Abstract
Background:
Early mobilization after total hip replacement (THR) is key for fast recovery but is often limited by pain. Oral enzyme combinations (OECs) have demonstrated anti-inflammatory and pain-relieving effects.
Objectives and design:
This prospective, randomized, double-blind, placebo-controlled exploratory trial evaluated the effects of pre- and post-operative use of OEC (90 mg bromelain, 48 mg trypsin, 100 mg rutoside) following elective THR, on post-operative recovery.
Methods:
Candidates for primary elective cementless THR owing to osteoarthritis were eligible for participation [age ⩾50 years, body mass index 25–35 kg/m2, C-reactive protein (CRP) ⩽6 mg/L]. Following randomization to OEC or placebo, intervention started pre-operatively and continued onwards until day 42. Main outcomes included post-operative CRP levels (days 1–7), self-reported hip pain at rest (by 0–10 cm visual analogue scale on post-operative days 1–42), post-operative analgesic use [by cumulative analgesic consumption score (CACS) days 7–42], tolerability and adverse events.
Results:
Patients (N = 34) were recruited from a tertiary orthopaedic hospital in the Czech Republic, of whom 33 completed the study (OEC/placebo: n = 15/18). Baseline characteristics across the groups were comparable. Compared with placebo, the OEC group had numerically lower CRP levels on post-operative days 1–7, including peak level [mean (standard deviation) OEC versus placebo: 81.4 (28.3) versus 106.7 (63.3) mg/L], which translated into a significant 32% lower CRP area under the curve (p = 0.034). The OEC group reported significantly less pain during post-operative days 1–7 versus placebo (analysis of variance treatment × visit [F(4) = 3.989]; p = 0.005). Analgesic use was numerically reduced as assessed through an accumulated CACS. No deleterious effects on haemorheological parameters were observed in either group.
Conclusions:
Pre- and post-operative use of OEC significantly reduced CRP levels and patient self-reported pain. OEC may be an efficacious and safe treatment option to facilitate post-operative recovery following THR.
Trial registration:
EudraCT number 2016-003078-41
Keywords: bromelain, C-reactive protein, hip surgery, inflammation, oral enzyme combination, rutoside, total hip arthroplasty, trypsin
Background
Total hip replacements (THRs) are among the most common types of surgery, and the number of procedures performed per year is increasing. The average number of procedures among the 38 member states of the Organization for Economic Co-operation and Development, which includes countries from Europe, Asia, Oceania and North and South America, was 182 per 100,000 population in 2017, an increase of 30% from 2007. 1 In the United States alone, nearly half a million THR procedures are being performed per year, and are predicted to rise to 1.4 million by 2040. 2
The increasing rates of THR are thought to be due to a combination of an ageing population, leading to an increasing incidence of osteoarthritis (OA), and a rise in the prevalence of obesity. 2 Obesity, which independently increases the risk of hip OA across all ages, 3 has tripled in global prevalence over the last four decades. 4 Beyond altered demographics, changes in clinical practice have also been implied as a reason for the increasing numbers of THR, driven by changes in policy and indications for surgery, and in improvements in prosthesis longevity and outcomes following surgery. 5
Early mobilization following elective hip surgery is strongly recommended,6,7 and recognized as an important element of ‘enhanced recovery after surgery’ protocols. 8 Patients receiving inpatient rehabilitation and physiotherapy within 24 h after surgery 7 typically have fewer post-operative complications and shorter length of hospital stay than patients receiving delayed physiotherapy.9,10 Reducing the length of stay remains an important target for procedure-level cost containment, especially in lieu of the rising healthcare costs associated with joint replacement surgeries. 11 Pain and stiffness are considered factors limiting early mobilization, 12 and are the main reasons why patients are not discharged early.12,13
A prerequisite for early mobilization is adequate pain management.6,14,15 Multimodal analgesia with paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) aims to reduce the use of opioids, which can cause drowsiness, nausea and vomiting, urinary retention and potentially, addiction. 6 NSAIDs, however, are also associated with contraindications and adverse events (AEs), especially in the elderly population, and have been linked to an increased risk of gastrointestinal damage, cardiovascular disease, renal insufficiency and to a lesser extent, hepatotoxicity. 16 Therefore, judicious use and appropriate patient selection are required in the post-operative period. 6
A potential worthwhile option for alleviating post-operative pain and reducing NSAID consumption is the use of oral enzyme combinations (OECs). The effect of OECs constituting proteases, such as bromelain (a proteolytic enzyme) and other ingredients like rutoside (a glycoside combining the flavonol quercetin and the disaccharide rutinose) on reducing pain, oedema and inflammation, has been demonstrated in animal models,17,18 in experimentally induced skin biopsies 19 or haematomas, 20 after septoplasty,21,22 orthognathic surgery 23 and dental surgery.24–26 However, there is limited evidence of the effect of systemic enzyme therapy in patients undergoing orthopaedic surgery.
The potential benefits of OEC with bromelain, trypsin (a serine protease) and rutoside have been suggested by previous open-label studies,27–30 but no randomized, placebo-controlled, double-blind trials have provided high-quality evidence for the effect of this treatment in the context of THR. Also, most studies have examined the effects of OEC on short-term swelling and pain, but not on serological markers of inflammation. Whether pre- and post-operative intervention with OEC could alleviate some of the hurdles for effective recovery in the context of elective THR is therefore unknown. The objective of this study was to investigate the impact of OEC therapy on the post-operative systemic inflammatory response, pain and patient rehabilitation.
Methods
Design
This was a randomized, double-blind, placebo-controlled, stratified, parallel-group exploratory study to explore the effects of pre- and post-operative OEC versus placebo on early and later outcomes with relevance for patient recovery: changes in systemic inflammation [C-reactive protein (CRP) levels], self-reported hip pain at rest, analgesic use, oedema (assessed by thigh and calf circumference), cumulative Redon drain discharge volume, temperature, Harris Hip Score (HHS),31,32 the patient-rated Patient Global Impression of Change (PGIC), 33 and the clinician-rated Clinical Global Impression – Improvement scale (CGI-I).34,35 AEs, as well as specific haemorheological parameters, were also recorded.
The study was approved by the Ethics Committee of Hospital Jihlava, Jihlava, Czech Republic (ref. 778). It was conducted in full compliance with the International Council for Harmonization Good Clinical Practice Guidelines, the principles of the Declaration of Helsinki and the laws and regulations of the Czech Republic, and was registered in the European Clinical Trial Database (EudraCT 2016-003078-41).
Patients
The key inclusion criteria of the patients, who were candidates for primary cementless THR via an anterolateral approach with spinal anaesthesia (subarachnoid block) owing to a primary diagnosis of non-inflammatory degenerative joint disease, were: age 50 years or older, body mass index (BMI) >25 to <35 kg/m2 and CRP ⩽ 6 mg/L. Key exclusion criteria were: active smoking, insulin-dependent diabetes mellitus, certain systemic or metabolic bone disorders (e.g. rheumatoid arthritis, lupus erythematosus, Paget’s disease), and patients receiving steroids (see Supplemental File 1 for full inclusion and exclusion criteria). The study was conducted at the Department of Orthopaedics and Traumatology of the Regional Hospital Jihlava, Czech Republic, which is responsible for the healthcare of 500,000 inhabitants. In 2019, the department was staffed by 18 doctors, and performed 874 trauma and 1227 orthopaedic surgeries.
Randomization and masking
A computer-generated block randomization sequence stratified by sex with a 1:1 allocation, using fixed block size of four, was prepared independently and kept confidential and not disclosed to the study staff, the clinical research organization, or the sponsor’s clinical staff. Randomization occurred at screening (5 days before scheduled surgery). The active (OEC) and placebo tablets were identical in appearance and delivered in identical boxes with only the randomization code printed on the package label. Patients, treating physicians, assessors and study staff were all blinded to the allocation.
The principal investigator received a set of sealed envelopes, marked with each participant’s assigned number, for medication identity disclosure in case of emergency. The integrity of the envelopes was verified at each monitoring visit.
Intervention and placebo
The total duration of the study was a maximum of 8 weeks, including screening. Following randomization, a pre-operation period of 4 days was planned, followed by the day of operation, and then 42 days of follow-up. Active intervention was the OEC Phlogenzym® in tablet form, 36 containing 48 mg trypsin (corresponds to 24 microkatal), 90 mg bromelain [corresponds to 450 International Pharmaceutical Federation (FIP) units], and 100 mg rutoside trihydrate per tablet. The placebo tablets contained the same excipients as the OEC without the active ingredients, which were substituted with microcrystalline cellulose.
Dosing of the OEC or placebo was scheduled according to the following regimen: three tablets twice daily (b.i.d) during the pre-operative days −4 to −2; three tablets in the morning on day −1 pre-operative; zero tablets on the day of THR surgery (day 0); six tablets b.i.d during the first post-operative week (days 1–7); and five tablets b.i.d until the end of the study (days 8–42). Tablets had to be swallowed with at least 250 mL of water on an empty stomach (earliest 2 h after the last meal and at least 30 min before the next meal).
Standard care
Each THR was performed with standard cementless cup and stem via the anterolateral approach according to Watson and Jones under spinal anaesthesia (subarachnoid block). 37 This anterolateral approach is relatively gentle on soft tissues but requires a partial incision of the glutaeus medius muscle. 37 All patients received standard pre-, peri- and post-operative care according to the local protocols and guidelines. Peri-operative analgesia administered to all participants was predefined to either metamizole [intravenous (i.v.)] or piritramide (i.v. or subcutaneous). Post-operative use of analgesics was limited to metamizole (oral), diclofenac (intramuscular), diclofenac and orphenadrine (i.v.) and paracetamol (i.v.). Each patient received the factor Xa inhibitor rivaroxaban 38 for prevention of thromboembolism, and antibiotics (i.v.). The minimum hospital stay was 7 days.
Outcomes
There were differing outcomes of interest for the early post-operative phase (days 1–7) and for the rehabilitation period (days 7–42). For days 1–7: serum CRP (analyzed at the local laboratory of the hospital), axillary temperature and cumulative Redon drain discharge volume; for days 1–42: daily mean local pain at rest [self-rated on a 0–10 cm visual analogue scale (VAS) in the morning (before mobilizing), and at night (before sleeping)], oedema (thigh and calf circumference); for days 7–42: analgesic consumption [overall and accumulated use assessed by the validated cumulative analgesic consumption score 39 (CACS)] and HHS;(Harris 1969) 32 the patient-rated PGIC 33 and the clinician-rated CGI-I.34,35 For a detailed description of the assessment tools, see Supplemental File 2.
Safety endpoints
Vital signs, physical examination data and AEs were documented as safety variables. The AEs were assessed for seriousness, severity/intensity, relation to the study drug and outcome. Since one of the OEC components, bromelain, may influence blood coagulation 40 and the study participants were treated prophylactically with rivaroxaban, four coagulation parameters were measured post-operatively on days 1–7 at the local hospital laboratory using standard assays: anti-Xa, Quick prothrombin time (PT) test, activated partial thromboplastin time (APTT) and fibrinogen. The blood sample for the estimation of anti-Xa was taken 4 h after rivaroxaban administration, at the expected maximum concentration of rivaroxaban. Serious adverse events (SAEs) were reported according to the State Institute for Drug Control guideline KLH-21 Version 7. 41 AEs were coded according to Medical Dictionary for Regulatory Activities (MedDRA®) version 19.0 terms.
Statistical analysis
This was an exploratory study and a sample of 40 patients was considered to provide a reassuring sample size to explore the outcomes of interest. The per-protocol and intention-to-treat analyses were both explored with descriptive statistics for continuous data: mean, standard deviation (SD), standard error and 95% confidence interval. For categorical data, absolute counts (N) and percentages (%) were reported. Quantitative data with expected monotonous change were analyzed with the mixed-effects analysis of variance (ANOVA) model with repeated measures. Ordinal data (e.g. frequency of analgesic use) and the CACS were analyzed using the Mann–Whitney U test.
For all analyses, group comparisons were performed via appropriate contrasts at the 5% significance level (two-sided). Missing data were not reconstructed, and statistical analysis was performed only on available data (available-case analysis) using STATISTICA (version 10, StatSoft, Tulsa, OK, USA).
Results
Patients
Recruitment took place from March 2019 to July 2020 but was prematurely terminated owing to the ongoing COVID-19 pandemic, reducing the rate of the planned THRs. A total of 33 patients (19 women) of 34 randomized patients completed the study (n = 15 OEC; n = 18 placebo; CONSORT diagram in Supplemental File 3). Mean (SD) age and BMI in the OEC group was 69.3 (6.4) years and 28.3 (3.4) kg/m2, respectively, which was comparable with the placebo group [67.8 (8.5) years and 29.9 (3.7) kg/m2, respectively]. Other characteristics between groups were also generally balanced (Table 1). The mean (SD) length of hospital stay in the OEC group was 10.6 (1.7) days and 10.3 (1.9) days in the placebo group. All patients had cementless THRs with standard cups and stems.
Table 1.
Baseline demographics and characteristics of participants by treatment group.
| Characteristic | OEC (n = 15) | Placebo (n = 18) |
|---|---|---|
| Age, years, mean (SD) | 69.3 (6.4) | 67.8 (8.5) |
| Sex, n (%) | ||
| Men | 6 (40.0) | 8 (44.4) |
| Women | 9 (60.0) | 10 (55.6) |
| Body mass index, kg/m2, mean (SD) | 28.3 (3.4) | 29.9 (3.7) |
| Blood pressure, mmHg, mean (SD) | ||
| Systolic | 144.7 (24.3) | 145.7 (18.1) |
| Diastolic | 84.7 (11.8) | 86.2 (11.7) |
| Pre-operative CRP, mg/L, mean (SD) | 2.3 (1.5) | 2.4 (1.7) |
| Pre-operative pain score, cm, mean (SD) | 4.2 (1.3) | 4.7 (2.7) |
| Pre-operative Harris Hip Score, mean (SD) | 60.1 (12.7) | 62.2 (15.5) |
| Pre-operative thigh circumference, cm, mean (SD) | 45.9 (4.9) | 48.5 (5.7) |
| Pre-operative calf circumference, cm, mean (SD) | 36.2 (3.8) | 38.4 (4.1) |
CRP, C-reactive protein; OEC, oral enzyme combination; SD, standard deviation.
CRP – post-operative days 1–7
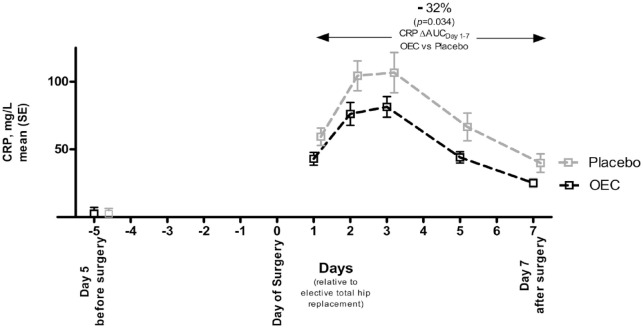
The mean CRP (SD) values on the fifth pre-operative day were similar between the two groups [OEC: 2.3 (1.5) mg/L; placebo: 2.5 (1.7)]. Following THR, CRP increased (Figure 1) with minimum and maximum levels observed in the OEC and the placebo groups of 9.2–152.3 and 11.9–245.9 mg/L, respectively. The mean levels were 81.4 (28.3) mg/L in the OEC group and 106.7 (63.3) mg/L in the placebo group (p = 0.102). The levels of CRP in the OEC group were consistently lower than in the placebo group, with a difference of >20% at all assessments (−27.4%, −27.0%, −23.7%, −33.8% and −36.8% on days 1, 2, 3, 5 and 7, respectively). This translated into a significant −32% lower area under the curve for CRP (CRPAUC) where OEC CRPAUC Days 1–7 was 222.0 (84.6) mg/L × days and placebo CRPAUC Days 1–7 was 327.3 [165.9] mg/L × days (p = 0.034).
Figure 1.
CRP trajectory following total hip replacement surgery from days 0 to 7 according to treatment groups. Values are shown as mean (SE).
AUC, area under the curve; CRP, C-reactive protein; OEC, oral enzyme combination; SE, standard error.
Pain at rest – post-operative days 1–42
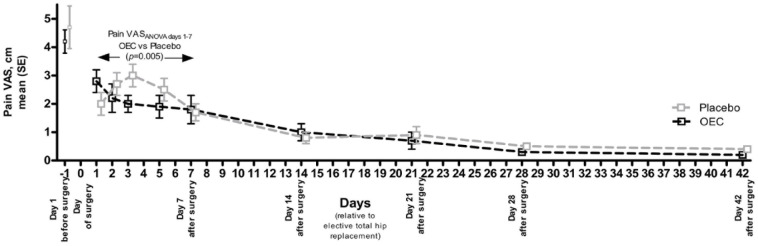
A greater degree of hip pain was reported by both groups on the first pre-operative day [OEC: 4.2 (1.3) cm, placebo: 4.7 (2.7) cm] relative to that reported after the procedure (Figure 2). Following THR, the evolution of the pain pattern in the placebo group resembled that of the CRP pattern with an initial increase followed by a gradual decline from day 3 onwards. In contrast, in the OEC group, a reduction was observed from post-operative day 1 onwards. This difference translated into a statistically significant difference between the treatment groups (ANOVA treatment × visit interaction, [F(4) = 3.989]; p = 0.005). When assessing the pain trajectory from day 7 onwards to day 42 during the rehabilitation period, there was a numerical difference favouring the OEC, but no significant difference (ANOVA treatment × visit interaction, [F(4) = 0.159]; p = 0.958). At the end of the study, patients reported little pain in both groups (Figure 2).
Figure 2.
Pain trajectory following total hip replacement surgery from first pre-operative day to post-operative day 42. Pain was assessed using a VAS. ANOVA with factors treatment × visit interaction. Values are shown as mean (SE).
ANOVA, analysis of variance; OEC, oral enzyme combination; SE, standard error; VAS, visual analogue scale.
Analgesic use – post-operative days 7–42
The use of patient-requested analgesics was highest in both groups between post-operative days 7 and 14, where the mean (SD) number of doses was 3.0 (4.0) in the OEC group and 6.2 (8.5) in the placebo group (p = 0.300). Analgesic use then gradually declined up to the end of the study, with no significant difference between groups (see Supplemental File 4), but was numerically lower in the OEC group during all, but one, visit. When considering the potency of the medications taken, using the CACS, we observed a numerical, but not statistically significant, lower cumulative CACS from the start of the rehabilitation period in the OEC group relative to those receiving placebo at post-operative days 14, 21, 28 and 42 (Figure 3).
Figure 3.
Cumulative use of patient-driven analgesics in the rehabilitation period from post-operative days 7 onwards to 42. Analgesic use was assessed by stepwise cumulative count of the CACS units administered.
Values are shown as mean (SE).
CACS, cumulative analgesic consumption score; OEC, oral enzyme combination; SE, standard error.
Oedema: Thigh and calf circumference – post-operative days 1–42
In both groups, thigh circumference increased as expected following the THR procedure, but then decreased over time (Table 2). Calf circumference, however, remained relatively stable over the full study period. No notable differences between the groups were observed for thigh or calf circumference.
Table 2.
Recovery outcomes during early (post-operative days 1–7) and rehabilitation (post-operative days 7–42) phases.
| Outcome | Day 1 (n = 33) | Day 2 (n = 33) | Day 3 (n = 32) | Day 5 (n = 31) | Day 7 (n = 30) | Day 14 (n = 29) | Day 21 (n = 28) | Day 28 (n = 28) | Day 42 (n = 28) |
|---|---|---|---|---|---|---|---|---|---|
| Redon drain, mL (SD) | |||||||||
| OEC | 398 (231) | 178 (144) | NA | NA | NA | NA | NA | NA | NA |
| Placebo | 428 (214) | 181 (151) | NA | NA | NA | NA | NA | NA | NA |
| Thigh circumference, cm (SD) | |||||||||
| OEC | 44.3 (7) | 47.0 (5) | 47.4 (4.9) | 48.0 (4.4) | 48.2 (5.5) | 45.7 (4.5) | 44.4 (4.7) | 44.8 (5.8) | 45.3 (5.9) |
| Placebo | 48.9 (7) | 49.6 (5.8) | 50.1 (5.6) | 50.7 (6) | 50.9 (6.1) | 49.2 (5.8) | 48.2 (6.1) | 47.5 (8.3) | 48.9 (6.2) |
| Calf circumference, cm (SD) | |||||||||
| OEC | 35.8 (3.1) | 36.0 (3.7) | 36.1 (3.6) | 36.7 (4.2) | 36.9 (4.7) | 35.7 (3.8) | 35.3 (3.3) | 35.7 (3.5) | 35.4 (3.2) |
| Placebo | 37.6 (4.0) | 37.6 (3.9) | 37.7 (3.8) | 38.8 (4.0) | 38.7 (4.1) | 38.9 (4.7) | 37.8 (3.8) | 38.0 (4.1) | 38.2 (4.4) |
| Temperature, °C (SD) | |||||||||
| OEC | 36.7 (0.3) | 36.6 (0.1) | 36.5 (0.2) | 36.5 (0.1) | 36.4 (0.1) | NA | NA | NA | NA |
| Placebo | 36.6 (0.2) | 36.7 (0.2) | 36.7 (0.3) | 36.6 (0.2) | 36.5 (0.2) | NA | NA | NA | NA |
| HHS, score (SD) | |||||||||
| OEC | NA | NA | NA | NA | 56.0 (8.7) | 66.5 (14.4) | 78.1 (11.4) | 80.4 (9.5) | 84.6 (5.4) |
| Placebo | NA | NA | NA | NA | 54.3 (7.7) | 63.0 (10.5) | 73.7 (9.9) | 78.4 (7.3) | 84.1 (2.7) |
| PGIC score (SD) | |||||||||
| OEC | NA | NA | NA | NA | 1.8 (0.6) | 1.6 (0.9) | 1.4 (0.7) | 1.3 (0.5) | 1.2 (0.4) |
| Placebo | NA | NA | NA | NA | 1.7 (0.8) | 1.6 (0.7) | 1.6 (1.0) | 1.3 (0.5) | 1.3 (0.6) |
| CGI-I score (SD) | |||||||||
| OEC | NA | NA | NA | NA | 1.4 (0.5) | 1.7 (1.0) | 1.3 (0.5) | 1.3 (0.5) | 1.2 (0.4) |
| Placebo | NA | NA | NA | NA | 1.6 (0.7) | 1.3 (0.5) | 1.3 (0.6) | 1.2 (0.4) | 1.1 (0.3) |
Values are shown as mean (SD).
CGI-I, Clinical Global Impression – Improvement scale; HHS, Harris Hip Score; NA, not assessed; OEC, oral enzyme combination; PGIC, Patient Global Impression of Change; SD, standard deviation.
HHS – post-operative days 7–42
The HHS significantly improved in both treatment groups during the rehabilitation period (Table 2), with numerical, albeit not statistically significant, differences between the treatment groups favouring the OEC.
PGIC and CGI-I–post-operative days 7–42
A significant number of patients reported that their condition was ‘much improved’ or ‘very much improved’ as assessed by PGIC on day 7 in both groups (OEC: 92%, 11/12; placebo: 83%, 15/18), and on day 42 (OEC: 100%, 10/10; placebo: 94%, 17/18). There was no significant effect of treatment between the groups on the PGIC score as rated by patients (Table 2). Correspondingly, a substantial improvement in patients’ condition reflected by the CGI-I as assessed by clinicians was noted (rating of condition as ‘much improved’ or ‘very much improved’ on day 7 was 100% (12/12) in the OEC group and 89% (16/18) in the placebo group, and 100% on day 42 in both groups). No significant difference in treatment effect across the groups was observed for the CGI-I.
Other outcomes – post-operative days 1–7
The drain volume markedly reduced from post-operative day 1 to day 2 in both groups (Table 2), without any significant between-group difference. Also, no significant difference between the groups in mean axillary temperature was noted (Table 2).
Safety evaluation
A total of 15 AE episodes were reported from n = 10 (66%) patients in the OEC group, and eight AE episodes were reported from n = 7 (39%) patients in the placebo group. The most frequent AE was irritation of the upper gastrointestinal tract (nausea and/or vomiting), with equal frequency (n = 4 patients in each group) (Table 3). Most AEs occurred early (at day 1 following surgery, four out of 15 patients in the OEC group and four out of 18 patients in the placebo group). At the remaining visits, the absolute number of AEs was relatively low (between 0 and 3). One SAE of a urinary tract infection was reported, which led to hospitalization of one woman in the OEC group. The patient fully recovered, and the SAE was not deemed related to the study product by the investigator; hence, unblinding was not performed. The SAE was considered related to limited access to outpatient care during the Christmas holidays, and the patient was withdrawn from the study. There were two additional premature withdrawals from the study related to AE, both in the OEC group; in one, study treatment was discontinued owing to diarrhoea (deemed possibly related to treatment), and study treatment was discontinued owing to urticaria in the other (deemed unrelated to treatment). No AEs associated with laboratory abnormalities were detected throughout the whole study.
Table 3.
Number of patients with AEs during the full study based on treatment groups.
| Adverse event | OEC (n = 15) | Placebo (n = 18) |
|---|---|---|
| Any AE | 10 | 7 |
| Nausea and/or vomiting | 4 | 4 |
| Prolonged secretion from the wound | 3 | 1 |
| Diarrhoea | 2 | 0 |
| Pneumonia | 1 | 0 |
| Urticaria | 1 | 0 |
| Palpitation | 1 | 0 |
| Greater than expected blood loss during surgery a | 1 | 0 |
| Urinary tract infection | 1 | 0 |
| Loss of appetite | 1 | 0 |
| Back pain | 0 | 1 |
| Gingivitis | 0 | 1 |
| Bradycardia | 0 | 1 |
Subjective assessment.
AE, adverse event; OEC, oral enzyme combination.
Coagulation
Anti-Xa values during post-operative days 1–7 did not significantly differ between treatment groups, as was the case for the PT values and the APTT values (Supplemental Figure S2a–c, Supplemental File 5). Fibrinogen values increased from screening, which was expected as this is also an acute-phase protein, and over the immediate post-operative phase; however, no significant difference between treatment groups was observed (Supplemental Figure S2d, Supplemental File 5).
Discussion
The purpose of this study was to evaluate the effect and safety of pre- and post-operative OEC therapy on several patient-relevant outcomes following elective THR surgery, including effects on systemic inflammation (measured by CRP), pain (measured by VAS and use of analgesia) and oedema (measured by changes in thigh and calf thickness). Despite COVID-related recruitment challenges and a limited sample size, this study observed several interesting differences between the treatment groups, such as reduced levels of CRP and less patient self-reported pain during the early phase (days 1–7), favouring the OEC group.
CRP typically reaches a peak on the second or third post-operative day following hip or knee arthroplasties,42–44 and reflects the extent of surgical trauma, as well as type of tissue injured. 43 In a study of THR after femoral neck fracture, CRP levels notably increased in the first post-operative week after surgery, and gradually normalized in the following weeks. 45 After uncomplicated THR, the CRP levels in one study involving 30 patients was reported to reach a mean value of 204.88 mg/L at day 2.42 In our study, the CRP levels also exhibited an early post-operative peak (at day 3), but at a slightly lower level (mean 81.4 mg/L in the OEC group and mean 106.7 mg/L in the placebo group) than in the mentioned study. Nevertheless, in the OEC group, CRP levels were reduced by 32% relative to the placebo group over the 7-day observation period, indicating a reduction in trauma-induced inflammation with the OEC. This reduction of the post-operative inflammation may translate into improved clinical outcomes and faster recovery, as observed in a study where dexamethasone, administered to reduce inflammation following primary THR, led to improved range of movement. 46 It could be speculated that the beneficial effect of OEC on inflammation reduction may also be important in other situations where CRP levels follow a similar trajectory.
Another important aspect of THR post-operative management is pain. It is reported that adequate analgesia can lead to earlier mobilization and shorter length of hospital stay,12,13 although this does not always correlate with improved functional performance, for example, knee-extension strength. 47 Furthermore, indiscriminate analgesic use should be avoided because it is associated with side effects such as constipation, nausea, confusion and indigestion, particularly in the elderly. 48 Similar to our observations in THRs, OECs are associated with pain reduction in orthopaedic surgeries outside of THR. In a randomized, open-label trial in 60 patients that underwent internal fixation of long bone fractures, OEC treatment resulted in less pain, reduced use of analgesics and less swelling, compared with the anti-oedematous substance aescin. 27 The specific OEC used in the current study has also been used successfully in the rehabilitation of children with long tubular bone fractures as a part of a rehabilitation programme, 28 after operation for disc prolapse 29 and in patients with ankle sprain, treated conservatively. 30
Moreover, there is an increasing amount of literature suggesting that OECs can serve a role in post-operative rehabilitation or recovery outside of the orthopaedic context. A combination of bromelain, rutoside and other ingredients appeared to accelerate wound healing in healthy volunteers who had small skin biopsies, 19 and reduce pain after induced haematomas. 20 Severity of pain, swelling, nasal obstruction and nasal discharge after septoplasties have also been reported to be reduced following OEC treatment versus placebo;21,22 a 2020 systematic review supported the use of oral bromelain in decreasing pain, trismus and to a lesser degree, swelling after molar extraction, despite heterogeneous study designs and dosing regimens. 26
The effects observed in our study, in the context of other literature, indicate that OEC treatments have the potential to improve rehabilitation and patient outcomes. The absence of statistically significant differences between groups in analgesic use, HHS, CGI-I or PGIC scores may be related to the small sample size. There was, however, a numerical tendency in favour of the OEC for analgesic use, the HHS and the PGIC, and this should be further explored in an adequately powered study.
This study involved patients that underwent surgery, and thus reflects a population with an elevated risk for AEs. There were, however, no AEs considered to be definitely related to the study procedures, and no unexpected events were encountered in association with the OEC treatment. Results from the anti-Xa, Quick PT test, APTT and fibrinogen analyses did not notably differ between the treatment groups, suggesting that interactions between OEC and rivaroxaban are unlikely. The lack of an effect on coagulation parameters observed in this study is important, given that bromelain has been reported to affect coagulation parameters in ex vivo and in vivo animal models.40,49 The present observation is consistent with other clinical studies using OECs, even when used in combination with low-molecular-weight heparin. 50 Overall, the OEC was well tolerated and showed a safety and tolerability profile similar to that of placebo.
Limitations
The relatively small sample size, which was unintentionally restricted owing to the COVID-19 pandemic, represents a limitation for generalizability, in addition to a slightly skewed number of participants in the OEC and the placebo groups owing to an asymmetrical dropout rate. However, a strength is the randomized and blinded nature of the study, with an 8-week follow-up period. Furthermore, only standard cementless cups and stems were used in this study. Although other types of stems, such as short or ultra-short stems, may influence individual pain and other post-operative outcomes differently compared with standard stems, 51 we would expect a similar treatment effect of the OEC. Additional limitations include the single-centre design and most of the study population being Caucasian. However, given that previous studies do not suggest a difference in response across baseline characteristics, we believe that the results of this study also represent what could reasonably be expected outside of the study population. Lastly, there were no adjustments for the multiple statistical tests performed, which are therefore all considered to be of exploratory nature.
Conclusions
To the best of our knowledge, this exploratory study is the first double-blind, randomized, placebo-controlled trial to investigate the effects of pre- and post-operative OEC therapy constituting trypsin, bromelain and the flavonoid rutoside in a controlled post-operative setting following THR. Pre- and post-operative use of OEC was associated with significantly reduced CRP levels and patient self-reported pain during the first post-operative week. Reduced inflammation is related to better outcomes after hip surgery, and pain is a major limitation factor for potential earlier mobilization. Thus, the OEC may be an efficacious and safe treatment option to facilitate post-operative recovery; however, further investigations are warranted.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a ranmizedod exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-6-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors acknowledge Constantinos Bezos, MCh, of 3 Stories High (3SH), UK, for medical writing support, which was funded by Nestlé Health Science, in accordance with Good Publication Practice guidelines (Ann Intern Med 2022;175:1298-1304). The authors have authorized the submission of the manuscript by 3SH and approved all statements and declarations. We also thank Václav Filip at pharmnet.cz for statistical analysis and statistical advice, funded by Nestlé Health Science. We acknowledge Maximillian von Eynatten from Nestlé Health Science for critical comments, and Pharmnet, the clinical research organization, for operational work. MedDRA® trademark is registered by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use.
Footnotes
ORCID iDs: Stefanie M. Rau  https://orcid.org/0000-0002-9872-2399
https://orcid.org/0000-0002-9872-2399
Odd Erik Johansen  https://orcid.org/0000-0003-2470-0530
https://orcid.org/0000-0003-2470-0530
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jiří Vosáhlo, Orthopaedic and Traumatology Department, Jihlava Hospital, Jihlava, Czech Republic.
Adam Salus, Orthopaedic and Traumatology Department, Jihlava Hospital, Jihlava, Czech Republic.
Michael Smolko, Orthopaedic and Traumatology Department, Jihlava Hospital, Jihlava, Czech Republic.
Barbora Němcová, Rehabilitation Department, Jihlava Hospital, Jihlava, Czech Republic.
Veit Nordmeyer, Clinical Department for Trauma Surgery, University Hospital Tulln, Tulln, Austria.
Milos Mikles, Clinical Department for Trauma Surgery, University Hospital Tulln, Tulln, Austria.
Stefanie M. Rau, Nestlé Health Science, Vevey, Switzerland
Odd Erik Johansen, Nestlé Health Science, Avenue Nestle 55, Vevey, Vaud 1800, Switzerland.
Declarations
Ethics approval and consent to participate: All patients signed an informed consent form prior to participation in the study. The study was approved by the Ethics Committee of Hospital Jihlava, Jihlava, Czech Republic (ref. 778). It was conducted in full compliance with the International Council for Harmonization Good Clinical Practice Guidelines, the principles of the Declaration of Helsinki, and the laws and regulations of the Czech Republic.
Consent for publication: The signed consent form included a clause for agreement to publication.
Author contributions: Jiří Vosáhlo: Conceptualization; Investigation; Methodology; Project administration; Resources; Validation; Writing – review & editing.
Adam Salus: Investigation; Methodology; Project administration; Resources; Validation; Writing – review & editing.
Michael Smolko: Investigation; Methodology; Project administration; Resources; Validation; Writing – review & editing.
Barbora Němcová: Investigation; Methodology; Project administration; Resources; Validation; Writing – review & editing.
Veit Nordmeyer: Investigation; Validation; Writing – review & editing.
Milos Mikles: Investigation; Validation; Writing – review & editing.
Stefanie M. Rau: Conceptualization; Funding acquisition; Project administration; Resources; Validation; Writing – review & editing.
Odd Erik Johansen: Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Nestlé Health Science (NHSc), the manufacturer of the oral enzyme combination Phlogenzym®, which was used in this study. NHSc was involved in study design, data interpretation and writing of the manuscript. Data collection was performed by the Department of Orthopaedics and Traumatology of the Regional Hospital Jihlava, Czech Republic, which received study funding from NHSc.
Competing interests: JV: received funding from Nestlé Health Science to complete this trial as the Primary Investigator. AS: received funding from Nestlé Health Science to complete this trial. MS: received funding from Nestlé Health Science to complete this trial. BN: received funding from Nestlé Health Science to complete this trial. VN: has received consultancy fees from Nestlé Health Science. MM: has received consultancy fees from Nestlé Health Science. SMR: is employed by Nestlé Health Science. OEJ: is employed by Nestlé Health Science.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. OECD. Chapter 9: Healthcare activities: Hip and knee surgery. Health at a Glance 2019: OECD indicators. Paris: OECD Publishing, 2019, pp.198–199. [Google Scholar]
- 2. Singh JA, Yu S, Chen L, et al. Rates of total joint replacement in the United States: Future projections to 2020–2040 using the National Inpatient Sample. J Rheumatol 2019; 46: 1134–1140. [DOI] [PubMed] [Google Scholar]
- 3. Reyes C, Leyland KM, Peat G, et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol 2016; 68: 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Lancet Gastroenterology & Hepatology. Obesity: another ongoing pandemic. Lancet Gastroenterol Hepatol 2021; 6: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackerman IN, Bohensky MA, de Steiger R, et al. Lifetime risk of primary total hip replacement surgery for osteoarthritis from 2003 to 2013: a multinational analysis using national registry data. Arthritis Care Res 2017; 69: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 6. Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: enhanced recovery after surgery (ERAS(®)) Society recommendations. Acta Orthop 2020; 91: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institute for Health and Care Excellence. Joint replacement (primary): hip, knee and shoulder NICE guideline [NG157], https://www.nice.org.uk/guidance/ng157 (2020, accessed 20 December 2021). [PubMed]
- 8. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth 2016; 117: iii62–iii72. [DOI] [PubMed] [Google Scholar]
- 9. Chua MJ, Hart AJ, Mittal R, et al. Early mobilisation after total hip or knee arthroplasty: A multicentre prospective observational study. PLoS One 2017; 12: e0179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guerra ML, Singh PJ, Taylor NF. Early mobilization of patients who have had a hip or knee joint replacement reduces length of stay in hospital: a systematic review. Clin Rehabil 2015; 29: 844–854. [DOI] [PubMed] [Google Scholar]
- 11. Molloy IB, Martin BI, Moschetti WE, et al. Effects of the length of stay on the cost of total knee and total hip arthroplasty from 2002 to 2013. J Bone Joint Surg 2017; 99: 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gautreau S, Haley R, Gould ON, et al. Predictors of farther mobilization on day of surgery and shorter length of stay after total joint arthroplasty. Can J Surg 2020; 63: e509–e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Husted H, Lunn TH, Troelsen A, et al. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop 2011; 82: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu S, Qian W, Jiang C, et al. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta-analysis. Postgrad Med J 2017; 93: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frassanito L, Vergari A, Nestorini R, et al. Enhanced recovery after surgery (ERAS) in hip and knee replacement surgery: description of a multidisciplinary program to improve management of the patients undergoing major orthopedic surgery. Musculoskelet Surg 2020; 104: 87–92. [DOI] [PubMed] [Google Scholar]
- 16. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 2020; 180: 114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lotz-Winter H. On the pharmacology of bromelain: an update with special regard to animal studies on dose-dependent effects. Planta Med 1990; 56: 249–253. [DOI] [PubMed] [Google Scholar]
- 18. Uhlig G, Seifert J. The effect of proteolytic enzymes (traumanase) on posttraumatic edema. Fortschr Med 1981; 99: 554–556. [PubMed] [Google Scholar]
- 19. Brown SA, Coimbra M, Coberly DM, et al. Oral nutritional supplementation accelerates skin wound healing: a randomized, placebo-controlled, double-arm, crossover study. Plast Reconstr Surg 2004; 114: 237–244. [DOI] [PubMed] [Google Scholar]
- 20. Kleine M. Evidence of the efficacy of an enzyme combination preparation using the method of artificial hematomas in combination with a pressure meter: A placebo controlled, randomised, prospective, double blind study. J Clin Res 1998; 1: 87–102. [Google Scholar]
- 21. Nanda MS, Kaur M. Role of oral enzymes in post operative septoplasty cases. Indian J Otolaryngol Head Neck Surg 2019; 71: 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lukáš J, Hroboň M, Kaňa R. Phlogenzym, systémová enzymoterapie u septoplastik. Choroby hlavy a krku 2000; 1: 7–9. [Google Scholar]
- 23. Shetty V, Mohan A. A prospective, randomized, double-blind, placebo-controlled clinical trial comparing the efficacy of systemic enzyme therapy for edema control in orthognathic surgery using ultrasound scan to measure facial swelling. J Oral Maxillofac Surg 2013; 71: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 24. Vinzenz K. Edema therapy in dental interventions with hydrolytic enzymes. Quintessenz 1991; 42: 1053–1064. [PubMed] [Google Scholar]
- 25. Tharani Kumar S, Ashok Prasanna R, Kirubanandan J, et al. Postoperative healing after surgical removal of mandibular third molar: a comparative study between two proteolytic enzymes. J Pharm Bioallied Sci 2020; 12: S289–S294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knackstedt R, Gatherwright J. Perioperative homeopathic arnica and bromelain: current results and future directions. Ann Plast Surg 2020; 84: e10–e15. [DOI] [PubMed] [Google Scholar]
- 27. Kameníček V, Holáň P, Franěk P. Systemic enzyme therapy in the treatment and prophylaxis of posttraumatic swelling. Acta Chir Orthop Traumatol Cech 2001; 68: 45–49. [PubMed] [Google Scholar]
- 28. Isaeva AV, Minaev SV, Sternin. Modern approach to the rehabilitation of children with fractured long tubular bones. Vopr Kurortol Fizioter Lech Fiz Kult 2009; 3: 29–31. [PubMed] [Google Scholar]
- 29. Pekař L, Steindler J. Systémová enzymoterapie po operacích výhřezu bederní meziobratlové ploténky. Klin Farmakol Farm 2009; 23: 166–170. [Google Scholar]
- 30. Kerkhoffs GMMJ, Struijs PAA, de Wit C, et al. A double blind, randomised, parallel group study on the efficacy and safety of treating acute lateral ankle sprain with oral hydrolytic enzymes. Br J Sports Med 2004; 38: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg 1969; 51: 737–755. [PubMed] [Google Scholar]
- 32. Söderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res 2001; 384: 189–197. [DOI] [PubMed] [Google Scholar]
- 33. Swanenburg J, Gruber C, Brunner F, et al. Patients’ and therapists’ perception of change following physiotherapy in an orthopedic hospital’s outpatient clinic. Physiother Theory Pract 2015; 31: 293–298. [DOI] [PubMed] [Google Scholar]
- 34. Guy W. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health Education and Welfare, 1976, pp.217–222. [Google Scholar]
- 35. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007; 4: 28–37. [PMC free article] [PubMed] [Google Scholar]
- 36. MUCOS Pharma GmbH. Phlogenzym, https://prehledy.sukl.cz/prehledy/v1/dokumenty/16280 (2022, accessed 10 July 2023).
- 37. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplasty 2008; 23: 64–68. [DOI] [PubMed] [Google Scholar]
- 38. Bayer AG. Xarelto (rivaroxaban) summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf (2021, accessed 9 September 2021).
- 39. Frank AHR, Groene P, von Ehrlich-Treuenstatt V. Evaluation of pain relief sufficiency using the cumulative analgesic consumption score (CACS) and its modification (MACS). Wideochir Inne Tech Maloinwazyjne 2017; 12: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaur H, Corscadden K, Lott C, et al. Bromelain has paradoxical effects on blood coagulability: a study using thromboelastography. Blood Coagul Fibrinolysis 2016; 27: 745–752. [DOI] [PubMed] [Google Scholar]
- 41. State Institute for Drug Control. KLH-21 version 21: Reporting adverse reactions to medicinal products for human use in a clinical trial and to medicinal products without marketing authorisation, https://www.sukl.eu/medicines/klh-21-version-7 (2018, accessed 9 September 2021).
- 42. Krishna A, Garg S, Ghupta S, et al. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) trends following total hip and knee arthroplasties in an Indian population – a prospective study. Malays Orthop J 2021; 15: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen H, Zhang N, Zhang X, et al. C-reactive protein levels after 4 types of arthroplasty. Acta Orthop 2009; 80: 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Azboy I, Çatal B, Başarır K, et al. The natural course of serum D-Dimer, C-reactive protein, and erythrocyte sedimentation rate levels after uneventful primary total joint arthroplasty. J Arthroplasty 2021; 36: 3118–3122. [DOI] [PubMed] [Google Scholar]
- 45. Cho MR, Choi WK, Jun CM, et al. The natural trends of C-reactive protein after hip arthroplasty for femoral neck fracture without infection. Med 2021; 100: e27299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lei Y, Huang Q, Xu B, et al. Multiple low-dose dexamethasone further improves clinical outcomes following total hip arthroplasty. J Arthroplasty 2018; 33: 1426–1431. [DOI] [PubMed] [Google Scholar]
- 47. Holm B, Kristensen MT, Husted H, et al. Thigh and knee circumference, knee-extension strength, and functional performance after fast-track total hip arthroplasty. PMR 2011; 3: 117–124. [DOI] [PubMed] [Google Scholar]
- 48. O’Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother 2012; 10: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Azarkan M, González MM, Esposito RC, et al. Stem bromelain proteolytic machinery: study of the effects of its components on fibrin (ogen) and blood coagulation. Protein Pept Lett 2020; 27: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 50. Johann K, Eschmann K, Meiser P. [No clinical evidence for an enhanced bleeding tendency due to perioperative treatment with bromelain]. Sportverletz Sportschaden 2011; 25: 108–113. [DOI] [PubMed] [Google Scholar]
- 51. Melišík M, Hrubina M, Heřt J, et al. [Mid-term results of Proxima ultra-short anatomical stem: analysis of 130 cases]. Acta Chir Orthop Traumatol Cech 2021; 88: 50–57. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a ranmizedod exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-6-tab-10.1177_1759720X231186875 for Oral enzyme combination with bromelain, trypsin and the flavonoid rutoside reduces systemic inflammation and pain when used pre- and post-operatively in elective total hip replacement: a randomized exploratory placebo-controlled trial by Jiří Vosáhlo, Adam Salus, Michael Smolko, Barbora Němcová, Veit Nordmeyer, Milos Mikles, Stefanie M. Rau and Odd Erik Johansen in Therapeutic Advances in Musculoskeletal Disease