Abstract
High Energy Musculoskeletal Traumas (HEMTs) represent a relevant problem for healthcare systems, considering the high social costs, and both the high morbidity and mortality. The poor outcomes associated with HEMT are related to the high incidence of complications, including bone infection, fracture malunion and non-union. The treatment of each of these complications could be extremely difficult. Limb reconstruction often needs multiple procedures, rising some questions on the opportunity in perseverate to try to save the affected limb. In fact, theoretically, amputation may guarantee better function and lower complications. However, amputation is not free of complication, and a high long-term social cost has been reported. A comprehensive literature review was performed to suggest possible ways to optimize the limb preservation surgeries of HEMT’s complications in order to ameliorate their management.
Keywords: fracture-related infection, high energy musculoskeletal traumas, limb reconstruction, limb salvage, mal union, non-union, osteomyelitis
Introduction
High Energy Musculoskeletal Traumas (HEMTs) are a prominent problem for healthcare systems, considering both the social cost and the high morbidity and mortality. 1 Several factors contribute to generate the considerable healthcare cost associated with HEMT, that was estimated to be between 1% and 3% of the gross domestic product in the United States of America.1,2 Indeed, only 58% of patients with a HEMT are able to return to their work activities in the first year after the injury. Moreover, disability, patient death and some other direct medical costs also act in increasing the reported economic burden. 2
In many cases, HEMTs severely affect also the vascular, nervous, and other soft tissues. Therefore, a multidisciplinary evaluation of HEMTs is often necessary to plan the limb reconstruction, generally involving the orthopedic, the plastic and the vascular surgeons.2–4 The occurrence of a severe vascular injury may be a dreadful event, often representing an emergency. In fact, in these cases the revascularization must be performed in the first 6 h considering the relevant risk of necrosis after this time point. An appropriate evaluation of the vascular injury should be constantly performed by looking for high suspicious signs (such as pallor, decreased temperature, reduced pulses, delayed capillary refill), also after gross realignment of the skeletal segment. A strict collaboration between the trauma leader and vascular surgeon is essential in case of HEMTs to organize the most convenient sequence of surgical procedures to minimize the risk of long ischemia.2,3 Another actor in this multidisciplinary framework is the plastic surgeon, preferably with experience in microsurgery. In fact, the use of complex reconstructive techniques, such as muscle flaps, free flaps, vascularized bone graft, may considerably improve the outcomes, thanks to the notable effects of filling bone and/or skin defects using vascularized tissues. Furthermore, some techniques are able to provide for the association of muscle-cutaneous flaps containing nervous structures to be anastomosed with peripheral injured nerves.5–7
Anyway, the high morbidity and poor outcomes associated with HEMT are mostly related to the high incidence of complications, including wound infection (28.3%) and fracture non-union (23.7%). 8 Treatment decision-making of HEMT-related complications may be extremely difficult. Limb preservation options often need multiple surgical procedures, rising some questions on the opportunity in perseverate to try to save the affected limb. In fact, amputation has proven to be a valid alterative that guarantees good function and reduced complications. 9 Moreover, some studies have shown that quality of life is not directly dependent on limb salvage. 9 Several parameters have been described to help in the decision to save or amputate a severely injured limb.10–12 The main criteria to be considered may be age, general condition, time of ischemia, extent of soft tissue damage and bilateral amputations. These criteria are part of some scores that may further aid in the treatment decision-making, reducing the rate of complications following a limb salvage attempt. Particularly, Battiston et al. 10 proposed a modified mangled extremity severity score (MESS) in which a score <5 clearly indicate a reimplantation, while a score >8 an amputation (see Table 1). 10 Moreover, the authors described a high rate of secondary complications requiring a subsequent amputation, in a case series of 12 patients with a reimplanted limb. 10 Chen and Chen described a grade system, according to which it is possible to foresee the functional outcomes of a reimplanted limb. 11
Table 1.
MESS score modified by Battiston et al. 10
| Age Score |
>50 2 |
30–50 1 |
<30 0 |
||
|---|---|---|---|---|---|
| General conditions Score |
Shock 4 |
Systemic disease, diabetes, hypertension, heart problems 2 |
Good conditions 0 |
||
| Ischemia time Score |
6 h, cold ⩽4 h, warm 4 |
3–6 h, cold 2 |
⩽3 h, cold 0 |
||
| Local conditions Score |
Severe contamination, comminution, bone loss 2 |
Complex fracture without severe contamination 1 |
Neat lesions, no bone loss contamination 0 |
||
| Soft-tissue problems Score |
Severe lesions of posterior tibial nerve 4 |
Large skin and muscular-tendon loosening 3 |
Severe skin problems but good muscle conditions 2 |
Partial skin necrosis 1 |
Good conditions 0 |
If the score is >8, contraindication for replantation; 6–7, possible replantation, poor functional result; ⩽5, indication for replantation.
MESS, mangled extremity severity score.
However, amputee long-term management is not cost and risk free.13,14 Recently, in the United Kingdom, a total 40-year £288 million cost (equal to USD 444 million) has been estimated among the UK Afghanistan lower limb amputee cohort. 13 Moreover, a loss of function related to a gait imbalance, was demonstrated even among patients with transtibial amputation (one of the most efficient level of amputation). 14
Therefore, to ameliorate patients’ outcomes, the limb salvage procedures of HEMT complications should be carried out carefully and patients appropriately selected.
Considering the multiple unmet needs related to the surgical procedures available to treat HEMT-related complications, we decided to perform a comprehensive evaluation of the available literature to guide the orthopedic surgeon throughout the treatment decision-making.
Fracture Non-Union
The failure of the bone healing process occurs in up to 10% of patients with a fracture, 15 but HEMTs consistently raises up the probability of observing this complication. The incidence of non-unions depends also on the bone involved. In fact, carpal scaphoid fractures are associated with a risk of non-union of 15.5%, whereas tibial and femoral ones of 14.4% and 13.9% respectively. 15
Multiple definitions of fracture non-union were reported in the current literature. Indeed, the Food and Drug Administration defined a non-union as a fracture that was not healed at 9 months after the injury. 16 Some other authors, instead, described it as a fracture that was not healed in the expected time and/or with no healing progression on plain radiographs, or a fracture that needs additional procedures to achieve union. 17
Both patient- and surgical-related factors could negatively affect the bone healing and they must all be considered before starting to treat a fracture non-union. 18 The evaluation of the previous treatment, the morphology of the original fracture, the appropriateness of the previous fracture fixation and the quality of reduction are all surgical-related factors that have to be taken into account. Moreover, a global health status evaluation of the patient-related factors, including comorbidities, life habits, as well as bone metabolism and bone quality should be performed. Obviously, bone and soft tissue infections must be excluded, considering that they lead to a different diagnosis and dramatically change the management.
Classification of Non-Union
The classification of non-union is not univocal. The most popular was described by Weber and Cech, 19 that distinguished three types of non-unions, basically biologically active (hypertrophic) and inactive (oligotrophic and atrophic) (see Table 2). However, available evidence did not completely support this classification. In fact, some histological studies did not report relevant differences between hypertrophic and atrophic non-unions. 20 Moreover, this kind of classification did not consider patients’ comorbidities.
Table 2.
Most widely used classifications of fracture non-unions. In both of them, reading from the left to the right, a greater need for biological supplementation is required.
| Classification of non-unions | ||||
|---|---|---|---|---|
| Weber and Cech 19 |
Hypertrophic
Hypervascular fracture site (good biology, poor mechanical stability) |
Oligotrophic
Adequate vascularization with minimal callus formation |
Atrophic
Impaired vascularity, no callus formation (poor biology) |
|
| NUSS 21 |
0–25 pts
Major mechanical problem |
26–50 pts
Minor mechanical + minor biological problem |
51–75 pts
Combination of a Major and a Minor problem |
75–100 pts
Major mechanical + Major biological problem |
NUSS, Non Union Scoring System.
In bold there are reported the categories of each classification.
For this reasons, other authors proposed more complex classification systems.19,22 One of the most used is the Non Union Scoring System. 21 This classification included patient-related factors, soft tissue status, Weber and Cech classification, previous treatment, bone quality and fracture-related factors (see Table 2 for further details).
Non-Surgical Treatments
If a biologically active non-union is suspected, some non-surgical treatments may be attempted.
Recently, a relevant role has been suggested for bone anabolic drugs for the treatment of delayed consolidations and non-unions. Specifically, teriparatide, a parathyroid hormone (PTH) derivate, has been suggested to be able to improve bone healing, 23 although the scientific evidence is still poor and mainly based on case reports and small clinical series.24–29 Particularly, Gariffo et al. 27 in a recent case series of 20 delayed/non-unions reported a union rate of 85%, most of which occurred after 3 months of teriparatide use.
Abaloparatide, a synthetic analog of a PTH-related peptide with a weaker binding to the R° conformation of the PTH receptor compared to teriparatide, has been recently proposed as a more effective anabolic drug in osteoporotic patients. 30 Recent preclinical studies supported its role also on fracture healing. 31
Physical agent modalities too may act as non-surgical means to improve bone healing. In fact, a recent systematic review on Pulsed Electromagnetic Fields demonstrated a low to moderate quality of evidence supporting its role in increasing bone healing rate and accelerating time to healing. 32
Low-intensity pulsed ultrasound (LIPUS) is another physical-agent modality with promising results in the treatment of non-unions. 33 The authors of a recent systematic review reported a success rate of 82% and proposed LIPUS as an alternative to surgery in fracture non-union in elderly, or in patients with severe comorbidities. 34 Moreover, some evidence supported their use also in case of hypertrophic non-unions. 34
Surgical Management of Non-Unions
The surgical management of non-unions may be extremely difficult, and often requires a comprehensive approach focused on all those factors that could affect bone healing. This kind of approach has been recently summarized in the ‘diamond concept’ conceived by Andrzejowski and Giannoudis 35 According to this concept, a successful fracture healing depends on both the biological and the mechanical environment observable at the fracture site as well as the vascularization. In fact, according to the authors, a viable biological environment is essential to provide mediators and cells, as well as osteoconductive matrix necessary to the osteoprogenitors to act, while the need of a consistent mechanical environment, underline the relevance of a proper fracture stability to aid cells in appropriately complete the callus formation. 36 Andrzejowski and Giannoudis 35 affirmed that a rigorous application of all the aspects of the diamond concept could ensure a success rate of 89–100% in the treatment of long-bone non-union.
To achieve the needed focal bone revitalization for the bone union, some biologically active materials and techniques are available. 37 These materials and techniques may be divided into two main categories: vascularized and non-vascularized. The first ones are represented by autologous vascularized graft and distraction osteogenesis, while the second typically include allogenic graft, autologous non-vascularized graft, and bone substitutes (scaffolds). 37
In the context of vascularized grafts, the use of the vascularized fibular graft can provide both an adequate mechanical stability and biology in case of non-unions with large segmental defects. 38 Moreover, its use is possible also for the management of infected non-unions. However, the harvesting of a vascularized bone graft may be extremely difficult and requires microvascular environment. When biological support is strongly recommended but microsurgical supplies are not available, the historical Huntington’s ipsilateral vascularized fibular transposition is still a viable option in selected patients.39–42 Figure 1 shows a clinical case of a recalcitrant middle tibia non-union treated using the Huntington’s procedure.
Figure 1.
A clinical case of a 42 years old male with a middle shaft tibia septic non-union following an open fracture treated with intramedullary nailing (a). The patient was treated through septic bone resection and subsequent bone transport (b). In (c) non-union of the docking point, treated through a new bone resection and bone transport for definitive infection control (d). In (e) X-ray at 7 months suggestive of docking point union. In (f) recurrence of an aseptic atrophic non-union treated using the Huntington’s procedure (g). In (h) X-ray at 12 months after the procedure, note the full integration and partial hypertrophy of the fibula.
Moreover, recent studies supported the use of Bone Marrow (BM) Concentrate to treat long-bone non-unions, 43 especially when non-vascularized biological materials are used. In fact, as suggested by pre-clinical studies, the application of BM is able to enhance bone vascularization. 44 Moreover, the simultaneous use of internal fixation, cortical allograft and BM-stem cells has been recently demonstrated to be a viable option to treat humeral shaft non-union also in the elderly patient. 45
The management of non-unions with severe bone loss following HEMTs might be addressed also using an external fixation,17,20 considering its reported ability to provide stability, correct alignment and biological stimulation at the non-union site. Moreover, the massive bone loss might be replaced using the bone transport technique. Theoretically, it leaves intact the soft tissues around the fracture site, being a percutaneous device. This leads to a further preservation of the biological and vascularization environment, that might aid also for the reconstruction of any soft tissue injury. However, pin tract infections, docking-site non-union are among the complications reported for the external fixator use in fracture non-unions.46–48 To reduce their incidence an accurate surgical technique and post-operative follow-up is needed, especially for pin tract-related complications. 49 On the other hand, acute shortening or reintervention with the application of bone stimulating substances are among the proposed protocols to treat docking-site non-union.46,50
Recently, the adoption of a contemporary use of internal and external fixations in the same patient is gaining popularity, thanks also to the reported reduction in time of use of the external fixator.51–53 Particularly, Kadhim et al., 54 in their Systematic review, reported that the combined use of circular fixation and intramedullary nail provided the highest success rate for the treatment of tibial non-union with segmental bone defect.
Fracture Malunion
Malunion is an extremely common complication of HEMTs, with a reported incidence of about 22%.8,55
The treatment of malunion is often required because it may severely affect limb function or increase the risk of early osteoarthritis. Particularly, a 10° varus/valgus malunion has been observed to lead to a critical increase in medial and lateral knee cartilage stress, respectively. 56
Several techniques had been described to manage fracture malunion, substantially based on corrective osteotomy and application of both internal and external fixation.57,58 Infection, delayed healing and non-union are among the reported complications of corrective osteotomies of the lower limbs.58,59 In order to reduce bone healing complications and improving patients’ comfort the clamshell and chipping corrective osteotomies had been proposed.60–63 Clamshell osteotomy requires the identification of the malunited segment and to perform two subsequent osteotomies, one proximally and one other distally the center of the deformity. After that, the malunited segment is again osteotomized along its axis and wedged open. The three resulting fragments are then aligned with an intramedullary rod.
The efficacy of chipping corrective osteotomy (CCO) was recently reported in a small series in which a bone healing was achieved after a mean of 3.5 months. 63 The technique requires the use of a temporary external fixator, followed by a definitive osteosynthesis using a locking plate. CCO improves the bone healing probably thanks to a sort of biological chamber created by the chipping in fragments of the malunion site. Figure 2 shows a clinical case treated with chipping osteotomy.
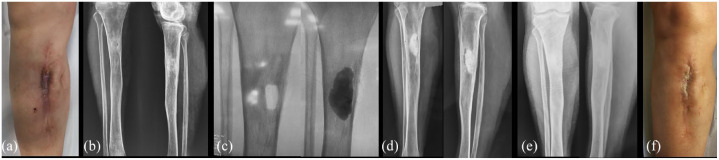
Figure 2.
A clinical case of a 38 years old male with a tibial varus malunion (a) after a road accident. Initially the malunion was treated using an exapodalic external fixator (b). In (c) the reoccurrence of varus deformity related to osteotomy partial non-union. In (d) intraoperative picture of the chipping osteotomy, leading to a good alignment and reliable healing; in (e) the post-operative X-ray; in (f) full length lower limb X-ray at 9 months after the surgery.
Fracture-Related Infections
Fracture-related infections (FRI) are a challenging and demanding complication with a severe impact on the healthcare systems, 64 considering the length of hospitalization, rehospitalization rate, 65 and the infection recurrence that may lead to a subsequent limb amputation in 3–5% of cases. 66
The reported rate of FRI for closed fractures was 1–2%, but it grew up to 30% in cases of open fractures.67,68
Theoretically, to reduce FRI occurrence, the treatment of an open fracture should be conducted in less than 6 h from the trauma, 69 and should be based on debridement, wound irrigation and antibiotic therapy. 69 However, current evidence reduced the impact of the 6-h rule on the infection rate, underlying, instead, the more relevant role of a careful surgical debridement and appropriate antibiotic treatement.70–73
In 2018, the International Consensus Meeting held in Philadelphia, proposed some criteria to define FRI, differentiating them in ‘confirmatory’ and ‘suggestive’ (see Table 3). 74
Table 3.
International Consensus Meeting (ICM) 2018 criteria for the diagnosis of FRI. 74
| Confirmative criteria | Suggestive criteria |
|---|---|
| Fistula, sinus or wound breakdown | Clinical signs: pain increasing over time, local redness, local swelling, increased local temperature or fever |
| Purulent drainage or presence of pus | Radiological and nuclear imaging signs |
| Phenotypically indistinguishable pathogens identified by culture from at least two separate deep tissue/implant specimens | Pathogenic organism identified by culture from a single deep tissue/implant specimen |
| Presence of more than five polymorphonuclear neutrophil per high power field, confirmed by histopathological examination | Elevated serum inflammatory markers: erythrocyte sedimentation rate (ESR), white blood cells count (WBC), C-reactive protein (CRP) |
| Persistent or increasing wound drainage | |
| New-onset of joint effusion in fracture patients |
FRI, Fracture-related infections.
The treatment of FRI may be extremely difficult and frustrating for both the patient and the surgeon. One of the main aspects that may explain the poor outcomes of FRI is the formation of the bacterial biofilm on the foreign material represented by the fixation devices. 75 The biofilm is a polymeric matrix mainly consisting of bacterial products that protects them from harmful environmental conditions, including the host immune responses and antimicrobial agents.76–79 Moreover, the formation of a biofilm may lead also to improper diagnosis since bacteria in the biofilm are in a somewhat anergic state. A recent Systematic Review proposed that sonication of fluid culture might improve diagnostic abilities, especially in those patients already treated with antibiotic therapy. 80
Very often, to effectively treat FRI, the biofilm must be surgically excised. In this perspective, the use of an antiseptic/antibiofilm wound lavage may be of aid, as recently underlined by Whitely et al. 81 in their delayed debridement animal model of open fractures.
However, the timely identification of a FRI remains relevant to appropriately guide the surgeon in the treatment decision-making.74,82 The relevance of timing is underlined by one of the most widely used classification of FRI, based on the time elapsed between the fracture fixation and the onset of the infection. In an early onset FRI (between 0 and 1 week after surgery) the fracture is supposed to be not healed yet and the surgeon should try to retain the fixation implant. For this reason, in most cases surgical debridement with deep samples for cultures, and subsequent specific antibiotic therapy is often the treatment of choice [debridement, antimicrobial therapy and implant retention (DAIR)]. The antibiotic therapy may be prolonged until the bone healing occurred and then the implant should be removed. 83 In case of delayed onsets FRI (between 2 and 10 weeks after surgery), and in late/chronic onsets the removal of the hardware is always necessary, followed by surgical debridement and antibiotic therapy. There is no clear recommendation for the timing of an effective DAIR, but the rate of success decreases constantly with time. In fact, clinical studies reported a success rate of 90% when applied within 3 weeks,84,85 70% within 6 weeks,86,87 51–67% over 10 weeks after surgery.65,85,88 However, time, fracture healing status and implant stability are not the unique factors to consider when treating a FRI. In this context the Cierny’s classification may be of aid in the treatment decision-making, especially in case of late/chronic infections (see Tables 4 and 5). 89
Table 4.
Cierny’s classification.
| Anatomic type | |
|---|---|
| I | Medullary osteomyelitis |
| II | Superficial osteomyelitis |
| III | Localized osteomyelitis |
| IV | Diffuse osteomyelitis |
| Physiologic classes | |
| A-Host | Good immune system and Delivery |
| B-Host | Compromised locally (B L) or Systemically (B S) |
| C-Host | Requires suppressive or no treatment; minimal disability; treatment worse than disease; not a surgical candidate |
| Clinical Stage | |
| Type + Class = Clinical Stage. Example: Stage IVB S osteomyelitis = a diffuse lesion in a systemically compromised host | |
Source: Adapted from Cierny et al. 89
Table 5.
Proposed intervention according to the Cierny’s classification.
| Clinical stage | Proposed intervention |
|---|---|
| Stage I | Systemic antimicrobial therapy at early stage. Debridement may be needed at late stage. |
| Stage II | Systemic antimicrobial therapy at early stage. Debridement may be needed at late stage. |
| Stage III | Antimicrobial therapy at early stage in addition to limited surgical procedures. |
| Stage IV | Requires surgical and antimicrobial therapy in addition to post-surgery stabilization. |
Source: Adapted from Wassif et al. 90
After a surgical debridement and especially in case of chronic/late osteomyelitis or in case of infected non-union, one of the main critical issues to face up might be the reconstruction of the subsequent bone loss. Several techniques are available, including the use of autologous or heterologous bone graft, bone substitute or bone transport with external fixator.17,20,37,91
The ideal bone substitute should present osteoinduction (the process by which osteogenesis is induced), osteoconduction (the process through which the bone grows on a surface) and osseointegration (the stable anchorage of an implant achieved by direct bone-to-implant contact) properties. Only autografts (bone graft obtained from the affected patient) present all these three characteristics, but their availability is very limited. 92 The non-biological materials, such as ceramics, metals, alloys, polymers, composites, and hydrogels are generally referred as alloplasts and synthetic materials. 92 Considering their wide availability, synthetic bone substitute (i.e. calcium sulfate, calcium phosphate, hydroxyapatite) are generally used with the purpose to achieve bone loss reconstruction exploiting their reported osteoconduction and osteointegration properties. 93 However, both may be severely impaired by the persistence of infection. Therefore, several antibiotic-loaded bone substitutes had been proposed with encouraging results. 94
A case series of 100 patients with chronic osteomyelitis treated though a single-stage debridement and the application of an absorbable, gentamicin-loaded, calcium suphate/hydroxyapatite biocomposite, reported a healing rate of 96%, with rare adverse events. 95 Figure 3 shows a case of a chronic tibial osteomyelitis treated using an antibiotic-loaded bone substitute.
Figure 3.
A clinical case of a 61 years old female with a tibial chronic osteomyelitis following a road accident occurred 40 years before our observation. In (a) and (b) clinics and antero-posterior and latero-lateral X-rays at the time of our observation. The patient was treated through a sequestrectomy and application of an antibiotic-loaded bone substitute. In (c) the intraoperative fluoroscopy before (left) and after (right) the application of the bone substitute. In (d) antero-posterior and latero-lateral X-rays at 1 month after the surgery. Note the partial bone substitute reabsorption. In (e) and (f) X-rays and clinics at 4 months after the surgery.
Anyway, also in the case of using antibiotic-loaded bone substitutes, prolonged antimicrobial therapy is recommended (at least 6 weeks after implant removal or 12 weeks in case of implant retention).96,97 One issue that limits the antibiotic use for FRI is also the poor bone penetration of most antibiotics. Currently there is a relevant effort to achieve a high bone antibiotic concentration using also local delivery agents.98–100 However a satisfactory kinetics of these local antibiotic-loaded carriers is not reported yet.
Conclusions
HEMTs are often associated with severe complications, especially non-union, malunion and FRI, that may lead to limb amputation. Although good outcomes were reported with limb amputation, this treatment still presents high long-term costs and disputable functional results. The limb preservation surgery for HEMTs-related complications may be frustrating and difficult, and often requires multiple/staged procedures. The orthopedic surgeon must use several techniques to tailorize-to-the-patient the treatment. In order to improve patients’ outcomes while reducing costs, an appropriate limb salvage technique selection is mandatory, even combining them. Moreover, the surgeon must keep in mind that bone biology is as essential as fracture stability and bone stock preservation/restoration. Finally, in our opinion the pivotal factor to effectively treat the HEMTs-related complications is the engagement of a close cooperative relationship between the patient and the surgeon.
Acknowledgments
None.
Footnotes
ORCID iDs: Giuseppe Toro  https://orcid.org/0000-0002-8560-721X
https://orcid.org/0000-0002-8560-721X
Giovanni Iolascon  https://orcid.org/0000-0002-0976-925X
https://orcid.org/0000-0002-0976-925X
Contributor Information
Giuseppe Toro, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Antonio Benedetto Cecere, Unit of Orthopaedics and Traumatology, San Giuliano Hospital, Giugliano in Campania, Naples, Italy.
Adriano Braile, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Via L. De Crecchio, 4. Naples 80138, Italy Unit of Orthopaedics and Traumatology, Ospedale del Mare, Naples, Italy.
Annalisa De Cicco, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy Unit of Orthopaedics and Traumatology, Santa Maria delle Grazie Hospital, Pozzuoli, Italy.
Sara Liguori, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Umberto Tarantino, Department of Clinical Sciences and Translational Medicine, University of Rome Tor Vergata, Rome, Italy.
Giovanni Iolascon, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Declarations
Ethics approval and consent to participate: As a standard protocol, all patients provided written and informed consent allowing to undergo surgery and to have their data collected for scientific and audit purposes. The present study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. According to Italian law, formal ethics approval was not required for this study, as it involved routine tests and clinical evaluations.
Consent for publication: All patients provided written and informed consent allowing to have their data collected for scientific and audit purposes.
Author contributions: Giuseppe Toro: Conceptualization; Data curation; Writing – review & editing.
Antonio Benedetto Cecere: Investigation; Writing – original draft.
Adriano Braile: Data curation; Investigation; Methodology.
Annalisa De Cicco: Data curation; Investigation.
Sara Liguori: Formal analysis; Investigation; Writing – review & editing.
Umberto Tarantino: Data curation; Supervision; Writing – review & editing.
Giovanni Iolascon: Funding acquisition; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data are given in the present manuscript.
References
- 1. Hoogervorst P, Shearer DW, Miclau T. The burden of high-energy musculoskeletal trauma in high-income countries. World J Surg 2020; 44: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 2. Stinner DJ, Edwards D. Surgical management of musculoskeletal trauma. Surg Clin N Am 2017; 97: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 3. Marchesini A, Senesi L, De Francesco F, et al. Efficacy of the arteriovenous loop for free flap reconstruction in patients with complex limb trauma: case series and literature review. Medicina (Kaunas) 2020; 56: 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Francesco F, Marchesini A, Campodonico A, et al. A multistep iter for functional reconstruction in mangled upper limb: a retrospective analysis of integrated surgical and medical approach. Medicinar 2020; 56: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gravina P, De Francesco F, Pangrazi PP, et al. A case report of upper limb loss of substance: use of functional gracilis free flap, brachioradialis transposition and bioglass for bone regeneration. Trauma Case Rep 2022; 38: 100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riccio M, Zingaretti N, Verdini F, et al. Functional donor-site morbidity after soleus muscle-flap procedure in the treatment of lower limb severe injuries. Handchir Mikrochir Plast Chir 2019; 51: 453–463. [DOI] [PubMed] [Google Scholar]
- 7. Tos P, Antonini A, Pugliese P, et al. Below knee stump reconstruction with a foot fillet flap. J Reconstr Microsurg 2017; 33: S20–S26. [DOI] [PubMed] [Google Scholar]
- 8. Harris AM, Althausen PL, Kellam J, et al. Complications following limb-threatening lower extremity trauma. J Orthop Trauma 2009; 23: 1–6. [DOI] [PubMed] [Google Scholar]
- 9. Fioravanti M, Maman P, Curvale G, et al. Amputation versus conservative treatment in severe open lower-limb fracture: a functional and quality-of-life study. Orthop Traumatol Surg Res 2018; 104: 277–281. [DOI] [PubMed] [Google Scholar]
- 10. Battiston B, Tos P, Pontini I, et al. Lower limb replantations: indications and a new scoring system. Microsurgery 2002; 22: 187–192. [DOI] [PubMed] [Google Scholar]
- 11. Chen ZW, Chen LE. Textbook of microsurgery. In: Brunelli G. (ed.) Milan, Masson, 1988. [Google Scholar]
- 12. Battiston B, Santoro D, Baido RL, et al. Treatment of acute bone defects in severe lower limb trauma. Injury 2019; 50(Suppl. 5): S40–S45. [DOI] [PubMed] [Google Scholar]
- 13. Edwards DS, Phillip RD, Bosanquet N, et al. What is the magnitude and long-term economic cost of care of the British military Afghanistan amputee cohort? Clin Orthop Relat Res 2015; 473: 2848–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eshraghi A, Safaeepour Z, Geil MD, et al. Walking and balance in children and adolescents with lower-limb amputation: a review of literature. Clin Biomech 2018; 59: 181–198. [DOI] [PubMed] [Google Scholar]
- 15. Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg 2016; 151: e162775. [DOI] [PubMed] [Google Scholar]
- 16. Calori GM, Mazza EL, Mazzola S, et al. Non-unions. Clin Cases Miner Bone Metab 2017; 14: 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simpson AHRW, Robiati L, Jalal MMK, et al. Non-union: indications for external fixation. Injury 2019; 50(Suppl. 1): S73–S78. [DOI] [PubMed] [Google Scholar]
- 18. Bell A, Templeman D, Weinlein JC. Nonunion of the Femur and Tibia: an update. Orthop Clin North Am 2016; 47: 365–375. [DOI] [PubMed] [Google Scholar]
- 19. Calori GM, Colombo M, Mazza EL, et al. Validation of the non-union scoring system in 300 long bone non-unions. Injury 2014; 45(Suppl. 6): S93–S97. [DOI] [PubMed] [Google Scholar]
- 20. Rupp M, Biehl C, Budak M, et al. Diaphyseal long bone nonunions - types, aetiology, economics, and treatment recommendations. Int Orthop 2018; 42: 247–258. [DOI] [PubMed] [Google Scholar]
- 21. Calori GM, Phillips M, Jeetle S, et al. Classification of non-union: need for a new scoring system? Injury 2008; 39(Suppl. 2): S59–S63. [DOI] [PubMed] [Google Scholar]
- 22. van Basten Batenburg M, Houben IB, Blokhuis TJ. The non-union scoring system: an interobserver reliability study. Eur J Trauma Emerg Surg 2019; 45: 13–19. [DOI] [PubMed] [Google Scholar]
- 23. Canintika AF, Dilogo IH. Teriparatide for treating delayed union and nonunion: a systematic review. J Clin Orthop Trauma 2020; 11: S107–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciurlia E, Leali PT, Doria C. Use of teriparatide off-label: our experience and review of literature. Clin Cases Miner Bone Metab 2017; 14: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coppola C, Del Buono A, Maffulli N. Teriparatide in fracture non-unions. Transl Med UniSa 2015; 12: 47–53. [PMC free article] [PubMed] [Google Scholar]
- 26. Garg B, Batra S, Dixit V. An unexpected healing of an established non union of the radial neck through teriparatide: a case report and review of literature. J Clin Orthop Trauma 2018; 9: S103–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gariffo G, Bottai V, Falcinelli F, et al. Use of teriparatide in preventing delayed bone healing and nonunion: a multicentric study on a series of 20 patients. BMC Musculoskelet Disord 2023; 24: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai L, Li Y, Shen M, et al. Treatment of postoperative non-union with internal fixation loosening of Garden IV femoral neck fracture with teriparatide in a young adult: a case report. Front Surg 2022; 9: 938595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emanuele C, Leonardo P, Gianfilippo C, et al. Peri-prosthetic humeral non-union: where biology meets bio-mechanic. A case report. Int J Surg Case Rep 2017; 39: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iolascon G, Moretti A, Toro G, et al. Pharmacological therapy of osteoporosis: what’s new? Clin Interv Aging 2020; 15: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brent MB. Abaloparatide: a review of preclinical and clinical studies. Eur J Pharmacol 2021; 909: 174409. [DOI] [PubMed] [Google Scholar]
- 32. Peng L, Fu C, Xiong F, et al. Effectiveness of pulsed electromagnetic fields on bone healing: a systematic review and meta-analysis of randomized controlled trials. Bioelectromagnetics 2020; 41: 323–337. [DOI] [PubMed] [Google Scholar]
- 33. Romanò CL, Kirienko A, Sandrone C, et al. Low-intensity pulsed ultrasound in the treatment of nonunions and fresh fractures: a case series. Trauma Care 2022; 2: 174–184. [Google Scholar]
- 34. Leighton R, Watson JT, Giannoudis P, et al. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): a systematic review and meta-analysis. Injury 2017; 48: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 35. Andrzejowski P, Giannoudis PV. The ‘diamond concept’ for long bone non-union management. J Orthop Traumatol 2019; 20: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Augat P, Simon U, Liedert A, et al. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int 2005; 16(Suppl. 2): S36–S43. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto N, Hayashi K, Tsuchiya H. Progress in biological reconstruction and enhanced bone revitalization for bone defects. J Orthop Sci 2019; 24: 387–392. [DOI] [PubMed] [Google Scholar]
- 38. Soucacos PN, Dailiana Z, Beris AE, et al. Vascularised bone grafts for the management of non-union. Injury 2006; 37(Suppl. 1): S41–S50. [DOI] [PubMed] [Google Scholar]
- 39. Vi HT. Case of bone transference: use of a segment of fibula to supply a defect in the tibia. Ann Surg 1905; 41: 249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agarwal P, Savant R, Sharma D. Huntington’s procedure revisited. J Clin Orthop Trauma 2019; 10: 1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan AQ, Siddiqui YS, Julfiqar, et al. Role of Huntington procedure as a limb salvage surgery for complex gap nonunion of tibia in children. J Clin Orthop Trauma 2021; 18: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kundu ZS, Gupta V, Sangwan SS, et al. Gap nonunion of tibia treated by Huntington’s procedure. Indian J Orthop 2012; 46: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palombella S, Lopa S, Gianola S, et al. Bone marrow-derived cell therapies to heal long-bone nonunions: a systematic review and meta-analysis-which is the best available treatment? Stem Cells Int 2019; 2019: 3715964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mousaei Ghasroldasht M, Matin MM, Kazemi Mehrjerdi H, et al. Application of mesenchymal stem cells to enhance non-union bone fracture healing. J Biomed Mater Res A 2019; 107: 301–311. [DOI] [PubMed] [Google Scholar]
- 45. Toro G, Lepore F, Calabrò G, et al. Humeral shaft non-union in the elderly: results with cortical graft plus stem cells. Injury 2019; 50(Suppl. 2): S75–S79. [DOI] [PubMed] [Google Scholar]
- 46. Giotakis N, Narayan B, Nayagam S. Distraction osteogenesis and nonunion of the docking site: is there an ideal treatment option? Injury 2007; 38(Suppl. 1): S100–S107. [DOI] [PubMed] [Google Scholar]
- 47. Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res 1990; 250: 81–104. [PubMed] [Google Scholar]
- 48. Jilani LZ, Shaan ZH, Ranjan R, et al. Management of complex non union of tibia using rail external fixator. J Clin Orthop Trauma 2020; 11: S578–S584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferreira N, Marais LC. Prevention and management of external fixator pin track sepsis. Strategies Trauma Limb Reconstr 2012; 7: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tetsworth K, Paley D, Sen C, et al. Bone transport versus acute shortening for the management of infected tibial non-unions with bone defects. Injury 2017; 48: 2276–2284. [DOI] [PubMed] [Google Scholar]
- 51. Lu V, Zhang J, Zhou A, et al. Management of post-traumatic femoral defects with a monorail external fixator over an intramedullary nail. Eur J Orthop Surg Traumatol 2022; 32: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gulabi D, Erdem M, Cecen GS, et al. Ilizarov fixator combined with an intramedullary nail for tibial nonunions with bone loss: is it effective? Clin Orthop Relat Res 2014; 472: 3892–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Biz C, Iacobellis C. Nailing treatment in bone transport complications. Strategies Trauma Limb Reconstr 2014; 9: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kadhim M, Holmes L, Jr, Gesheff MG, et al. Treatment options for nonunion with segmental bone defects: systematic review and quantitative evidence synthesis. J Orthop Trauma 2017; 31: 111–119. [DOI] [PubMed] [Google Scholar]
- 55. Daley DN, Westbrook PA, Barfield WR, et al. The rates of nonunion and Malunion in lower extremity fractures: experience in South Carolina over 17 years. J Surg Orthop Adv 2020; 29: 129–134. [PubMed] [Google Scholar]
- 56. Ding K, Yang W, Wang H, et al. Finite element analysis of biomechanical effects of residual varus/valgus malunion after femoral fracture on knee joint. Int Orthop 2021; 45: 1827–1835. [DOI] [PubMed] [Google Scholar]
- 57. Neiman R. Focal dome osteotomy for the treatment of diaphyseal malunion of the lower extremity. Medicinar 2022; 58: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg 2003; 11: 302–311. [DOI] [PubMed] [Google Scholar]
- 59. Tunggal JA, Higgins GA, Waddell JP. Complications of closing wedge high tibial osteotomy. Int Orthop 2010; 34: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pires RE, Gausden EB, Sanchez GT, et al. Clamshell osteotomy for acute fractures in the malunion setting: a technical note. J Orthop Trauma 2018; 32: e415–e420. [DOI] [PubMed] [Google Scholar]
- 61. Pires RE, Reis IGN, Santana Eo., Jr. Treatment of a diaphyseal tibial malunion with use of the clamshell osteotomy. JBJS Essen Surg Tech. 2021; 11: e20.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Russell GV, Graves ML, Archdeacon MT, et al. The clamshell osteotomy: a new technique to correct complex diaphyseal malunions: surgical technique. J Bone Joint Surg Am 2010; 92(Suppl. 1): 158–175. [DOI] [PubMed] [Google Scholar]
- 63. Miyamoto W, Watanabe Y, Kawano H, et al. Chipping corrective osteotomy for reconstruction of malunion with angular deformity of the lower extremity: technical tips and preliminary clinical results. Injury 2021; 52: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 64. Baecker H, Frieler S, Schildhauer TA, et al. [Fracture-related infections in traumatology: current standards and new developments in diagnostics and treatment]. Orthopade 2020; 49: 702–709. [DOI] [PubMed] [Google Scholar]
- 65. Depypere M, Morgenstern M, Kuehl R, et al. Pathogenesis and management of fracture-related infection’ – Author’s reply. Clin Microbiol Infect 2020; 26: 652–653. [DOI] [PubMed] [Google Scholar]
- 66. Bezstarosti H, Van Lieshout EMM, Voskamp LW, et al. Insights into treatment and outcome of fracture-related infection: a systematic literature review. Arch Orthop Trauma Surg 2019; 139: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Metsemakers W-J, Onsea J, Neutjens E, et al. Prevention of fracture-related infection: a multidisciplinary care package. Int Orthop 2017; 41: 2457–2469. [DOI] [PubMed] [Google Scholar]
- 68. Ktistakis I, Giannoudi M, Giannoudis PV. Infection rates after open tibial fractures: are they decreasing? Injury 2014; 45: 1025–1027. [DOI] [PubMed] [Google Scholar]
- 69. Elniel AR, Giannoudis PV. Open fractures of the lower extremity: current management and clinical outcomes. EFORT Open Rev 2018; 3: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ali AM, Noyes D, Cogswell LK. Management of open fractures of the lower limb. Br J Hosp Med 2013; 74: 577–580. [DOI] [PubMed] [Google Scholar]
- 71. Schenker ML, Yannascoli S, Baldwin KD, et al. Does timing to operative debridement affect infectious complications in open long-bone fractures?: a systematic review. J Bone Joint Surg 2012; 94: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 72. Werner CM, Pierpont Y, Pollak AN. The urgency of surgical débridement in the management of open fractures. J Am Acad Orthop Surg 2008; 16: 369–375. [DOI] [PubMed] [Google Scholar]
- 73. British Orthopaedic Association Trauma Committee. Electronic address: william.eardley@nhs.net. British orthopaedic association standard for trauma (BOAST): open fracture management. Injury 2020; 51: 174–177. [DOI] [PubMed] [Google Scholar]
- 74. Metsemakers W, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury 2018; 49: 505–510. [DOI] [PubMed] [Google Scholar]
- 75. Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS 2017; 125: 353–364. [DOI] [PubMed] [Google Scholar]
- 76. Blanchette KA, Prabhakara R, Shirtliff ME, et al. Inhibition of fracture healing in the presence of contamination by Staphylococcus aureus: effects of growth state and immune response. J Orthop Res 2017; 35: 1845–1854. [DOI] [PubMed] [Google Scholar]
- 77. Sanchez CJ, Jr, Mende K, Beckius ML, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis 2013; 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kwiecinski J, Kahlmeter G, Jin T. Biofilm formation by Staphylococcus aureus isolates from skin and soft tissue infections. Curr Microbiol 2015; 70: 698–703. [DOI] [PubMed] [Google Scholar]
- 79. Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet 2001; 358: 135–138. [DOI] [PubMed] [Google Scholar]
- 80. Onsea J, Depypere M, Govaert G, et al. Accuracy of tissue and sonication fluid sampling for the diagnosis of fracture-related infection: a systematic review and critical appraisal. JBone Jt Infect 2018; 3: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Whitely ME, Helms SM, Muire PJ, et al. Preclinical evaluation of a commercially available biofilm disrupting wound lavage for musculoskeletal trauma. J Orthop Surg Res 2022; 17: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Metsemakers W-J, Morgenstern M, Senneville E, et al. General treatment principles for fracture-related infection: recommendations from an international expert group. Arch Orthop Trauma Surg 2020; 140: 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury 2018; 49: 511–522. [DOI] [PubMed] [Google Scholar]
- 84. Tschudin-Sutter S, Frei R, Dangel M, et al. Validation of a treatment algorithm for orthopaedic implant-related infections with device-retention-results from a prospective observational cohort study. Clin Microbiol Infect 2016; 22: 457.e1–457.e9. [DOI] [PubMed] [Google Scholar]
- 85. Kuehl R, Tschudin-Sutter S, Morgenstern M, et al. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: an observational prospective study with 229 patients. Clin Microbiol Infect 2019; 25: 76–81. [DOI] [PubMed] [Google Scholar]
- 86. Barberán J, Aguilar L, Giménez M-J, et al. Levofloxacin plus rifampicin conservative treatment of 25 early staphylococcal infections of osteosynthetic devices for rigid internal fixation. Int J Antimicrob Agents 2008; 32: 154–157. [DOI] [PubMed] [Google Scholar]
- 87. Depypere M, Kuehl R, Metsemakers W-J, et al. Recommendations for systemic antimicrobial therapy in fracture-related infection: a consensus from an international expert group. J Orthop Trauma 2020; 34: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop Relat Res 2008; 466: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cierny G, 3rd, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res 2003; 414: 7–24. [DOI] [PubMed] [Google Scholar]
- 90. Wassif RK, Elkayal M, Shamma RN, et al. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv 2021; 28: 2392–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nicholson JA, Makaram N, Simpson AHRW, et al. Fracture nonunion in long bones: a literature review of risk factors and surgical management. Injury 2021; 52: S3–S11. [DOI] [PubMed] [Google Scholar]
- 92. Wickramasinghe ML, Dias GJ, Premadasa KMGP. A novel classification of bone graft materials. J Biomed Mater Res Part B Appl Biomater 2022; 110: 1724–1749. [DOI] [PubMed] [Google Scholar]
- 93. Giannoudis PV, Calori GM, Bégué T, et al. Tissue loss and bone repair: time to develop an international strategy? Injury 2015; 46(Suppl. 8): S1–S2. [DOI] [PubMed] [Google Scholar]
- 94. Ferguson J, Diefenbeck M, McNally M. Ceramic biocomposites as biodegradable antibiotic carriers in the treatment of bone infections. J Bone Jt Infect 2017; 2: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McNally MA, Ferguson JY, Lau AC, et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J 2016; 98-B: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 96. Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep 2008; 10: 394–403. [DOI] [PubMed] [Google Scholar]
- 97. Pesch S, Hanschen M, Greve F, et al. Treatment of fracture-related infection of the lower extremity with antibiotic-eluting ceramic bone substitutes: case series of 35 patients and literature review. Infection 2020; 48: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tanaka KS, Dietrich E, Ciblat S, et al. Synthesis and in vitro evaluation of bisphosphonated glycopeptide prodrugs for the treatment of osteomyelitis. Bioorg Med Chem Lett 2010; 20: 1355–1359. [DOI] [PubMed] [Google Scholar]
- 99. Thabit AK, Fatani DF, Bamakhrama MS, et al. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis 2019; 81: 128–136. [DOI] [PubMed] [Google Scholar]
- 100. Urish KL, Cassat JE. Staphylococcus aureus osteomyelitis: bone, bugs, and surgery. Infect Immun 2020; 88: e00932. [DOI] [PMC free article] [PubMed] [Google Scholar]