Abstract
BACKGROUND:
Real-world evidence on the comparative effectiveness of pegfilgrastim biosimilars compared with the originator product is limited.
OBJECTIVE:
To compare the risk of febrile neutropenia (FN) among users of pegfilgrastim biosimilars (pegfilgrastim-jmdb and pegfilgrastim-cbqv) and the originator product.
METHODS:
A retrospective cohort study was conducted using 2019 IBM MarketScan databases to assess comparative effectiveness of pegfilgrastim originator and biosimilars for prevention of FN among patients receiving myelosuppressive chemotherapy. Patients with cancer, including breast, lung, colorectal, esophageal and gastric, pancreatic, prostate, ovarian, and non-Hodgkin lymphomas, initiating myelosuppressive chemotherapy courses were selected. We further selected patients who used pegfilgrastim originator and biosimilars within 3 days of chemotherapy completion. FN-associated hospitalizations were measured by International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis codes. After 1:1 propensity score matching, we used equivalence (with a margin of 6%) hypothesis tests to compare FN-related hospitalization risk in the first cycle and across all cycles between biosimilars and originator users.
RESULTS:
A total of 2,045 patients were included, of which 445 (21.8%) used pegfilgrastim-jmdb, 636 (31.1%) used pegfilgrastim-cbqv, and 964 (47.1%) used pegfilgrastim originator. After matching, 13 out of 445 originator users and 17 out of 445 pegfilgrastim-jmdb users developed FN after the first chemotherapy cycle (risk difference was 0.9%; P < 0.001 for equivalence test indicating statistical equivalence). After matching, 14 out of 633 originator users and 16 out of 633 pegfilgrastim-cbqv users developed FN (risk difference was 0.32%; P < 0.001 for equivalence test indicating statistical equivalence). Results across all cycles (including the first cycle) were consistent with that in the first cycle.
CONCLUSIONS:
In this real-world study of patients with cancer receiving myelosuppressive chemotherapy, there was no difference in FN risk between patients receiving pegfilgrastim originator and biosimilars in the first cycle and across all cycles. These results add further to the current evidence on pegfilgrastim biosimilars and support wider adoption of pegfilgrastim biosimilars among payers, providers, and patients. Future studies assessing the tolerability, side effects, and other safety issues of pegfilgrastim biosimilars are needed.
Plain language summary
Febrile neutropenia is a serious side effect of chemotherapy that results in low white blood cell count and fever among patients with cancer. Pegfilgrastim is a preventative treatment for febrile neutropenia (FN), and new biosimilar options may provide lower costs compared with the original treatment (originator). This real-world study shows that the pegfilgrastim originator and biosimilars resulted in similar rates of FN in privately insured US patients who received chemotherapy treatment.
Implications for managed care pharmacy
In a cohort of patients with breast, lung, colorectal, esophageal and gastric, pancreatic, prostate, and ovarian cancer and non-Hodgkin lymphomas, risks for FN between patients who used the pegfilgrastim originator and biosimilars were statistically equivalent across all chemotherapy cycles. This analysis supports adoption of pegfilgrastim biosimilars based on effectiveness; however, other nonclinical factors, such as a stable supply chain, patient out-ofpocket costs, and acquisition costs, are additional determining factors related to biosimilar adoption.
Biosimilars are biologic products that are highly similar to and have no clinically meaningful difference in terms of safety, purity, and potency compared with the existing US Food and Drug Administration (FDA)–approved originator products.1 In 2010, an abbreviated approval pathway for biosimilars in the United States was established through the Biologic Price Competition and Innovation Act to encourage innovation and development of biosimilars. Biosimilars were also intended to provide competition to originators and reduce the cost of therapy. Since the Biologic Price Competition and Innovation Act was enacted, the FDA approved 34 biosimilars for 11 originator products.
Granulocyte colony-stimulating factors (G-CSFs) are one of earliest drug classes with biosimilars available, including filgrastim and its long-acting version, pegfilgrastim.2 G-CSFs reduce the duration of neutropenia and the risk of febrile neutropenia (FN) in patients with cancer receiving myelosuppressive chemotherapy. Currently, there are 3 filgrastim biosimilars (filgrastim-sndz, filgrastim-aafi, and filgrastim-ayow) and 4 pegfilgrastim biosimilars (pegfilgrastim-jmdb, pegfilgrastim-cbqv, pegfilgrastim-bmez, and pegfilgrastim-apgf) approved by the FDA and available in the US market.
The approval of pegfilgrastim-jmdb, the first pegfilgrastim biosimilar, was based on study MYL-1401H-3001, a phase 3, multicenter, randomized, double-blind, parallel-group equivalence trial conducted in patients with breast cancer receiving a single chemotherapy regimen (docetaxel, doxorubicin, cyclophosphamide).3 The primary endpoint in MYL-1401H-3001 was the duration of severe neutropenia in cycle 1; subsequent cycles (up to cycle 4) were only investigated as secondary endpoints.3 Moreover, in that study, the biosimilar was compared with the European Union–sourced reference pegfilgrastim, not the US-sourced reference pegfilgrastim.3 The similarity of pegfilgrastim-jmdb with the US-sourced reference pegfilgrastim were demonstrated through comparison on pharmacokinetic and pharmacodynamic endpoints among healthy participants in a separate study.4 For pegfilgrastim-cbqv, there are no trials assessing efficacy endpoints among patients with cancer receiving chemotherapy. FDA approval of pegfilgrastim-cbqv was based on pharmacokinetic and pharmacodynamic data among healthy participants5 as well as other nonclinical data.
Previous studies comparing a filgrastim biosimilar (filgrastim-sndz) to its originator (Neupogen) using real-world data showed no clinically meaningful differences between the products.6-9 However, filgrastim (including originator and biosimilars) accounts for only 3% of all G-CSF use.10 Although pegfilgrastim dominates the G-CSF market, data stemming from clinical trials for pegfilgrastim biosimilars are limited to certain patient population and study end-points.3-5 Currently, there are no real-world studies that compare pegfilgrastim biosimilars with their originator (Neulasta).
Investigating the comparative effectiveness of pegfilgrastim biosimilars using real-world data and addressing this knowledge gap are needed to strengthen the confidence of providers, patients, and payers in adopting pegfilgrastim biosimilars. Thus, the objective of this study was to compare the risk of FN among patients with cancer receiving myelosuppressive chemotherapy among users of the originator pegfilgrastim prefilled syringe (PFS) and biosimilars pegfilgrastim-cbqv and pegfilgrastim-jmdb.
Methods
STUDY DESIGN AND DATA SOURCE
This was a retrospective cohort study using the 2019 IBM MarketScan Commercial and Medicare Supplemental database during which all 3 pegfilgrastim products were available (pegfilgrastim was approved on June 4, 2018; pegfilgrastim-cbqv was approved on November 2, 2018). The MarketScan database includes individuals with employer-based insurance. The MarketScan Medicare Supplemental database includes Medicare beneficiaries who possess supplemental insurance. The University of Florida Institutional Review Board approved the use of the data for this study.
STUDY POPULATION
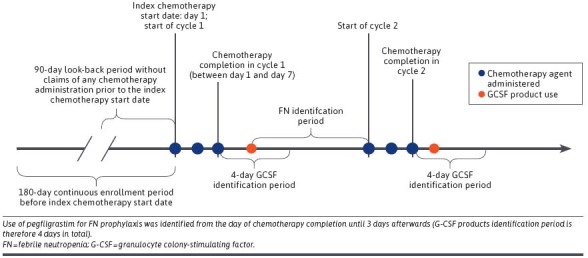
A cohort of adult patients (aged ≥ 18 years) with cancer (breast, lung, colorectal, esophageal and gastric, pancreatic, prostate, ovarian, or non-Hodgkin lymphomas) who received their first cycle of myelosuppressive chemotherapy and pegfilgrastim originator or biosimilars for FN prophylaxis between January 1, 2019, and November 30, 2019, were selected (the end of cohort selection period was intentionally set to 1 month before the end of study period to ensure a minimum of 1 month of potential follow-up). Chemotherapy agents were identified by Healthcare Common Procedure Coding System level 2 codes (Supplementary Table 1 (315.5KB, pdf) , available in online article). The index chemotherapy start date (day 1) was defined as the administration date of the first chemotherapy agent. Patients were censored at the occurrence of FN, end of cycle 8, disenrollment, the end of the study data (December 31, 2019), or until end of the last qualified cycle, whichever occurred first.
Chemotherapy regimens were ascertained based on all chemotherapy claims within 7 days after the index chemotherapy start date (days 1-7) (Figure 1). Commonly used regimens were selected and further categorized into low (<10%), intermediate (10%-20%), or high (>20%) risk for FN according to National Comprehensive Cancer Network guidelines11 and clinical expert opinion (Supplementary Table 2 (315.5KB, pdf) ).10
FIGURE 1.

Study Population Selection Criteria
INCLUSION AND EXCLUSION CRITERIA
We required patients to have 180 days of continuous enrollment and a 90-day look-back period without claims for any chemotherapy administration prior to the index chemotherapy start date (Figure 1). For each patient, 2 or more diagnoses of the same cancer type, at least 7 days apart within ±30 days of the index chemotherapy start date, were required. This criterion excluded patients with rule-out cancer diagnosis and ensured patients were receiving chemotherapy treatment for the specific type of cancer. Cancer types included breast, lung, colorectal, esophageal and gastric, pancreatic, prostate, ovarian, and non-Hodgkin lymphomas (Supplementary Table 3 (315.5KB, pdf) ). To be included in the study, patients were required to use pegfilgrastim-PFS, pegfilgrastim-jmdb, or pegfilgrastim-cbqv in the first chemotherapy cycle for FN prophylaxis. All G-CSF products were identified by National Drug Code numbers in pharmacy claims and Healthcare Common Procedure Coding System in medical claims (Supplementary Table 4 (315.5KB, pdf) ), from the day of chemotherapy completion until 3 days afterward (G-CSF products identification period is therefore 4 days in total) (Figure 1).
Patients were excluded if they had a chemotherapy agent administered between day 8 and day 11 because pegfilgrastim is not recommended for chemotherapy regimens with weekly administration schedules.11
The following additional exclusion criteria were applied: (1) having 2 or more different primary solid cancers or acute myeloid leukemia within ±30 days of index chemotherapy start date; (2) receiving autologous peripheral blood progenitor cell collection, bone marrow transplantation, or hematopoietic stem cell transplantation during the period beginning 30 days before the index chemotherapy start date and ending 10 days after that; or (3) using more than 1 G-CSF product, pegfilgrastim on-body-injector, or pegfilgrastim with unknown route. Diagnosis and procedure codes for the exclusion criteria are shown in Supplementary Table 5 (315.5KB, pdf) . Pegfilgrastim on-body-injector and those with unknown route were excluded to remove any possible effect due to differences in administration route.
CHEMOTHERAPY CYCLE
Each patient was followed for up to 8 consecutive qualifying cycles. The first cycle began on the index chemotherapy start date and ended on the day prior to the next chemotherapy administered between day 12 and day 60. The second cycle followed immediately beginning on the day of the next chemotherapy administration. If no chemotherapy was administered between day 12 and day 60, the cycle and follow-up were considered completed on day 35, disenrollment, or end of study period, whichever came first. All subsequent cycles were defined by the same rules. To be a qualified cycle, there needed to be no change in chemotherapy agents administered, compared with the first cycle, and patients were required to use the same pegfilgrastim product as the first cycle. Cycles ended because of disenrollment or end of study period were considered incomplete cycles and excluded from the study.
OUTCOME MEASURES
Risk of FN-related hospitalizations among patients receiving pegfilgrastim-jmdb or pegfilgrastim-cbqv were compared against patients receiving pegfilgrastim-PFS in the first cycle and across all subsequent cycles. FN was measured from the date pegfilgrastim was administered (index date) to the end of the cycle (Figure 1) using International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis codes (Supplementary Table 6 (315.5KB, pdf) ) by the following 2 definitions: (1) neutropenia, fever, or infection diagnosis code in any position (“broad definition”) and (2) neutropenia diagnosis code in any position (“narrow definition”). These 2 definitions for FN were validated in administrative claims databases12 and used by previous studies.13,14 Risk of FN (both broad and narrow definitions) were compared in the first cycle and across all cycles between pegfilgrastim-PFS and pegfilgrastim-jmdb as well as between pegfilgrastim-PFS and pegfilgrastim-cbqv. No comparison was made between the biosimilars.
STATISTICAL ANALYSES
To reduce potential selection bias, propensity score (PS) models were developed using logistic regression to estimate the probability of patients receiving pegfilgrastim-PFS or pegfilgrastim biosimilars (pegfilgrastim-jmdb and pegfilgrastim-cbqv). PS models included all variables shown in Supplementary Tables 7 and 8 (315.5KB, pdf) (diagnosis codes shown in Supplementary Table 9 (315.5KB, pdf) ). Based on the PS, pegfilgrastim-PFS users were 1:1 matched to pegfilgrastim-jmdb or pegfilgrastim-cbqv users using the greedy nearest neighbor algorithm.15 Balances between groups were assessed by standardized difference (covariates with ≥10% were considered imbalanced).
In the main analysis, equivalence tests were conducted based on risk difference between groups. The null hypothesis assumes risk difference between 2 groups is lower than the lower margin (-6%) or higher than the upper margin (+6%). Rejection of the null hypothesis indicates statistical equivalence between the 2 groups. The equivalence margin defines a range of value, in this case from −6% to 6%, for which the efficacy of 2 groups are close enough to be considered equivalent.16 Using the 2-1-sided t-tests procedure, equivalence is established at the α significance level if a (1-2α) × 100% CI for the difference in efficacy is contained within the equivalence margin.16 To yield a 0.05 significance level for testing equivalence, 90% CIs for the risk difference were calculated using Farrington-Manning method.16 Evidence of equivalence is declared when the 90% CI lies wholly within the equivalence margin (-6%, +6%) and the P value is less than 0.05 (Figure 2).16 The larger of the 2 P values of each of the 1-side tests was reported.
FIGURE 2.
Pegfilgrastim Product Identification Period
For the sensitivity analyses, superiority tests (McNemar test) were conducted in which the null hypothesis assumes that the risks of developing FN are the same between biosimilar and originator users; the alternative hypothesis assumes the risks of developing FN are different between biosimilar and originator users. Furthermore, risk of FN (both broad and narrow definition) within each cycle was compared among the matched cohorts of pegfilgrastim-PFS and pegfilgrastim-jmdb users and between pegfilgrastim-PFS and pegfilgrastim-cbqv users. Both superiority tests and equivalence tests were performed based on risk differences between groups. Analyses were stratified by prophylaxis type (primary vs secondary). Primary prophylaxis is defined as no FN occurrence (broad definition) in the prior cycles; secondary prophylaxis is defined as having FN occurrence (broad definition) in the prior cycles.
Results
STUDY POPULATIONS AND CHARACTERISTICS
A total of 2,045 patients with cancer receiving selected myelosuppressive chemotherapy and any of the 3 pegfilgrastim products were included. Among them, 445 (21.8%) used pegfilgrastim-jmdb, 636 (31.1%) used pegfilgrastim-cbqv, and 964 (47.1%) used pegfilgrastim originator. Before PS matching, multiple covariates were imbalanced between pegfilgrastim-PFS and pegfilgrastim biosimilars users. All covariates were balanced after PS matching (Supplementary Tables 7 and 8 (315.5KB, pdf) ).
MAIN ANALYSES
First Cycle. Among the matched cohort for the comparison in the first cycle between pegfilgrastim-PFS and pegfilgrastim-jmdb, 2.92% of pegfilgrastim-PFS users and 3.82% of pegfilgrastim-jmdb users developed FN based on the broad outcome definition (risk difference, 0.9%; P < 0.001 for equivalence test) (Table 1); 1.8% of both pegfilgrastim-PFS and pegfilgrastim-jmdb users developed FN based on the narrow outcome definition (risk difference, 0%; P < 0.001 for equivalence test) (Table 1).
TABLE 1.
FN Incidence in Cycle 1 Between Pegfilgrastim Originator and Biosimilars (Pegfilgrastim-jmdb and Pegfilgrastim-cbqv) Among PS-Matched Cohort
| Pegfilgrastim originator | Pegfilgrastim-jmdb | Pegfilgrastim originator | Pegfilgrastim-cbqv | |
|---|---|---|---|---|
| Number of patients | 445 | 445 | 633 | 633 |
| Number of patients with broad FN | 13 | 17 | 14 | 14 |
| Cumulative incidence of broad FN, % | 2.92 | 3.82 | 2.21 | 2.53 |
| Risk difference for broad FN (90% CI) | 0.90 (–1.54 to 3.34) | 0.32 (–1.57 to 2.20) | ||
| P value for equivalence test | <0.001a | <0.001a | ||
| Number of patients with narrow FN | 8 | 8 | 9 | 7 |
| Cumulative incidence of narrow FN, % | 1.80 | 1.80 | 1.42 | 1.11 |
| Risk difference for narrow FN (90% CI) | 0 (–2.14 to 2.14) | –0.31 (–2.05 to 1.42) | ||
| P value for equivalence testa | <0.001a | <0.001a | ||
a P < 0.05 indicates pegfilgrastim-PFS and pegfilgrastim-jmdb are statistically equivalent. Using the 2-1-sided t-tests procedure, equivalence is established at the α significance level if a (1-2α) × 100% CI for the difference in efficacy is contained within the equivalence margin. To yield a 0.05 significance level for testing equivalence, 90% CIs for the risk difference were calculated using Farrington-Manning method. Evidence of equivalence is declared when the 90% CI lies wholly within the equivalence margin (−6%, +6%) and P value is less than 0.05. The larger of the 2 P values of each of the 1-side tests was reported.
FN = febrile neutropenia; PFS = pegfilgrastim-prefilled syringe; PS = propensity score.
For the comparison between pegfilgrastim-PFS and pegfilgrastim-cbqv, 2.21% of pegfilgrastim-PFS users and 2.53% of pegfilgrastim-cbqv users developed FN based on the broad outcome definition (risk difference, 0.32%; P < 0.001 for equivalence test) (Table 1); 1.42% of pegfilgrastim-PFS users and 1.11% of pegfilgrastim-cbqv users developed FN based on the narrow outcome definition (risk difference, −0.31%; P < 0.001 for equivalence test) (Table 1).
Across All Cycles. Among the matched cohort for the comparison across all cycles between pegfilgrastim-PFS and pegfilgrastim-jmdb, 5.84% of pegfilgrastim-PFS users and 6.74% of pegfilgrastim-jmdb users developed FN based on the broad outcome definition (risk difference, 0.90%; P = 0.002 for equivalence test) (Table 2); 2.70% of pegfilgrastim-PFS users and 3.60% of pegfilgrastim-jmdb users developed FN based on the narrow outcome definition (risk difference, 0.90%; P < 0.001 for equivalence test) (Table 2).
TABLE 2.
FN Incidence Across All Cycles Between Pegfilgrastim Originator and Biosimilars (Pegfilgrastim-jmdb and Pegfilgrastim-cbqv) Among PS-Matched Cohort
| Pegfilgrastim originator | Pegfilgrastim-jmdb | Pegfilgrastim originator | Pegfilgrastim-cbqv | |
|---|---|---|---|---|
| Number of patients | 445 | 445 | 633 | 633 |
| Number of patients with broad FN | 26 | 30 | 32 | 46 |
| Cumulative incidence of broad FN, % | 5.84 | 6.74 | 5.06 | 7.27 |
| Risk difference for broad FN (90% CI) | 0.90 (−2.00 to 3.80) | 2.21 (−0.23 to 4.65) | ||
| P value for equivalence testa | 0.002a | 0.004a | ||
| Number of patients with narrow FN | 12 | 16 | 16 | 25 |
| Cumulative incidence of narrow FN, % | 2.70 | 3.60 | 2.53 | 3.95 |
| Risk difference for narrow FN (90% CI) | 0.90 (−1.50 to 3.30) | 1.42 (−0.63 to 3.47) | ||
| P value for equivalence testa | <0.001a | <0.001a | ||
a P < 0.05 indicates pegfilgrastim-PFS and pegfilgrastim-jmdb are statistically equivalent. Using the 2-1-sided t-tests procedure, equivalence is established at the α significance level if a (1-2α) × 100% CI for the difference in efficacy is contained within the equivalence margin. To yield a 0.05 significance level for testing equivalence, 90% CIs for the risk difference were calculated using Farrington-Manning method. Evidence of equivalence is declared when the 90% CI lies wholly within the equivalence margin (−6%, +6%) and P value is less than 0.05. The larger of the 2 P values of each of the 1-side tests was reported.
FN = febrile neutropenia; PFS = pegfilgrastim-prefilled syringe; PS = propensity score.
For the comparison between pegfilgrastim-PFS and pegfilgrastim-cbqv, 5.06% of pegfilgrastim-PFS users and 7.27% of pegfilgrastim-cbqv users developed FN based on the broad outcome definition (risk difference, 2.21%; P = 0.004 for equivalence test) (Table 2); 2.53% of pegfilgrastim-PFS users and 3.95% of pegfilgrastim-cbqv users developed FN based on the narrow outcome definition (risk difference, 1.42%; P < 0.001 for equivalence test) (Table 2).
SENSITIVITY ANALYSES
Results from the sensitivity analysis were consistent with that from the main analysis. Superiority tests (McNemar tests) showed no statistically significant difference between the biosimilars and originator (results not shown). Furthermore, risk of FN within each cycle was statistically equivalent between biosimilar and originator users. Superiority tests also showed no difference in risk of FN (Supplementary Table 10 (315.5KB, pdf) ).
In the stratified analyses by prophylaxis type, restricting to cycles with primary prophylaxis showed consistent results in which equivalence was established and no difference was observed (Supplementary Table 11 (315.5KB, pdf) ). However, the number of cycles with secondary prophylaxis were too small that equivalence could not be established. Statistically significant difference was observed between pegfilgrastim-PFS and pegfilgrastim-jmdb (Supplementary Table 11 (315.5KB, pdf) ).
Discussion
To the best of our knowledge, this is the first US study confirming the clinical equivalence by evaluating occurrence of FN between pegfilgrastim biosimilars and originator using real-world data obtained from a large national insurance database. This is also the first US study to assess the occurrence of FN associated with pegfilgrastim biosimilars among patients with cancer types other than breast cancer.
In this study, the risk of FN-associated hospitalizations among patients with cancer undergoing chemotherapy was statistically equivalent between those who received pegfilgrastim-jmdb and pegfilgrastim originator, as well as between pegfilgrastim-cbqv and pegfilgrastim originator, in the first chemotherapy cycle and across all chemotherapy cycles.
This demonstration of clinical equivalence has important implications for enhancing the use of pegfilgrastim biosimilars in clinical practice and the quality of oncology supportive care. FN is associated with great clinical and economic burden. It was reported that the inpatient fatality rate for FN is around 10%, with a mean total hospitalization cost of $18,880-$22,086 USD.17-19 Use of G-CSFs to prevent FN among eligible patients can both improve patient outcomes and be cost saving. However, studies reported that many patients who were candidates for G-CSF did not receive it.20,21 High costs of these G-CSF products have been reported as a primary reason for suboptimal utilization patterns.22 The availability of pegfilgrastim biosimilars can potentially fill these gaps with increased competition and ultimately improve the quality of care.
In the present analysis, the risk of FN in the first cycle among patients with cancer receiving chemotherapy and prophylactic pegfilgrastim originator ranged from 1.35% to 2.59%. The FN incidence ranged from 1.10% to 2.53% for pegfilgrastim-cbqv and from 1.80% to 3.83% for pegfilgrastim-jmdb. The observed risk of FN was similar to that reported in a randomized clinical trial (RCT) of pegfilgrastim originator (2% incidence of FN) and pegfilgrastim-cbqv (4%),3 as well as previous observational studies (pegfilgrastim originator, 1.55%),23 despite the differences in the population and assessed chemotherapy regimens.
A difference in the FN incidence of less than 6% was selected as the margin of equivalence for this study. Determination of the equivalence margins is not well defined, especially for observational studies. Guidelines put forward by the International Conference on Harmonization stated that the equivalence margin is the largest difference that can be judged as being clinically acceptable and should be smaller than differences observed in superiority trials of the active comparator.24 In a meta-analysis of placebo-controlled trials, risk differences for FN between patients who received pegfilgrastim and placebo were 11.3%.25 RCTs comparing pegfilgrastim vs filgrastim also found the risk difference for FN to be 7%.26 Furthermore, the 6% margin was also used by a prior study to establish clinical equivalence between filgrastim biosimilar and originator.8 Given the rationale above, we considered 6% an appropriate margin.
LIMITATIONS
Our study has several limitations. First, FN was identified by diagnosis codes using a previously validated algorithm. Although we examined FN using 2 different definitions (broad and narrow definitions), misclassification of FN is possible. The broad FN definition has a higher sensitivity (87%) and lower specificity (53%) reported in the original validation study,12 which could result in patients without FN misclassified as having FN. On the other hand, the narrow FN definition has a nearly perfect specificity (94%) and lower sensitivity (67%), which could result in patients with FN misclassified as not having FN. In both situations, if the mis-classification is nondifferential, there will be either no bias in risk difference estimation or an underestimation of the risk difference depending on the assumption made on the true risk difference between the originator and biosimilar users. Second, the study was conducted using a commercial claims database; thus, the results can only be generalized to the commercially insured population in the US Third, small sample size limits our ability to conduct stratified analysis by cancer type or FN risk level. Lastly, switching between pegfilgrastim originator and biosimilars was not assessed in the present study. Thus, safety and effectiveness of switching between pegfilgrastim originator and biosimilars cannot be assumed.
Conclusions
In this real-world study of patients with cancer receiving myelosuppressive chemotherapy, the risk of FN was statistically equivalent between patients receiving pegfilgrastim originator and biosimilars in the first chemotherapy cycle and across all chemotherapy cycles. The results add further to the current evidence on pegfilgrastim biosimilars and support wider adoption of pegfilgrastim biosimilars among payers, providers, and patients.
REFERENCES
- 1.US Food and Drug Administration. Biosimilar and interchangeable products. Accessed September 12, 2022. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchange-able-products#biosimilar
- 2.US Food and Drug Administration. Biosimilar product information. FDA-approved biosimilar products. Accessed September 12, 2022. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
- 3.Waller CF, Ranganna GM, Pennella EJ, et al. Randomized phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H in the prophylactic treatment of chemotherapy-induced neutropenia. Ann Hematol. 2019;98(5):1217-24. doi:10.1007/s00277-019-03639-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waller CF, Tiessen RG, Lawrence TE, et al. A pharmacokinetics and pharmacodynamics equivalence trial of the proposed pegfilgrastim biosimilar, MYL-1401H, versus reference pegfilgrastim. J Cancer Res Clin Oncol. 2018;144(6):1087-95. doi:10.1007/s00432-018-2643-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finck B, Tang H, Civoli F, et al. Pharmacokinetic and pharmacodynamic equivalence of pegfilgrastim-cbqv and pegfilgrastim in healthy subjects. Adv Ther. 2020;37(10):4291-307. doi:10.1007/s12325-020-01459-y [DOI] [PubMed] [Google Scholar]
- 6.Schwartzberg LS, Lal LS, Balu S, et al. Incidence of febrile neutropenia during chemotherapy among patients with nonmyeloid cancer receiving filgrastim vs a filgrastim biosimilar. Clinicoecon Outcomes Res. 2018;10:493-500. doi:10.2147/CEOR.S168298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas AG, Schwab P, Lane D, et al. A comparison of brand and biosimilar granulocyte-colony stimulating factors for prophylaxis of chemotherapy-induced febrile neutropenia. J Manag Care Spec Pharm. 2017;23(12):1221-6. doi:10.18553/ jmcp.2017.23.12.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartzberg LS, Lal LS, Balu S, et al. Clinical outcomes of treatment with filgrastim versus a filgrastim biosimilar and febrile neutropenia-associated costs among patients with nonmyeloid cancer undergoing chemotherapy. J Manag Care Spec Pharm. 2018;24(10):976-84. doi:10.18553/jmcp.2018.17447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Agiro A, Barron J, et al. Early adoption of biosimilar growth factors in supportive cancer care. JAMA Oncol. 2018;4(12):1779-81. doi:10.1001/ jamaoncol.2018.5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CY, Heldermon CD, Vouri SM, et al. Trends in use of granulocyte colony-stimulating factor following introduction of biosimilars among adults with cancer and commercial or Medicare insurance from 2014 to 2019. JAMA Netw Open. 2021;4(11):e2133474. doi:10.1001/ jamanetworkopen.2021.33474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford J, Becker PS, Armitage JO, et al. Myeloid growth factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(12):1520-41. doi:10.6004/jnccn.2017.0175 [DOI] [PubMed] [Google Scholar]
- 12.Weycker D, Sofrygin O, Seefeld K, et al. Technical evaluation of methods for identifying chemotherapy-induced febrile neutropenia in healthcare claims databases. BMC Health Serv Res. 2013;13:60. doi:10.1186/1472-6963-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weycker D, Li X, Tzivelekis S, et al. Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer. 2017;25(2):439-47. doi:10.1007/s00520-016-3421-x [DOI] [PubMed] [Google Scholar]
- 14.Weycker D, Doroff R, Hanau A, et al. Use and effectiveness of pegfilgrastim prophylaxis in US clinical practice: A retrospective observational study. BMC Cancer. 2019;19(1):792. doi:10.1186/s12885-019-6010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-69. doi:10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-6. doi:10.1007/s11606-010-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulisse B, Li X, Gayle JA, et al. A retrospective study of the clinical and economic burden during hospitalizations among cancer patients with febrile neutropenia. J Med Econ. 2013;16(6):720-35. doi:10.3111/13696998.2013.782034 [DOI] [PubMed] [Google Scholar]
- 18.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-66. doi:10.1002/cncr.21847 [DOI] [PubMed] [Google Scholar]
- 19.Michels SL, Barron RL, Reynolds MW, et al. Costs associated with febrile neutropenia in the US. Pharmacoeconomics. 2012;30(9):809-23. doi:10.2165/11592980-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 20.Ramsey SD, McCune JS, Blough DK, et al. Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care. 2010;16(9):678-86. [PubMed] [Google Scholar]
- 21.Sosa R, Li S, Molony JT, et al. Use of prophylactic growth factors and antimicrobials in elderly patients with cancer: A review of the Medicare database. Support Care Cancer. 2017;25(10):3123-32. doi:10.1007/s00520-017-3720-x [DOI] [PubMed] [Google Scholar]
- 22.Hawkins A, Murphy A, McNamara M, et al. A survey of oncologists’ perceptions and opinions regarding the use of granulocyte colony-stimulating factors. J Cancer Educ. 2020;35(1):178-86. doi:10.1007/s13187-019-01638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride A, Campbell K, Li E, et al. Economic and clinical outcomes of pegfilgrastim via prefilled syringe vs on-body injector: A real-world data analysis. J Manag Care Spec Pharm. 2021;27(9): 1230-8. doi:10.18553/jmcp.2021.21010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. Guidance Document: E10 Choice of control group and related issues in clinical trials. August 24, 2018. (updated). Accessed March 30, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ e10-choice-control-group-and-related-issues-clinical-trials
- 25.Wang L, Baser O, Kutikova L, et al. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: A systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23(11):3131-40. doi:10.1007/s00520-015-2686-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose singleadministration pegfilgrastim versus daily filgrastim in patients receiving myelosup-pressive chemotherapy. Ann Oncol. 2003;14(1):29-35. doi:10.1093/annonc/mdg019 [DOI] [PubMed] [Google Scholar]


