.2KB, pdf)