Abstract
BACKGROUND:
Continuous glucose monitoring (CGM) can improve behavioral and clinical outcomes. The use of CGM in real-world practice appears to be increasing. However, actual prevalence and characteristics of using CGM in real-world practice are unknown.
OBJECTIVE:
To investigate the prevalence of CGM use by American adults with diabetes mellitus and differences in demographics and health-related quality of life (HRQOL) between users of CGM and self-monitoring of blood glucose (SMBG).
METHODS:
This serial cross-sectional study using 2014-2020 Behavioral Risk Factor Surveillance System data included nonpregnant adults with self-reported diabetes using CGM or 4-15 times daily SMBG. Outcomes were prevalence of CGM use, demographics, and the 4-item Centers for Disease Control and Prevention HRQOL (CDC HRQOL-4). Unadjusted analysis was performed using univariable regression, and adjusted analysis was performed using nearest neighbor matching to compare CDC HRQOL-4 between SMBG and CGM groups in SAS Studio version 5.2.
RESULTS:
Among 12,053 included respondents, 231 (1.9%) reported using CGM, and prevalence increased from 0.4% in 2014 to 4.1% in 2020. Compared with SMBG users, CGM users were more likely to be younger (50.3 years vs 56.1 years; P < 0.001), employed (59.6% vs 30.6%; P = 0.001), earn at least $75,000/year (48.5% vs 22.0%, P < 0.001), have insurance coverage (99.7% vs 95.4%; P = 0.005), and report fewer comorbidities (history of myocardial infarction, stroke, arthritis, depressive disorder, and kidney disease; all P < 0.05). After nearest neighbor matching, diabetes management-related characteristics were shown to have statistically significant differences between CGM and SMBG users including: age at diabetes diagnosis (30.6 vs 35.6 years; P = 0.005), not seeing a doctor because of cost concern (2.6% vs 7.8%; P = 0.011), checking hemoglobin A1c biannually (91.3% vs 86.6%; P = 0.047), performing daily foot self-examination (58.9% vs 69.6%; P = 0.028), receiving foot examination by a health care professional annually (87.9% vs 93.5%; P = 0.048), and receiving a shingles vaccine in the past (16.5% vs 10.1%; P = 0.024). CDC HRQOL-4 were shown to be similar between the 2 groups across the 4 domains (general health, physical, mental, and combined physical and mental health).
CONCLUSIONS:
An increased trend in CGM use was observed from 2014 to 2020. Economic factors were associated with CGM use over SMBG, and CGM use did not show a difference in HRQOL measured across the 4 domains.
Plain language summary
Continuous glucose monitoring (CGM) is a glucose monitoring device to help improve and manage diabetes. CGM use rose from 0.4% to 4.1% from 2014 to 2020. CGM is still used much less than the traditional finger prick method to monitor blood glucose level, called self-monitoring of blood glucose (SMBG). Compared with people using SMBG, people using CGM were shown to be younger, be employed, have higher income, and be more likely to have health insurance.
Implications for managed care pharmacy
Although prevalence of CGM use increased from 2014 to 2020, it remained far less than that for SMBG. Socioeconomic factors associated with CGM use over SMBG included employment, income, and health insurance status. Financial barriers may exist to CGM use that should be addressed to increase the use of CGM for glycemic monitoring.
Controlling and monitoring glucose levels is imperative in the management of diabetes mellitus. Self-monitoring of blood glucose (SMBG) and hemoglobin A1c (A1c) have historically been the mainstays of monitoring glycemic levels to provide clinical and behavioral feedback to patients to help them achieve better disease management.1 Although SMBG provides a snapshot of blood glucose levels at a single point in time and A1c correlates to an average of glycemia over a 3-month period, nonimplantable continuous glucose monitoring (CGM) provides insight into glucose levels around the clock for a period of 7 to 14 days per device. Since the early 2000s, CGM has shown benefits complementary to and beyond the use of conventional methods of glycemic monitoring.2 Literature has shown that CGM is associated with improved diabetes outcomes including reduced A1c by up to 0.9% more than traditional SMBG.3-5 In addition to clinical outcomes, CGM use has also demonstrated favorable behavioral outcomes, such as reduction in caloric intake and increase in exercise.6
Over the past decade, CGM device accuracy has improved substantially.3,4 The mean absolute relative difference (MARD) is an indicator to describe CGM device accuracy of the measured glucose level compared with the reference measure, in which a lower MARD indicates higher accuracy and a device with a MARD less than 10% is considered to be a well-performing system.7 In 2013, a study by Luijf and colleagues showed the MARDs of 3 CGM devices to be 16.5%, 16.4%, and 20.5%,3 whereas modern-day CGM devices achieve MARD values as low as 8.8%.4 Improved accuracy of CGM devices allows people with diabetes using these devices to better match their decisions regarding their medication therapy and lifestyle measures to their actual level of glycemic control.8 Parallel to the technological development of yielding more accurate CGM devices, many private and public health insurance plans have increased coverage of CGM for people with diabetes. In 2017, the Centers for Medicare and Medicaid Services proposed coverage of CGM for people with type 1 and type 2 diabetes receiving 3 or more daily injections of insulin or using continuous subcutaneous insulin infusion therapy and performing SMBG at least 4 times daily.9 With the increase in accuracy and accessibility of CGM devices, it is hypothesized that the prevalence of CGM use has increased in recent years. However, because insurance coverage still varies greatly and may have strict eligibility requirements for CGM use, it is also hypothesized that health insurance coverage status may play a role in CGM uptake, and demographic factors may differ between CGM and SMBG users.
As noted above, CGM use has led to improvements in behavioral outcomes.6 Positive change in behavior among people with diabetes is key to achieving successful diabetes management. Health-related quality of life (HRQOL) is defined as one’s perceived physical and mental health over time,10 and it has been shown that HRQOL is a powerful predictor of morbidity and mortality in people with diabetes.11 Low HRQOL has been identified as an independent predictor for amputation and death in people with diabetic foot ulcers, and high HRQOL has been associated with providing 10% relative risk reduction in cardiovascular mortality in people with diabetes.12,13 Therefore, understanding the association with CGM and physical and mental health is important, given the direct relationship to health outcomes and survival in people with diabetes. The wealth of information that CGM provides based on the minute-by-minute serial sequence of glucose level changes may serve as an important profiling tool to achieve individualized care for people with varying eating and exercising habits, and it is hypothesized that the mechanism of positive change in health behavior among people with diabetes using CGM may be associated with higher HRQOL compared with SMBG. Currently, no studies have examined the HRQOL of people with diabetes who are using CGM.
In this study, we examined the yearly prevalence rate of CGM use over a 7-year timespan from 2014 to 2020 in the United States among a large sample of adults aged 18 years or older using data from the Behavioral Risk Factor Surveillance System (BRFSS). We compared demographic characteristics of survey participants using CGM compared with participants performing SMBG 4-15 times per day. Finally, we assessed HRQOL among CGM and SMBG users by examining the results of the 4-item Centers for Disease Control and Prevention HRQOL (CDC HRQOL-4) questionnaire included within the BRFSS.14
Methods
This study received exempt status approval by the local institutional review board. This was a serial cross-sectional study using 2014-2020 BRFSS data, which is a publicly available collection of health-related telephone survey results among community-dwelling adult Americans. The design and sampling method used in the BRFSS is reported and available on the Centers for Disease Control and Prevention website.15 The BRFSS includes self-reported demographic, socioeconomic, and dietary data, as well as that for health-related risk behavior, chronic health conditions, and use of preventive services. CGM use began to be collected during the BRFSS interview in 2014; therefore, we have used the BRFSS dataset starting from 2014 to the most recent year available to the investigators at the point of analysis.15
The datafile from each year was downloaded and combined following the weight adjustment calculation published by the BRFSS Data User Guide.15 To calculate the adjusted weight, the number of eligible samples was counted for each year, and the sum of the number of eligible samples for the 2014-2020 dataset was calculated. To complete the data combining for 2014 to 2020 datasets, each year’s sample count was then divided by the total sample count and multiplied by the weight of each year to create a new value for adjusted weight.
The samples were eligible to be included in the study based on answering “yes” to “(Ever told) (you had) diabetes?” Samples reporting gestational diabetes, prediabetes, or borderline diabetes were excluded. The BRFSS dataset does not include a question that distinguishes between type 1 and type 2 diabetes mellitus; hence, the grouping of SMBG vs CGM was used for comparison among adults with type 1 or type 2 diabetes that are not gestational diabetes, prediabetes, and borderline diabetes. Next, “About how often do you check your blood for glucose or sugar?” was used to categorize respondents into 1 of 2 groups: (1) CGM group or (2) SMBG group. Respondents were categorized to the CGM group if the answer to the above question was coded as “198” based on interviewer prompt of “If the respondent uses a continuous glucose monitoring system (a sensor inserted under the skin to check glucose levels continuously), fill in ‘98 times per day.’” Respondents were categorized to the SMBG group if the answer to the frequency of checking blood glucose level was between 4 and 15 times per day. Respondents indicating SMBG 1-3 times per day were excluded to assimilate participants who would be more likely to be offered CGM in alignment with Medicare coverage criteria.9 In July 2021, Medicare announced the removal of frequency of checking SMBG at least 4 times daily to qualify for coverage of a CGM; however, given that the regulatory change happened after the data collection period of 2014 to 2020, we have set the lower threshold of 4 times per day SMBG to correlate to eligibility criteria during the time of data collection in this study.9
The prevalence of CGM use was calculated and reported in yearly ratios of the number of CGM users per each year divided by the number of respondents meeting the study eligibility criteria (ie, sum of number of people included in the CGM group and the SMBG group). In addition, the overall prevalence ratio among study participants from 2014 to 2020 was calculated based on the total number of samples included in the CGM group from 2014 to 2020 divided by the total number of samples included in the CGM and SMBG groups from 2014 to 2020. Finally, yearly prevalence ratios of the number of CGM users per the total number of respondents with self-reported diabetes were calculated.
The following variables were used to capture respondent characteristics and sociodemographic factors between the CGM and SMBG groups: age (in years), sex (male, female), race (White, African American/Black, Hispanic, Asian American/Native Hawaiian/Pacific Islander, multiracial/other), marital status (never married, previously married, currently married), education level (less than high school, graduated high school, more than high school), employment status (employed, not employed), income status (earning <$75,000, earning ≥$75,000), health plan coverage (yes, no), comorbidity status (myocardial infarction [MI], angina or coronary heart disease, stroke, asthma, arthritis, depressive disorder, chronic kidney disease [CKD]), body mass index (underweight, normal weight, overweight, obese), and current smoker (yes, no).
The following variables were captured and reported on self-reported diabetes-related disease knowledge and self-care behavior: age when diagnosed with type 1 or type 2 diabetes (in years), taking insulin (yes, no), having not seen a doctor because of cost concern (yes, no), seen a health care professional in general at least 1 time in the past 12 months (yes, no), seen a health care professional for diabetes at least 2 times in the past 12 months (yes, no), had A1c checked at least 2 times in the past 12 months (yes, no), performed daily foot self-examination (yes, no), had foot examination by health care professional at least 1 time in the past year (yes, no), status of diabetes affecting eyes (yes, no), had eyes checked by a health care professional at least 1 time in the past year (yes, no), taking classes in managing diabetes (yes, no), done any exercise in the past month (yes, no), and vaccine status for shingles, pneumonia, and influenza (received, not received).
The standard CDC HRQOL-4 was used to capture the outcome related to HRQOL. The validity of the CDC HRQOL-4 has been documented in the literature.16 The 4 items assessed (1) self-rated health; (2) number of unhealthy days due to physical illness or an injury during the past 30 days; (3) number of unhealthy days due to stress, depression, and problems with emotions during the past 30 days; and (4) number of days with limitation in self-care, work, or recreation due to poor physical or mental health during the past 30 days.
The nearest neighbor matching estimation for average treatment effects was used to compare CDC HRQOL-4 items between the 2 groups. These factors could be associated with HRQOL levels and may serve as confounders in the relationship between CGM use and HRQOL. The nearest neighbor matching was performed based on factors included in Table 1, including age, sex, race, marital status, education, employment status, income, insurance status, comorbidity status (MI, angina or coronary heart disease, stroke, asthma, arthritis, depressive disorder, and CKD), body mass index, and smoking status. Indicator variable method was used to handle any missing variables. Demographic characteristics were compared to assess the validity of the matching procedure. Univariable logistic regression was used to compare dichotomized outcomes on 4 questions on the CDC HRQOL-4 between the CGM users and matched SMBG users. Linear regression was used to assess items 2-4 of the CDC HRQOL-4. Diabetes management–related behavioral characteristics were also compared between the CGM users and matched SMBG users in Table 2.
TABLE 1.
Demographic Characteristics of Adults by Primary Use of Glucose Monitoring Device, BRFSS
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| CGM n = 231 | SMBGn = 11,822 | P value | CGMn = 231 | SMBGn = 217 | P value | |
| Demographic characteristics | ||||||
| Age, mean (SE), years | 50.3 (1.3) | 56.1 (0.3) | <0.001 | 56.9 (15.1) | 57.9 (13.9) | 0.49 |
| Sex, % | ||||||
| Male | 49.6 | 45.7 | 0.4606 | 49.8 | 51.2 | 0.77 |
| Female | 50.4 | 54.3 | 50.2 | 48.8 | ||
| Race, % | ||||||
| White | 71.0 | 72.6 | 0.7691 | 82.7 | 86.6 | 0.58 |
| African American/Black | 12.5 | 13.9 | 7.8 | 5.1 | ||
| Hispanic | 11.7 | 8.4 | 3.0 | 3.2 | ||
| AANHPI | 4.0 | 3.3 | 2.2 | 0.9 | ||
| Multiracial or other | 0.7 | 1.8 | 4.3 | 4.1 | ||
| Marital status, % | ||||||
| Never been married | 16.8 | 15.9 | 0.7985 | 12.6 | 12.0 | 0.80 |
| Previously been married | 16.6 | 29.1 | 21.6 | 24.4 | ||
| Currently married | 66.6 | 55.0 | 65.8 | 63.6 | ||
| Education, % | ||||||
| Less than high school | 9.4 | 16.6 | 0.3066 | 3.9 | 2.3 | 0.61 |
| High school | 15.3 | 30.3 | 16.0 | 17.1 | ||
| More than high school | 75.4 | 53.1 | 80.1 | 80.6 | ||
| Employment status, % | ||||||
| Employed | 59.6 | 30.6 | <0.001 | 48.9 | 47.5 | 0.69 |
| Income, % | ||||||
| <$75,000 | 51.5 | 78.0 | <0.001 | 42.4 | 43.3 | 0.82 |
| ≥$75,000 | 48.5 | 22.0 | 48.6 | 56.7 | ||
| Health plan coverage, % | ||||||
| Yes | 99.7 | 95.4 | 0.0048 | 99.6 | 99.5 | 0.96 |
| Comorbidity, % | ||||||
| MI | 10.5 | 18.0 | 0.0437 | 14.7 | 12.9 | 0.57 |
| Angina or CHD | 11.8 | 18.5 | 0.1281 | 13.9 | 14.3 | 0.92 |
| Stroke | 3.7 | 12.3 | 0.0009 | 5.2 | 4.6 | 0.77 |
| Asthma | 17.4 | 23.6 | 0.1428 | 17.3 | 17.5 | 0.97 |
| Arthritis | 32.6 | 49.8 | 0.0010 | 37.7 | 38.7 | 0.82 |
| Depressive disorder | 19.9 | 34.1 | 0.0017 | 21.2 | 20.3 | 0.81 |
| Kidney disease | 7.3 | 17.7 | 0.0009 | 10.8 | 9.7 | 0.69 |
| Body mass index, % | ||||||
| Underweight | 0.5 | 0.9 | 0.4870 | 0.9 | 0.0 | 0.53 |
| Normal weight | 28.3 | 18.8 | 25.1 | 23.5 | ||
| Overweight | 35.2 | 28.5 | 35.1 | 35.5 | ||
| Obese | 36.0 | 51.8 | 33.3 | 35.9 | ||
| Currently smoke, % | ||||||
| Yes | 2.6 | 3.7 | 0.4575 | 3.0 | 3.2 | 0.91 |
AANHPI = Asian American, Native Hawaiian, and Pacific Islander; BRFSS = Behavioral Risk Factor Surveillance System; CGM = continuous glucose monitoring; CHD = coronary heart disease; MI = myocardial infarction; SMBG = self-monitoring of blood glucose.
TABLE 2.
Diabetes-Specific Characteristics and Management-Related Behaviors of Adults by Primary Use of Glucose Monitoring Device, BRFSS
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| CGM n = 231 | SMBG n = 11,822 | P value | CGM n = 231 | SMBG n = 217 | P value | |
| Behavior related to diabetes self-management | ||||||
| Age when first diagnosed with diabetes, mean (SD), years | 27.8 (1.2) | 35.9 (0.3) | 0.0050 | 30.6 (18.3) | 35.6 (18.3) | 0.005 |
| Currently on insulin therapy, % | 81.8 | 87.6 | 0.2260 | 88.7 | 86.6 | 0.580 |
| Not seen doctor because of cost concern, % | 6.0 | 14.8 | 0.0500 | 2.6 | 7.8 | 0.011 |
| Had routine check-up in the past 12 months, % | 90.0 | 91.9 | 0.5179 | 92.6 | 92.2 | 0.860 |
| Seen provider for diabetes ≥2 times in the past 12 months, % | 89.4 | 89.1 | 0.9075 | 92.2 | 87.1 | 0.150 |
| Check hemoglobin A1c ≥2 times in the past 12 months, % | 91.6 | 87.2 | 0.2181 | 91.3 | 86.6 | 0.047 |
| Feet check, self-examination daily, % | 53.3 | 69.4 | 0.0019 | 58.9 | 69.6 | 0.028 |
| Feet check, health care professional examination ≥1 time in the past 12 months, % | 87.7 | 86.7 | 0.7952 | 87.9 | 93.5 | 0.048 |
| Was told to have retinopathy, % | 25.8 | 33.6 | 0.0870 | 31.2 | 31.3 | 0.970 |
| Eye check, health care professional screened ≥1 time in the past 12 months, % | 84.1 | 75.2 | 0.0404 | 85.3 | 85.7 | 0.990 |
| Took a course on how to manage diabetes, % | 21.7 | 27.0 | 0.2095 | 72.7 | 77.4 | 0.280 |
| Done any exercise in the past month, % | 78.4 | 60.8 | 0.0005 | 71.0 | 70.0 | 0.720 |
| Received vaccines in the past | ||||||
| Shingles, % | 39.9 | 30.5 | 0.2174 | 16.5 | 10.1 | 0.024 |
| Pneumonia, % | 66.6 | 70.5 | 0.3960 | 59.3 | 64.1 | 0.700 |
| Annual influenza, % | 66.9 | 59.5 | 0.1263 | 62.3 | 60.8 | 0.670 |
Behavioral Risk Factor Surveillance System; CGM = continuous glucose monitoring; SMBG = self-monitoring of blood glucose.
Data combining and analyses were performed using SAS Studio Version 5.2. For any missing variables, multiple imputation was performed to retain the original sample size. All analytical comparisons used survey procedures that accounted for the complex survey design of the BRFSS dataset. That is, the total sample adjusted weight, stratification, and clustering variables were applied to compare demographic and socioeconomic factors between the 2 groups. Regression with complex survey design was used to compare outcomes between the 2 groups. The nearest neighbor matching was performed to find matched comparator arm (SMBG) accounting for 17 demographic characteristics. Matched samples were then compared to assess the average treatment effects using CDC HRQOL-4 responses between the 2 groups accounting for the survey procedures. Significance was set as a 2-tailed test with a P value less than 0.05.
Results
PREVALENCE OF CGM USE
A total of 3,100,101 respondents participated in the BRFSS survey from 2014 to 2020, and 415,065 respondents reported being diagnosed with diabetes (13.4%). The yearly prevalence of diabetes was steady from 13.2% in 2014 to 13.0% in 2020. Of those with self-reported diabetes, 231 respondents reported using CGM (5.6 users per 10,000 people with self-reported diabetes), and 11,822 respondents reported frequency of checking blood glucose level 4-15 times daily (290 users per 10,000 people with self-reported diabetes) (Supplementary Table 1 (76.8KB, pdf) , available in online article). Of the 12,053 respondents with self-reported diabetes using CGM or 4-15 times daily SMBG from 2014 to 2020, the prevalence of using CGM was 1.9%, and an increased trend was observed in yearly prevalence rates of CGM use from 0.4% in 2014 to 4.1% in 2020 (Figure 1A). The prevalence of using CGM among all respondents with self-reported diabetes was 0.05%, with the increase in yearly prevalence rates of 1.3 persons using CGM in 10,000 people with diabetes in 2014, 16.6 person (per 10,000 people with diabetes) in 2019, and 7.3 persons (per 10,000 people with diabetes) in 2020 (Figure 1B).
FIGURE 1.
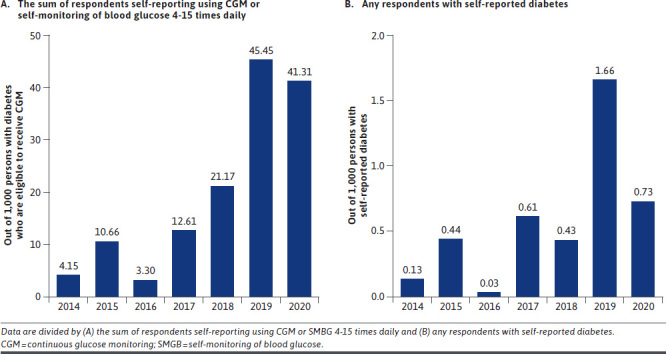
Yearly Prevalence of Self-Reported CGM Use
RESPONDENT CHARACTERISTICS AND SOCIODEMOGRAPHIC FACTORS
Of the 17 demographic characteristics, 9 parameters were significantly different between groups, meeting the a priori significance level of P < 0.05: age, employment status, income level, insurance status, and comorbidity status (MI, stroke, arthritis, depressive disorder, CKD) (Table 1). Compared with the SMBG group, the CGM group was more likely to be younger in age (50.3 [SD = 1.3] years in the CGM group compared with 56.1 [SD = 0.3] years in the SMBG group; P < 0.001), be employed (59.6% vs 30.6%, P < 0.001), earn at least $75,000 in income (48.5% vs 22.0%; P < 0.001), have health insurance coverage (99.7% vs 95.4%; P = 0.0048), and have fewer comorbid conditions such as MI (10.5% vs 18.0%; P = 0.0437), stroke (3.7% vs 12.3%; P = 0.0009), arthritis (32.6% vs 49.8%; P = 0.001), depressive disorder (19.9% vs 34.1%; P = 0.0017), and kidney disease (7.3% vs 17.7%; P = 0.0009).
Regarding items related to diabetes-specific characteristics and management-related behaviors (Table 2), the crude comparison revealed that the CGM users were younger in age when first diagnosed with diabetes (27.8 [SD = 1.2] vs 35.9 [SD = 0.3] years; P = 0.005), less likely to perform daily foot self-examinations (53.3% vs 69.4%; P = 0.0019), more likely to have had eyes checked by health care professional in the past 12 months (84.1% vs 75.2%; P = 0.0404), and more likely to have done any exercise in the past month (78.5% vs 60.8%; P = 0.0005). After performing the nearest neighbor matching to balance the demographic variables, diabetes-specific characteristics, and management-related behaviors were similar between groups, except answers to the questions on age when first diagnosed with diabetes, have not seen doctor because of cost concern, biannual A1c check, performing daily foot self-examinations, annual foot examination by health care professional, and status of receiving the shingles vaccine in the past. That is, the CGM group was younger in age when first diagnosed with diabetes (30.6 [SD = 18.3] vs 35.6 [SD = 18.3]; P = 0.005), less likely to report not seeing doctor because of cost concern (2.6% vs 7.8; P = 0.011), more likely to get biannual A1c screening (91.3% vs 86.6%; P = 0.047), less likely to perform daily foot self-examinations (58.9% vs 69.6; P = 0.028), and less likely to get an annual foot examination by a health care professional (87.9% vs 93.5; P = 0.048) and had received the shingles vaccine in the past (16.5% vs 10.1%; P = 0.024).
HRQOL
The crude comparison revealed that CGM users was associated with higher frequency of good-to-excellent general health status (62.8% vs 43.7%; P = 0.0011), was associated with fewer unhealthy days in a month due to physical reasons (6.6 vs 11.3 days; P = 0.0029), was associated with fewer unhealthy days in a month due to mental reasons (5.5 vs 6.7 days; P = 0.0003), and was associated with fewer unhealthy days due tocombined physical and mental health reasons (6.9 vs 10.4 days; P = 0.0016) (Table 3). After the nearest neighbor matching accounting for 17 demographic factors, each item on the CDC HRQOL-4 were answered in a similar manner, revealing no difference in HRQOL between the 2 groups.
TABLE 3.
Outcome Reporting of the CDC HRQOL-4 Before and After Matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| CGM n = 231 | SMBG n = 11,822 | P value | CGM n = 231 | SMBG n = 217 | P value | |
| CDC HRQOL-4 | ||||||
| #1 Self-rated health, % | ||||||
| Excellent, very good, good | 62.8 | 43.7 | 0.0011 | 61.9 | 68.7 | 0.12 |
| Poor or fair | 37.2 | 56.3 | 38.1 | 30.9 | ||
| #2 Unhealthy days, physical | ||||||
| Days, mean (SE) | 6.6 (1.0) | 11.3 (0.2) | 0.0029 | 6.6 (10.5) | 7.3 (11.1) | 0.49 |
| >14 days, % | 23.1 | 38.4 | 0.0022 | 21.2 | 24.0 | 0.82 |
| #3 Unhealthy days, mental | ||||||
| Days, mean (SE) | 5.5 (0.9) | 6.7 (0.2) | 0.0003 | 4.3 (8.8) | 3.7 (7.8) | 0.42 |
| >14 days, % | 18.8 | 22.0 | 0.4360 | 13.9 | 11.5 | 0.75 |
| #4 Unhealthy days, physical or mental | ||||||
| Days, mean (SE) | 6.9 (1.2) | 10.4 (0.2) | 0.0016 | 7.4 (10.9) | 9.4 (11.8) | 0.170 |
| >14 days, % | 16.5 | 26.0 | 0.0401 | 14.7 | 17.1 | 0.24 |
CDC = Centers for Disease Control and Prevention; CGM = continuous glucose monitoring; HRQOL = health-related quality of life; SMBG = self-monitoring of blood glucose.
Discussion
In this large cross-sectional study, we observed a steady increase in CGM use among people with self-reported diabetes. In 2014, the prevalence of CGM use was 4.1 cases per 1,000 persons with self-reported diabetes using either CGM or 4-15 times daily SMBG. Six years later in 2019, the prevalence of CGM use rose to 45.5 cases per 1,000 persons. This 11-fold increase in CGM use may reflect the start of a shift in patients’, providers’, and payors’ acceptance to use CGM devices for diabetes management because of the improved accuracy and accessibility of these devices over the years, which is consistent with the 3.8-fold rise in use from 2016 to 2017 when the Centers for Medicare and Medicaid Services proposed coverage of CGM.7 Additionally, several new devices were approved by the US Food and Drug Administration (FDA) over the course of the study period.17 In 2015, Dexcom’s G5 CGM device was approved; in 2017, Abbott’s Freestyle Libre was approved; and in 2018, Dexcom’s G6, Abbott’s Freestyle Libre 14 day, Senseonics’ Eversense, and Medtronic’s Guardian Connect were all granted FDA approval.17-20 The approval of 4 new devices in 2018 may help explain the drastic rise in CGM use in 2019. Although CGM use decreased slightly in 2020 down to 41.3 cases per 1,000 persons, this still reflects a 10-fold increase compared with 2014. The slight decrease in 2020 could be related to the COVID-19 pandemic and fewer health care visits to adjust diabetes management strategies.
Although CGM use rose during the study period, the total number of people self-reporting CGM use was only 231 counts in a large-scale survey interviewing 12,053 people between 2014 and 2020 with self-reported diabetes meeting eligibility criteria. As such, great opportunity exists to further expand the use of CGM among a large portion of CGM-naive people who may receive benefit from a CGM device, and profiling demographic characteristics that are associated with the likelihood of receiving CGM can help guide patients, providers, and payors to improve use of this technology. To the authors’ knowledge, no studies have been published regarding factors predictive of CGM use in the adult population.
In this study, we found that participants with self-reported diabetes using CGM appear to be younger and healthier (younger age at BRFSS survey completion and diabetes diagnosis and fewer comorbidities) while also exhibiting signs of financial stability (employed, higher income, insured, less instances of avoiding seeing a doctor because of cost). Although BRFSS is not able to distinguish between type 1 and type 2 diabetes, younger age at the time of the survey and at diabetes diagnosis, as well as fewer comorbidities, may suggest that CGM users may have been more likely to have type 1 diabetes, rather than type 2. This is important because insurance coverage for CGM devices has historically been better for type 1 diabetes, although eligibility restrictions have been slowly loosening. Younger age could also suggest greater acceptance of technological advances and ease of adaptability with frequently evolving technology. Greater financial stability in CGM users compared with SMBG users may indicate that cost is still a barrier to CGM device use and continued advocacy efforts are needed to provide access to this beneficial technology.
It is interesting to note that, after matching, CGM users were more likely to have A1c checked at least twice per year and receive a shingles vaccine (indicating higher health care utilization), but they were less likely to perform daily foot self-examinations or have an annual foot examination by a health care professional. Although the purpose of foot examinations is to screen for the diabetes-related complication of neuropathy, these behaviors may be lower in the CGM group because of the lower age and overall healthier status of this group. As noted above, the CGM group may have included more people with type 1 diabetes than the SMBG group, and screening for neuropathy is not indicated until 5 years after diagnosis of type 1 diabetes (as opposed to at the time of diagnosis for type 2 diabetes).21
HRQOL items favored CGM for all 4 items before nearest neighbor matching but showed no difference after matching (Table 2). Although the populations were inherently varied as indicated by the differences before matching noted in Table 1, once matching of 17 demographic characteristics was performed, no differences between groups in HRQOL were seen. One potential barrier to CGM use is the overload of information provided by these devices, which can be overwhelming to the person wearing the device; as such, it is reassuring to see that there was no change in the self-assessment of mentally unhealthy days between the CGM and SMBG groups.
LIMITATIONS
The results of this study warrant consideration for several limitations. The cross-sectional nature of the BRFSS data is not able to provide a causal relationship between the examined factors. The interviewer-led self-reported nature of the data collection process may leave room for recall bias or misclassification of the exposure status of CGM. Use of CGM and SMBG is not mutually exclusive monitoring therapy; hence, underreporting of CGM use may have been possible depending on the participants’ perceived notion of the dominant and/or usual way to monitor glucose levels at the time of the interview. Additionally, duration of CGM use and the time elapsed since the last time CGM was used to monitor glucose levels are not captured because of the nature of the survey. Differentiating type 1 and type 2 diabetes was not possible, and there was a lack of clinical outcome, such as A1c level. This information would have helped assess the disease control status and whether participants received CGM as a preventative measure to maintain control of their diabetes or as a last resort to manage uncontrolled diabetes.
There are several strengths to this study. The BRFSS is a large-scale survey capturing samples from a nationally representative participant pool. The multiple imputation methods with the BRFSS data have shown to improve the national level estimates to increase the generalizability of the findings.22 Additionally, the data reviewed from 2014 to 2020 reflects the comprehensive nature of the relevant and current datasets. The BRFSS interview began collecting information about CGM use since 2014, and examining all available data over the 7-year period helped depict relevant and meaningful information about the general patterns of CGM use from the public health point of view.
Conclusions
The results show that CGM use increased from 2014 to 2020 but remains far less than SMBG use. Economic factors appeared to predict CGM use over SMBG, indicating that cost may still limit access to CGM devices. Use of CGM neither negatively nor positively affected HRQOL compared with SMBG use.
REFERENCES
- 1.American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83-96. doi:10.2337/dc22-S006 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S97-112. doi:10.2337/dc22-S007 [DOI] [PubMed] [Google Scholar]
- 3.Luijf YM, Mader JK, Doll W, et al. . Accuracy and reliability of continuous glucose monitoring systems: A head-to-head comparison. Diabetes Technol Ther. 2013;15(8):722-7. doi:10.1089/dia.2013.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christiansen MP, Klaff LJ, Brazg R, et al. . A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther. 2018;20(3):197-206. doi:10.1089/dia.2017.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: Results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138-46. doi:10.1177/1932296817704445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo HJ, An HG, Park SY, et al. . Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73-9. doi:10.1016/j.diabres.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 7.Heinemann L, Schoemaker M, Schmelzeisen-Redecker G, et al. . Benefits and limitations of MARD as a performance parameter for continuous glucose monitoring in the interstitial space. J Diabetes Sci Technol. 2020;14(1):135-50. doi:10.1177/1932296819855670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller EM. Using continuous glucose monitoring in clinical practice. Clin Diabetes. 2020;38(5):429-38. doi:10.2337/cd20-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. Medicare coverage of diabetes supplies, services, & prevention programs. Accessed January 18, 2023. https://www.medicare.gov/Pubs/pdf/11022-Medicare-Diabetes-Coverage.pdf
- 10.Centers for Disease Control and Prevention. HRQOL concepts. Accessed January 18, 2023. https://www.cdc.gov/hrqol/concept.htm
- 11.Aschalew AY, Yitayal M, Minyihun A. Health-related quality of life and associated factors among patients with diabetes mellitus at the University of Gondar referral hospital. Health Qual Life Outcomes. 2020;18(1):62. doi:10.1186/s12955-020-01311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120-9. doi:10.4239/wjd.v8.i4.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siersma V, Thorsen H, Holstein PE, et al. . Health-related quality of life predicts major amputation and death, but not healing, in people with diabetes presenting with foot ulcers: The Eurodiale study. Diabetes Care. 2014;37(3):694-700. doi:10.2337/dc13-1212 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Healthy days core module (CDC HRQOL-4). Accessed January 18, 2023. https://www.cdc.gov/hrqol/hrqol14_measure.htm
- 15.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. Survey data and documentation. Accessed January 18, 2023. https://www.cdc.gov/brfss/data_documentation/index.htm
- 16.Ounpuu S, Chambers LW, Chan D, Yusuf S. Validity of the US Behavioral Risk Factor Surveillance System’s health related quality of life survey tool in a group of older Canadians. Chronic Dis Can. 2001;22(3-4):93-101. [PubMed] [Google Scholar]
- 17.Dexcom. FDA approves Dexcom G5 mobile continuous glucose monitoring system. Augus 24, 2015. Accessed Janurary 18, 2023. https://www.dexcom.com/it-CH/news/1257506247-fda-approves-dexcom-g5%C2%AE-mobile-continuous-glucose-monitoring-system
- 18.US Food and Drug Administration. FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. September 27, 2017. Accessed January 18, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-continuous-glucose-monitoring-system-adults-not-requiring-blood-sample [Google Scholar]
- 19.US Food and Drug Administration. FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. March 27, 2018. Accessed January 18, 2023. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-fully-interoperable-continuous-glucose-monitoring-system-streamlines-review [Google Scholar]
- 20.US Food and Drug Administration. Medical devices approval in 2018. Accessed June 30, 2021. https://www.fda.gov/medical-devices
- 21.Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol (Lausanne). 2021;12:671257. doi:10.3389/fendo.2021.671257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankel MR, Battaglia MP, Balluz L, Strine T. When data are not missing at random: Implications for measuring health conditions in the Behavioral Risk Factor Surveillance System. BMJ Open. 2012;2(4). doi:10.1136/bmjopen-2011-000696 [DOI] [PMC free article] [PubMed] [Google Scholar]


