Abstract
Mixed sex cord–stromal tumors, which consist of poorly differentiated Sertoli cells and Leydig cells and juvenile granulosa cell tumor tissue, are extremely rare. Most of these tumors are unilateral and stage I at the time of diagnosis; nonetheless, according to the available relevant English-language literature, these tumors maintain a malignant potential. We herein report a case involving a 15-year-old girl diagnosed with a mixed sex cord–stromal tumor (gynandroblastoma with juvenile granulosa cell tumor component). Left salpingo-oophorectomy was initially performed, and the diagnosis of a juvenile granulosa cell tumor was established. Right salpingo-oophorectomy was performed 1 year later, at which time the specimen showed a different growth pattern involving epithelioid cells and tubules, resembling a Sertoli–Leydig cell tumor. Immunohistochemical staining was performed and the specimen was compared with that obtained 1 year earlier. We concluded that the tumors were linked and most likely constituted a gynandroblastoma (mixed form of sex cord–stromal tumor). Although this is an extremely uncommon ovarian tumor, it should be considered when diverse tumor morphology is identified. Bilateral metachronous involvement of the ovaries is possible. The grade of the Sertoli–Leydig cell component may influence the prognosis of such a tumor.
Keywords: Granulosa cell tumor, Sertoli–Leydig cell tumor, sex cord–gonadal stromal tumor, DICER1, case report, gynandroblastoma
Introduction
Ovarian tumors in children and teenage girls are a rare but significant subset of gynecological cancers. They account for 1% of all malignant tumors and have an annual incidence of roughly 2.6 cases per 100,000 girls. 1 Most ovarian tumors fall under the categories of epithelial, mesenchymal, sex cord stromal, and germ cell tumors. 2 Whereas epithelial cell tumors account for 90% of ovarian cancers in all age groups, germ cell tumors predominate in children, accounting for around 70% of such ovarian neoplasms. Mature teratoma is the most common benign tumor, and dysgerminoma is the most common malignant tumor.1,3
Gynandroblastoma (mixed form of sex cord–stromal tumor) is a very rare ovarian tumor that has histological components of both a granulosa cell tumor (GCT) and Sertoli–Leydig cell tumor (SLT). 2 Gynandroblastoma comprising poorly differentiated Sertoli cells and Leydig cells and juvenile GCT tissue are tremendously rare, with only 6 of 28 such cases reported in the English-language literature to date. These tumors are usually found in adolescent girls.4–9
Patients with gynandroblastoma usually present with stage I disease. Most tumors are unilateral and behave in a benign manner, and only rare recurrences have been reported.2,10
We herein report a rare case of gynandroblastoma of the ovary composed of juvenile GCT and SLT elements with bilateral ovarian involvement and malignant behavior in an adolescent. The reporting of this study conforms to the CARE guidelines. 11
Case Report
A 15-year-old girl presented with left-sided abdominal pain associated with prolonged menstrual bleeding. She had undergone left nephrectomy at the age of 1 year because of a mass lesion; the pathology was unknown but the mass was reportedly benign. A first-degree relative had developed metastatic breast cancer at the age of 30 years. Physical examination was remarkable only for hirsutism.
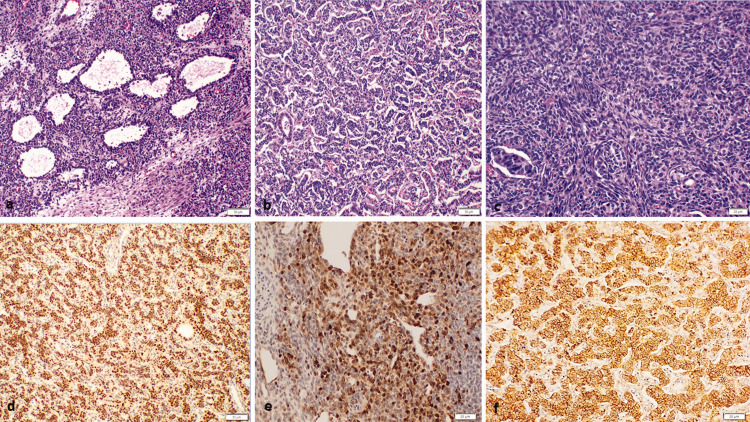
The patient underwent ultrasound examination, which showed a large mass in left ovary. Computed tomography confirmed this finding. Left salpingo-oophorectomy was performed, and the obtained specimen contained a left ovarian tumor measuring 18 × 14 × 7 cm with a smooth surface. Microscopic examination revealed nodular and diffuse growth of tumor cells with ovoid hyperchromatic nuclei, small nucleoli, amphophilic cytoplasm. Macrofollicle and microfollicle formations containing eosinophilic secretions were identified (Figure 1). The tumor cells were positive for steroidogenic factor 1 (SF-1) and inhibin, focally positive for cytokeratin (CK), and negative for SALL4. Reticulin stain highlighted groups/nests of tumor cells. B-catenin showed a positive membranous staining pattern. Therefore, a diagnosis of juvenile type GCT was established.
Figure 1.
Histological findings of left ovarian tumor. (a) The tumor was composed of cystic follicles (hematoxylin and eosin (H&E), 10×). (b) Focal tubule formation (H&E, 10×) and (c) focal spindle cell areas (H&E, 20×) were identified. The tumor cells were positive for (d) steroidogenic factor 1, (e) calretinin, and (f) membranous B-catenin.
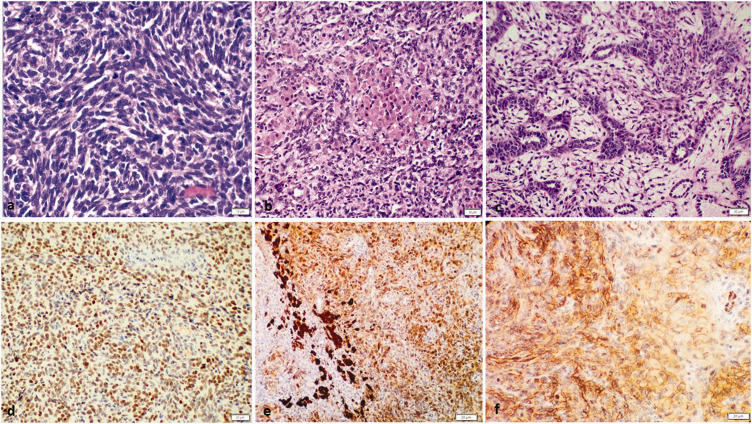
One year later, a right ovarian mass was found by ultrasound during a regular follow-up. Serum inhibin and anti-Müllerian hormone were negative. Pelvic magnetic resonance imaging revealed a pelvic mass measuring approximately 4.5 cm in axial diameter, located to the right of the rectum and probably originating from the right ovary. Right salpingo-oophorectomy was performed, and the specimen contained a tumor composed of poorly differentiated spindle/polygonal Sertoli cells admixed with fewer Leydig cells. Focal tubule formation, cystic follicles, and necrosis were also noted (Figure 2). The mitotic rate was 20 per 10 high-power fields. The tumor cells were positive for SF-1, B-catenin (membranous), alpha-inhibin, and calretinin and negative for SALL4, CK7, and epithelial membrane antigen (EMA).
Figure 2.
Histological findings of right ovarian tumor. The tumor was composed of (a) poorly differentiated spindle/polygonal Sertoli cells (hematoxylin and eosin (H&E), 40×) admixed with (b) fewer Leydig cells (H&E, 20×). (c) Focal tubule formation was identified (H&E, 20×). The tumor cells were positive for (d) steroidogenic factor 1, (e) calretinin, and (f) membranous B-catenin.
This tumor was compared with the first tumor diagnosed as juvenile GCT. Areas that were morphologically similar to the new tumor with tubules and cords of Sertoli cells were identified. We concluded that both tumors were related and most likely represented a gynandroblastoma (mixed form of sex cord–stromal tumor).
The patient underwent oocyte cryopreservation and started carboplatin/paclitaxel chemotherapy with no evidence of disease recurrence after 2 years of follow-up.
The patient was evaluated in the genetic clinic and discovered to have a pathogenic DICER1 mutation (Variant: c.3094-2_3096del). Therefore, she was placed on surveillance guidelines for individuals with a germline DICER1 mutation.
Materials and methods
In the pathology department of King Hussein Cancer Center, we identified a case of gynandroblastoma (mixed form of sex cord–stromal tumor). The excised tissue was serially sectioned and fixed in 10% buffered formalin overnight. The sections were routinely processed for paraffin embedding and stained using hematoxylin and eosin. Immunostaining was performed on the paraffin-embedded material using the avidin–biotin complex protocol with an iVIEW DAB detection kit (Ventana Medical Systems, Tucson, AZ, USA). We used monoclonal antibodies against SF-1 (Clone ERP19744; Abcam, Cambridge, UK), inhibin alpha (Clone R1; Ventana Medical Systems), pancytokeratin (Clone AE1/AE3/PCK26; Ventana Medical Systems), SALL4 (Clone 6E3; Abcam), B-catenin (Clone 14; Ventana Medical Systems), calretinin (Clone SP65; Ventana Medical Systems), CK7 (Clone B22.1; Ventana Medical Systems), and EMA (Clone E29; Ventana Medical Systems). All immunostains were performed on the Ventana Benchmark XT/Ultra automated immunostainer (Ventana Medical Systems).
Discussion
Gynandroblastoma is a mixed ovarian sex cord–stromal tumor with both male and female components. Two morphologic subtypes of the granulosa cell component can be defined when classifying these tumors, namely the juvenile and adult subtypes; this is important because these two subtypes may differ in behavior and prognosis. Juvenile GCT constitutes a relatively small percentage of all GCTs (about 5%). However, juvenile GCT accounts for approximately 90% of GCTs among prepubertal adolescents and women under the age of 30 years. Most juvenile GCTs are unilateral and appear at an early stage. These tumors are frequently functional and secrete estrogen, which leads to precocious puberty in approximately 80% of patients. Some patients may develop virilization due to the release of testosterone. The absence of a FOXL2 mutation in juvenile GCT can aid in differentiating it from adult GCT. 12
Gynandroblastoma generally exhibits adult-type GCT characteristics and is known to have malignant potential. Only a few cases of juvenile-type GCTs have been reported. These tumors are exceedingly rare. Therefore, there is no consensus on how to assess the likelihood of malignancy in this subtype of gynandroblastoma based on histology.
SLTs can be classified as well, moderately, or poorly differentiated based on the tubular differentiation of the Sertoli cell component. 2 Up to 22% of SLTs have heterologous mesenchymal or epithelial elements. These elements are only found in moderately and poorly differentiated tumors and in tumors with a retiform pattern. In rare cases, they can mimic aggressive tumors such as rhabdomyosarcomas.13,14 SLT staining is usually positive for inhibin, calretinin, WT-1, and CD56 and negative for EMA. Our patient was found to have a DICER1 mutation, which has been reported in up to 60% of SLTs in the literature. 15
DICER1 syndrome occurs in children and young adults. Its clinical presentation may include pleuropulmonary blastoma, cystic nephroma, ovarian SLT, and gynandroblastoma; multinodular goiter; embryonal rhabdomyosarcoma of the cervix; and sarcomas of different sites including the uterine cervix, kidney, and brain. 16 Our patient had a history of nephrectomy as a child, which was probably related to DICER1 syndrome, and she developed multinodular goiter during follow-up.
We believe that the features of malignant behavior (bilaterality and metachronicity) of the tumor in the present case may have been dictated by the grade of the SLT component (poorly differentiated histologic type 3). In tumors with a low-grade Sertoli cell component, however, whether the juvenile-type granulosa cells found in gynandroblastoma will have a poorer prognosis than the adult granulosa cell component found in classic gynandroblastoma remains unknown.
The pathogenesis and biologic behavior of these two subtypes are still unclear because of the rarity of these tumors and limited numbers of previous reports. More wide-range histologic and molecular studies are needed.
We found five cases of bilateral ovarian involvement by SLTs17–21 in the English-language literature, three of which were metachronous (asynchronous) and two of which were synchronous. However, we found no previous reports of bilateral ovarian involvement by gynandroblastomas with a juvenile GCT component. To our knowledge, ours is the only reported case of bilateral metachronous involvement of the ovaries by gynandroblastoma with poorly differentiated SLT and juvenile GCT components.
Ordulu and Young 22 reported 38 cases of SLTs that contained follicles resembling a juvenile GCT component. In their opinion, the presence of a juvenile granulosa-like morphology may simply represent another morphology of an SLT rather than a true gynandroblastoma. However, the authors described a multifocal origin within lobules of otherwise typical SLTs, in which the overall tumor features show unusual differentiation within these tumors.
In conclusion, gynandroblastoma with a juvenile GCT component is an extremely uncommon ovarian tumor. When diverse tumor morphology is identified, this type of tumor should be considered. Bilateral metachronous involvement of the ovaries is possible. The grade of the SLT component may influence the prognosis of such a tumor. More comprehensive histologic and molecular studies are required.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231187796 for Mixed sex cord–stromal tumor (gynandroblastoma) with malignant morphology involving both ovaries: a case report by Bayan Maraqa, Maxim Al-Ashhab, Nazmi Kamal, Maher Sughayer and Fareed Barakat in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231187796 for Mixed sex cord–stromal tumor (gynandroblastoma) with malignant morphology involving both ovaries: a case report by Bayan Maraqa, Maxim Al-Ashhab, Nazmi Kamal, Maher Sughayer and Fareed Barakat in Journal of International Medical Research
Author contributions: Bayan Maraqa was responsible for acquiring the data and drafting the manuscript. Maxim Al-Ashhab was responsible for reviewing the literature and drafting the manuscript. Nazmi Kamal and Maher Sughayer were responsible for critically revising the manuscript. Fareed Barakat designed, directed, and reviewed the manuscript.
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Bayan Maraqa https://orcid.org/0000-0001-9455-188X
Maher Sughayer https://orcid.org/0000-0002-9185-9616
Data availability statement
The original data presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
All treatments were carried out with written informed consent from the patient and her family members. The patient also provided written informed consent for the publication of this article related to her medical records. The study protocol was approved by the Ethics Committee of King Hussein Cancer Center.
References
- 1.Andrés MM, Costa E, Cañete A, et al. Solid ovarian tumours in childhood: a 35-year review in a single institution. Clin Transl Oncol 2010; 12: 287–291. DOI: 10.1007/s12094-010-0505-9. [DOI] [PubMed] [Google Scholar]
- 2.Folkins A, Palacios J, Cheng XW. WHO Classification of Tumours, 5th Edition, Volume 4: Female genital tumours. Geneva: WHO Classification of Tumours Editorial Board, 2020. ISBN 978-92-832-4504-9. [Google Scholar]
- 3.Boussios S, Karathanasi A, Zakynthinakis-Kyriakou N, et al. Ovarian carcinosarcoma: current developments and future perspectives. Crit Rev Oncol Hematol 2019; 134: 46–55. DOI: 10.1016/j.critrevonc.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 4.McCluggage WG, Sloan JM, Murnaghan M, et al. Gynandroblastoma of ovary with juvenile granulosa cell component and heterologous intestinal type glands. Histopathology 1996; 29: 253–257. DOI: 10.1111/j.1365-2559.1996.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 5.Broshears JR, Roth LM. Gynandroblastoma with elements resembling juvenile granulosa cell tumor. Int J Gynecol Pathol 1997; 16: 387–391. DOI: 10.1097/00004347-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Chan R, Tucker M, Russell P. Ovarian gynandroblastoma with juvenile granulosa cell component and raised alpha fetoprotein. Pathology 2005; 37: 312–315. DOI: 10.1080/00313020500169503. [DOI] [PubMed] [Google Scholar]
- 7.Wilberger A, Yang B. Gynandroblastoma with juvenile granulosa cell tumor and concurrent renal cell carcinoma: a case report and review of literature. Int J Surg Pathol 2015; 23: 393–398. DOI: 10.1177/1066896915573569. [DOI] [PubMed] [Google Scholar]
- 8.Takeda A, Watanabe K, Hayashi S, et al. Gynandroblastoma with a juvenile granulosa cell component in an adolescent: case report and literature review. J Pediatr Adolesc Gynecol 2017; 30: 251–255. DOI: 10.1016/j.jpag.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Talerman A. Gynandroblastoma with elements of juvenile granulosa cell tumor. Int J Gynecol Pathol 1998; 17: 190. DOI: 10.1097/00004347-199804000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Schultz KAP, Harris AK, Finch M, et al. DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol Oncol 2017; 147: 521–527. DOI: 10.1016/j.ygyno.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. DOI: 10.1111/head.12246. [DOI] [PubMed] [Google Scholar]
- 12.Cheung A, Shah S, Parker J, et al. Non-epithelial ovarian cancers: how much do we really know? Int J Environ Res Public Health 2022; 19: 1106. DOI: 10.3390/ijerph19031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnik Papler T, Frković Grazio S, Kobal B. Sertoli-Leydig cell tumor with retiform areas and overgrowth of rhabdomyosarcomatous elements: case report and literature review. J Ovarian Res 2016; 9: 46. DOI: 10.1186/s13048-016-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth LM. Recent advances in the pathology and classification of ovarian sex cord-stromal tumors. Int J Gynecol Pathol 2006; 25: 199–215. DOI: 10.1097/01.pgp.0000192271.22289.e6. [DOI] [PubMed] [Google Scholar]
- 15.Boussios S, Moschetta M, Zarkavelis G, et al. Ovarian sex-cord stromal tumours and small cell tumours: pathological, genetic and management aspects. Crit Rev Oncol Hematol 2017; 120: 43–51. DOI: 10.1016/j.critrevonc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Caroleo AM, De Ioris MA, Boccuto L, et al. DICER1 syndrome and cancer predisposition: from a rare pediatric tumor to lifetime risk. Front Oncol 2020; 10: 614541. DOI: 10.3389/fonc.2020.614541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latthe P, Shafi MI, Rollason TP. Recurrence of Sertoli-Leydig cell tumour in contralateral ovary. Case report and review of literature. Eur J Gynaecol Oncol 2000; 21: 62–63. [PubMed] [Google Scholar]
- 18.White LC, Buchanan KD, O'Leary TD, et al. Direct laparoscopic venous sampling to diagnose a small Sertoli-Leydig tumor. Gynecol Oncol 2003; 91: 254–257. DOI: 10.1016/s0090-8258(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 19.Warenik-Szymankiewicz A, Słopień R, Gaca M, et al. Bilateral androblastoma (Sertoli-stromal cell tumor) of the ovary: a rare cause of virilization in a teenager. Eur J Gynaecol Oncol 2012; 33: 217–218. [PubMed] [Google Scholar]
- 20.Dicker D, Dekel A, Feldberg D, et al. Bilateral Sertoli-Leydig cell tumor with heterologous elements: report of an unusual case and review of the literature. Eur J Obstet Gynecol Reprod Biol 1986; 22: 175–181. DOI: 10.1016/0028-2243(86)90064-x. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Peñaloza C, Cañavera-Constantino A, Aristi-Urista G. Bilateral, metachronic ovarian Sertoli–Leydig cell tumour in an 11-year-old patient: a case report. Revista Médica del Hospital General de México 2018; 81: 139–145. [Google Scholar]
- 22.Ordulu Z, Young RH. Sertoli-Leydig cell tumors of the ovary with follicular differentiation often resembling juvenile granulosa cell tumor: a report of 38 cases including comments on sex cord-stromal tumors of mixed forms (so-called gynandroblastoma). Am J Surg Pathol 2021; 45: 59–67. DOI: 10.1097/pas.0000000000001544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231187796 for Mixed sex cord–stromal tumor (gynandroblastoma) with malignant morphology involving both ovaries: a case report by Bayan Maraqa, Maxim Al-Ashhab, Nazmi Kamal, Maher Sughayer and Fareed Barakat in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231187796 for Mixed sex cord–stromal tumor (gynandroblastoma) with malignant morphology involving both ovaries: a case report by Bayan Maraqa, Maxim Al-Ashhab, Nazmi Kamal, Maher Sughayer and Fareed Barakat in Journal of International Medical Research
Data Availability Statement
The original data presented in the study are included in the article. Further inquiries can be directed to the corresponding author.