Abstract
BACKGROUND:
Prolonged, high-dose corticosteroid treatment for systemic lupus erythematosus (SLE) is associated with substantial health care costs, health care resource utilization (HCRU), and adverse events (AEs).
OBJECTIVE:
To compare all-cause health care costs, HCRU, and oral corticosteroid (OCS)–related AEs among patients with prevalent OCS use and patients without OCS use.
METHODS:
This retrospective, longitudinal cohort study (GSK study 214100) used claims data from the IQVIA Real-World Data Adjudicated Claims – US, IQVIA, Inc, database between January 1, 2006, and July 31, 2019, to identify patients with SLE. Patients with at least 1 OCS pharmacy claim during the study period and continuous OCS use during the 6-month pre-index (baseline) period (index date is the date of the first OCS claim following 6 months’ continuous use) formed the “prevalent OCS use cohort.” This cohort was subdivided based on the level of OCS exposure during the 12-month observation period, ie, the number of 6-month periods of greater than 5 mg/day OCS use (0, 1, or 2). Patients without OCS claims formed the “no OCS use cohort.” All patients had continuous enrollment during the baseline and observation periods, had at least 1 inpatient or at least 2 outpatient SLE diagnosis codes during baseline, and were aged at least 5 years at index. A 2-part model, a generalized linear regression model with a negative binomial distribution, and a multivariate logistic regression model were used to compare health care costs, HCRU, and the odds of developing an OCS-related AE between cohorts, respectively.
RESULTS:
The no OCS use and prevalent OCS use cohorts included 21,517 and 16,209 patients, respectively. Adjusted health care cost differences (95% CI) were significantly lower for the no OCS use cohort vs all prevalent OCS use exposure categories ($5,439 [$4,537-$6,371] vs $17,856 [$16,368-$19,498]), driven by inpatient stays and outpatient visits; HCRU was also significantly lower (adjusted incidence rate ratios vs no OCS use cohort [95% CI]: 1.20 [1.16-1.23] vs 1.47 [1.41-1.52]). Health care costs and HCRU increased with increasing length of OCS exposure. OCS-related AEs occurred more frequently for all prevalent OCS use exposure categories vs the no OCS use cohort (odds ratio [95% CI]: 1.39 [1.25-1.55] vs 2.32 [2.02-2.68]), driven by hematologic/oncologic and immune system–related AEs. The mean (SD) average daily dose of OCS increased with increasing periods of prevalent OCS use (2.5 [1.3], 6.9 [31.1], and 34.6 [1,717.3] mg/day, respectively, for patients with 0, 1, and 2 periods of OCS use).
CONCLUSIONS:
Prevalent OCS use incurs a substantial clinical and economic burden, highlighting the need for restricted OCS doses and durations.
Plain language summary
Steroids are often used in the treatment of lupus. In this study, patients with lupus who were treated with oral steroid medications had greater health care costs, greater use of health care resources, and more adverse events than patients not treated with steroids. This difference in clinical and economic burden is mostly due to an increased use of hospital facilities. These results support careful consideration of the risks and benefits of steroid treatments in lupus.
Implications for managed care pharmacy
The results of this study highlight the increased health care costs, health care resource utilization, and occurrence of oral corticosteroid (OCS)–related adverse events among patients with at least 6 months of OCS use. This burden increases with increasing duration of OCS use greater than 5 mg/day. An awareness of the risks of steroid treatment is essential to determine the most appropriate OCS dose and duration and ultimately improve the management of lupus and reduce the health care burden.
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disorder characterized by fluctuating periods of elevated disease activity (SLE flares) and reduced disease activity (remission).1 Manifestations of SLE vary widely, but they commonly affect the musculoskeletal, dermatologic, renal, neuropsychiatric, pulmonary, hematologic, and cardiovascular organ systems.2,3
Current treatment options in SLE include managing disease symptoms, reducing the frequency and severity of flares, and reducing the risk of organ damage.4,5 Corticosteroids are the cornerstone of SLE treatment because of their rapid and potent anti-inflammatory and immunosuppressive properties.6,7 In the United States, approximately 70% of patients with SLE will receive corticosteroids,8 with high doses frequently prescribed in response to flares.9
However, prolonged use of corticosteroids is associated with several adverse events (AEs). Although some of these AEs are potentially reversible (eg, type 2 diabetes mellitus and hypertension), others, including bone fractures caused by osteoporosis, avascular necrosis, and cataracts, represent irreversible damage.7,10 As a result, much of the organ damage observed in SLE can be attributed to corticosteroids, particularly following long-term use.11 The extent of corticosteroid-related organ damage is also dependent on the dose; data from the Hopkins Lupus Cohort demonstrated that each 1-mg increase in the average prednisone daily dose was associated with a 3% increase in the risk of developing new organ damage.12,13 Therefore, recommendations for SLE management aim to use the lowest possible prednisone-equivalent corticosteroid dose (<7.5 mg/day) and withdraw when possible.4
Additionally, corticosteroid use in SLE incurs a substantial economic burden,14-17 with health care costs and health care resource utilization (HCRU) increasing with increasing doses.15,16 The burden of long-term and short-term corticosteroid use in oral corticosteroid (OCS)–naive patients has previously been identified.16,18 However, an up-to-date and extensive analysis of the clinical and economic burden of prevalent (≥6 month) corticosteroid use in patients with SLE is lacking.
This study assessed the economic and clinical burden associated with prevalent OCS use in patients with SLE by evaluating health care costs among patients with at least 6 months of prednisone-equivalent OCS use greater than 5 mg/day compared with patients with no OCS use. Secondary objectives were to compare HCRU and the occurrence of OCS-related AEs in these cohorts and to describe treatment patterns.
Methods
STUDY DESIGN
This retrospective, longitudinal, observational study (GSK study 214100) utilized medical and pharmacy claims data from the IQVIA Real-World Data Adjudicated Claims – US, IQVIA, Inc, database from January 1, 2006, to July 31, 2019, to identify patients with SLE (Figure 1).
FIGURE 1.
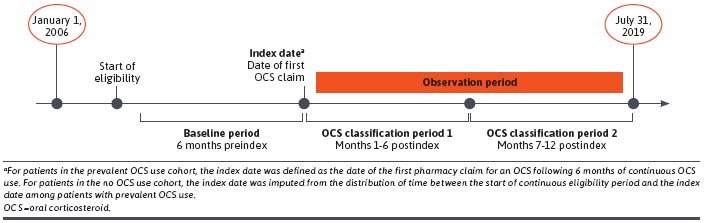
Study Design
Patients with SLE were categorized into 1 of 2 cohorts: the prevalent OCS use cohort and the no OCS use cohort. The index date of the prevalent OCS use cohort was defined as the date of the first OCS pharmacy claim following 6 months of continuous OCS use (no gaps in days supply >30 days); the index date of the no OCS use cohort was imputed based on the distribution of time between the start of the continuous eligibility period and the index date for the prevalent OCS use cohort. The baseline period was defined as the 6 months prior to the index date, and the observation period was defined as the 12 months following the index date.
ETHICAL APPROVAL AND INFORMED CONSENT
The IQVIA Real-World Data Adjudicated Claims – US, IQVIA, Inc, database is de-identified in compliance with the patient confidentiality requirement of the Health Insurance Portability and Accountability Act; therefore, no institutional review board or informed consent was required for this study.
STUDY POPULATION
Eligible patients were required to be aged at least 5 years at index with a diagnosis of SLE (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 710.0x or ICD-10-CM codes M32.0, M32.1x, M32.8, and M32.9; ≥2 outpatient medical claims or ≥1 inpatient/emergency department claim) during the baseline period and have continuous enrollment in a health plan for at least 6 months prior to index and at least 12 months following index.
Patients in the prevalent OCS use cohort were required to have at least 1 OCS pharmacy claim during the study period and continuous OCS use during the baseline period. The prevalent OCS use cohort was further categorized into 3 exposure categories based on the number of 6-month periods (0, 1, or 2) of average daily prednisone-equivalent OCS use greater than 5 mg/day during the observation period. Patients included in the no OCS use cohort were required to have no pharmacy claims for OCS at any time during the study period.
VARIABLES AND OUTCOMES
Patient demographic characteristics (sex, age at index, geographic region, health insurance type, and year of index), clinical characteristics (diagnosing physician specialty, Quan-Charlson comorbidity score, comorbidities [identified using ICD-9-CM and ICD-10-CM codes, Supplementary Table 1 (477.3KB, pdf) , available in online article], and concomitant medications commonly prescribed for SLE), and all-cause health care costs were captured during the baseline period.
SLE disease severity and the frequency/severity of flare episodes were assessed for each patient during the baseline period and the observation period and were classified in mutually exclusive categories as mild, moderate, or severe (definitions are provided in the footnotes of Table 1). Categories were determined using previously published health care utilization–based algorithms19-23 derived from the severity of diagnoses listed on administrative claims data using the ICD-9-CM diagnosis, Healthcare Common Procedure Coding System, and Current Procedural Terminology procedure codes. The disease severity algorithm combined elements of disease activity with cumulative damage and/or SLE medication utilization to determine severity. The flare algorithm was developed using definitions from the Lupus Foundation of America21 and criteria based on the British Isles Lupus Assessment Group index.24
TABLE 1.
Patient Baseline Demographics, Disease Characteristics, and Disease Severity for the Prevalent OCS Use Cohort Exposure Categories and the No OCS Use Cohort (N = 37,726)
| No OCS use (n = 21,517) | 0 periods of prevalent OCS use (n = 5,390) | 1 period of prevalent OCS use (n = 4,491) | 2 periods of prevalent OCS use (n = 6,328) | |
|---|---|---|---|---|
| Age, mean (SD), y | 49.4 (13.9) | 46.7 (13.7)a | 45.6 (13.9)a | 45.8 (13.6)a |
| Age category, n (%) | ||||
| 5-17 y | 368 (1.7) | 173 (3.2)a | 161 (3.6)a | 195 (3.1)a |
| ≥18 y | 21,149 (98.3) | 5,217 (96.8)a | 4,330 (96.4)a | 6,133 (96.9)a |
| Sex, n (%)b | ||||
| Female | 18,823 (87.5) | 4,801 (89.1)a | 3,925 (87.4)c | 5,468 (86.4)d |
| Male | 2,693 (12.5) | 589 (10.9)a | 566 (12.6) | 860 (13.6)d |
| Region, n (%) | ||||
| South | 6,590 (30.6) | 1,996 (37.0)a | 1,605 (35.7)a | 2,409 (38.1)a |
| Northeast | 5,514 (25.6) | 1,096 (20.3)a | 959 (21.4)a | 1,351 (21.3)a |
| Midwest | 5,048 (23.5) | 1,387 (25.7)a | 1,181 (26.3)a | 1,556 (24.6)c |
| West | 4,128 (19.2) | 864 (16.0)a | 704 (15.7)a | 947 (15.0)a |
| Unknown | 236 (1.1) | 47 (0.9)c | 42 (0.9)c | 65 (1.0)c |
| Insurance type, n (%)e | ||||
| Commercial | 12,591 (58.5) | 3,150 (58.4)c | 2,645 (58.9)c | 3,717 (58.7)c |
| Medicare | 596 (2.8) | 108 (2.0)a | 86 (1.9)a | 129 (2.0)a |
| Medicaid | 1,632 (7.6) | 366 (6.8)d | 310 (6.9)c | 462 (7.3)c |
| Self-insured | 6,585 (30.6) | 1,804 (33.5)a | 1,448 (32.2)d | 2,046 (32.3)a |
| Other/unknown | 204 (0.9) | 16 (0.3)a | 23 (0.5)a | 15 (0.2)a |
| Year of index date, n (%) | ||||
| 2006-2007 | 309 (1.4) | 728 (13.5)a | 607 (13.5)a | 1,121 (17.7)a |
| 2008-2009 | 3,621 (16.8) | 888 (16.5)c | 750 (16.7)c | 1,105 (17.5)c |
| 2010-2011 | 4,203 (19.5) | 824 (15.3)a | 694 (15.5)a | 976 (15.4)a |
| 2012-2013 | 3,911 (18.2) | 898 (16.7)a | 811 (18.1)c | 1,081 (17.1)d |
| 2014-2015 | 4,063 (18.9) | 1,145 (21.2)a | 906 (20.2)d | 1,119 (17.7)d |
| 2016-2017 | 4,609 (21.4) | 803 (14.9)a | 646 (14.4)a | 835 (13.2)a |
| 2018 | 801 (3.7) | 104 (1.9)a | 77 (1.7)a | 91 (1.4)a |
| Physician specialty, n (%)f | ||||
| Primary care physician | 2,358 (11.0) | 870 (16.1)a | 758 (16.9)a | 1,091 (17.2)a |
| Rheumatologist | 2,481 (11.5) | 1,383 (25.7)a | 1,103 (24.6)a | 1,501 (23.7)a |
| Quan-Charlson comorbidity index, mean (SD) | 0.9 (1.1) | 1.5 (1.1)a | 1.6 (1.2)a | 1.7 (1.3)a |
| Comorbidities, n (%)e,g | ||||
| Cardiovascular disease | 6,523 (30.3) | 2,054 (38.1)a | 1,932 (43.0)a | 2,915 (46.1)a |
| Hypertension | 5,936 (27.6) | 1,897 (35.2)a | 1,749 (38.9)a | 2,633 (41.6)a |
| Cerebrovascular disease | 773 (3.6) | 197 (3.7)c | 241 (5.4)a | 356 (5.6)a |
| Congestive heart failure | 532 (2.5) | 205 (3.8)a | 227 (5.1)a | 410 (6.5)a |
| Peripheral vascular disease | 589 (2.7) | 161 (3.0)c | 183 (4.1)a | 297 (4.7)a |
| Myocardial infarction | 187 (0.9) | 46 (0.9)c | 63 (1.4)a | 109 (1.7)a |
| Stroke | 201 (0.9) | 59 (1.1)c | 58 (1.3)d | 104 (1.6)a |
| Infections | 7,480 (34.8) | 2,012 (37.3)a | 1,858 (41.4)a | 2,691 (42.5)a |
| Immuno-inflammation–related | 1,876 (8.7) | 1,088 (20.2)a | 935 (20.8)a | 1,365 (21.6)a |
| Rheumatoid arthritis | 1,497 (7.0) | 975 (18.1)a | 819 (18.2)a | 1,175 (18.6)a |
| Thyroiditis | 275 (1.3) | 62 (1.2)c | 61 (1.4)c | 96 (1.5)c |
| Inflammatory bowel disease | 150 (0.7) | 77 (1.4)a | 92 (2.0)a | 153 (2.4)a |
| Renal disease | 1,130 (5.3) | 751 (13.9)a | 793 (17.7)a | 1,042 (16.5)a |
| Diabetes | 2,072 (9.6) | 489 (9.1)c | 472 (10.5)c | 832 (13.1)a |
| Osteoporosis | 1,108 (5.1) | 523 (9.7)a | 426 (9.5)a | 741 (11.7)a |
| Concomitant medications, n (%)e,h | ||||
| Antimalarials | 6,150 (28.6) | 3,567 (66.2)a | 2,879 (64.1)a | 3,846 (60.8)a |
| NSAIDs | 3,050 (14.2) | 1,177 (21.8)a | 1,068 (23.8)a | 1,598 (25.3)a |
| Immunosuppressants/biologics | 1,343 (6.2) | 2,489 (46.2)a | 2,374 (52.9)a | 3,315 (52.4)a |
| Costs, mean (SD), USD | ||||
| Total | 7,520 (23,406) | 13,668 (28,979)a | 18,992 (37,437)a | 22,761 (56,324)a |
| Medical | 6,242 (22,627) | 10,662 (27,268)a | 15,195 (35,430)a | 17,828 (44,690)a |
| Pharmacy | 1,278 (4,466) | 3,006 (7,044)a | 3,797 (8,758)a | 4,934 (22,355)a |
| Disease severity, n (%) | ||||
| Mildi | 17,208 (80.0) | 1,724 (32.0)a | 777 (17.3)a | 475 (7.5)a |
| Moderatej | 2,937 (13.6) | 2,943 (54.6)a | 2,797 (62.3)a | 4,423 (69.9)a |
| Severek | 1,372 (6.4) | 723 (13.4)a | 917 (20.4)a | 1,430 (22.6)a |
| SLE flares | ||||
| Patients with at least 1 SLE flare, n (%) | 10,017 (46.6) | 3,819 (70.9)a | 3,333 (74.2)a | 4,780 (75.5)a |
| Number of any SLE flares per patient, mean (SD) | 1.3 (0.6) | 1.5 (0.7)a | 1.6 (0.8)a | 1.6 (0.8)a |
| Patients with at least 1 mild SLE flare,l n (%) | 2,539 (11.8) | 1,091 (20.2)a | 939 (20.9)a | 1,411 (22.3)a |
| Number of mild SLE flares per patient, mean (SD) | 1.1 (0.3) | 1.1 (0.3)c | 1.1 (0.2)c | 1.1 (0.3)c |
| Patients with at least 1 moderate SLE flare,m n (%) | 8,346 (38.8) | 3,277 (60.8)a | 2,899 (64.6)a | 4,026 (63.6)a |
| Number of moderate SLE flares per patient, mean (SD) | 1.2 (0.4) | 1.3 (0.5)a | 1.4 (0.6)a | 1.4 (0.5)a |
| Patients with at least 1 severe SLE flare,n n (%) | 357 (1.7) | 225 (4.2)a | 314 (7.0)a | 615 (9.7)a |
| Number of severe SLE flares per patient, mean (SD) | 1.1 (0.4) | 1.1 (0.4)c | 1.2 (0.5)a | 1.2 (0.5)d |
| Patients with at least 1 moderate/severe SLE flare, n (%) | 8,524 (39.6) | 3,370 (62.5)a | 3,014 (67.1)a | 4,278 (67.6)a |
| Number of moderate/severe SLE flares per patient, mean (SD) | 1.2 (0.5) | 1.4 (0.6)a | 1.4 (0.6)a | 1.4 (0.7)a |
a P < 0.01 compared with the no OCS use cohort.
b One patient with unknown sex was excluded from subsequent multivariate analysis.
c P≥0.05 compared with the no OCS use cohort.
d P < 0.05 compared with the no OCS use cohort.
e Patients could have more than 1 value.
f Primary care included general practitioner/family practitioner, nurse practitioner, and internal medicine physician.
g Identified using the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification codes.
h Identified using Generic Product Identifier and Healthcare Common Procedure Coding System codes.
i Defined as eligible patients who did not meet the criteria for moderate or severe disease.
j Defined as eligible patients who did not meet the criteria for severe disease and had either at least 1 nonlaboratory claim with a diagnosis of any moderate conditions, in any position, or had at least 1 filled prescription for an OCS with a prednisone-equivalent dose of at least 7.5 mg/day and less than 60 mg/day or for an immunosuppressive agent (other than cyclophosphamide) during the baseline period.
k Defined as eligible patients who had at least 1 filled prescription for cyclophosphamide, rituximab, or OCS with a prednisone-equivalent dose of at least 60 mg/day during the baseline period, or those who had at least 1 nonlaboratory claim with a diagnosis of any severe conditions during the baseline period.
l Defined as the initiation of an antimalarial drug, an OCS with prednisone-equivalent dose of no more than 7.5 mg/day, or a nonimmunosuppressant drug (ie, NSAIDS or androgens) during the baseline period. Treatment was considered to be initiated if there were no filled prescriptions for that class of medication in the 60 days prior to the medication fill. The length of flare for each episode was set to 30 days. However, if a flare of higher severity (moderate or severe) occurred during those 30 days, the length of the flare was limited to the time between the start of the mild flare and the start of the higher-severity flare.
m Defined as the initiation of an OCS with a prednisone-equivalent dose of greater than 7.5 mg/day but no greater than 40 mg/day (if the patient had a prior fill within 60 days, treatment was considered initiated if the prior fill was for a prednisone-equivalent dose no more than 7.5 mg/day); an immunosuppressant drug (with the exception of cyclophosphamide); or a claim for an emergency department visit with a primary diagnosis of SLE with no inpatient admission within 1 day or a claim for an emergency department or office visit with a primary or secondary diagnosis for a specified SLE-related condition (if the diagnosis occurred during an office visit, the condition was required to be new, which was defined as no claims with this diagnosis during the previous 60 days) during the baseline period. The length of flare for each episode was set to 30 days; however, if a severe flare occurred during those 30 days, the length of the flare was limited to the time between the start of the moderate flare and the start of the severe flare.
n Defined as the initiation of an OCS with a prednisone-equivalent dose of greater than 40 mg/day (if the patient had a prior fill within 60 days, treatment was considered initiated if the prior fill was for a prednisone-equivalent dose of no more than 40 mg/day), cyclophosphamide (each prescription was counted as a new prescription if the prior fill was more than 100 days earlier), or admission for an inpatient hospital stay with a primary diagnosis of SLE or for a specified SLE-related condition during the baseline period. For flares based upon a hospitalization, the start date of the flare was the date that the patient was admitted to the hospital, unless the patient was admitted to the emergency department (with any diagnosis) during the previous day. If patients had an emergency department admission the day prior to the hospitalization, the date of the emergency department admission was considered to be the start date of the flare (note that only visits to an emergency department site were considered under this definition, and visits to urgent care or outpatient clinics were not included).
NSAID = nonsteroidal anti-inflammatory drug; OCS = oral corticosteroid; SLE = systemic lupus erythematosus; USD = US dollars; y = year.
Pharmacy claims were used to calculate the prednisone-equivalent OCS dose (see the Supplementary Material (477.3KB, pdf) for the list of OCS medications) during the observation period, for which the average daily dose (ADD; observation period) was based on the total number of days in the observation period and was calculated as follows:

For the sensitivity analysis, ADD (days supply) was defined based on the number of days of supply of OCS prescribed in the observation period and was calculated as follows:

All-cause health care costs (total [medical and pharmacy], medical [outpatient, inpatient, emergency department visits, and other encounters] and pharmacy costs) were reported during the baseline and observation periods.
All-cause and specific categories of HCRU were reported for the baseline and observation periods, including inpatient stays, emergency department visits, outpatient visits, and other encounters (including ambulance, assisted living facilities, comprehensive rehabilitation facilities, custodial care facilities, hospice/home care services, intermediate care facilities, psychiatric facilities, and skilled nursing facilities).
Incidence of OCS-related AEs (cardiovascular, metabolic and endocrine, central nervous system, bone and muscle, infections, ophthalmologic, gastrointestinal, dermatologic, and hematologic/oncologic) were reported during the observation period. Corticosteroid-related AEs were included based on package inserts for commonly prescribed corticosteroids or systematic literature searches.14,25
OCS treatment patterns were reported during each exposure category of the observation period. Treatment patterns included the cumulative OCS dose, ADD, and number of OCS claims per patient per 6 months.
STATISTICAL ANALYSIS
Patient demographic and clinical characteristics were summarized using means and SDs for continuous variables and relative frequencies and proportions for categorical variables. Comparisons between the prevalent OCS use cohort and the no OCS use cohort during the baseline period used chi-square tests and Wilcoxon rank sum tests for categorical and continuous variables, respectively.
All-cause, and individual medical and pharmacy health care costs were reported in US dollars and adjusted for inflation using the 2018 US Medical Care Consumer Price Index. Adjusted mean differences in health care costs for the prevalent OCS use cohort exposure categories compared with the no OCS use cohort were estimated using a 2-part model,26,27 adjusting for baseline covariates including sex, age on index date, geographic region, total health care costs, Quan-Charlson Comorbidity Index score, SLE medications (nonsteroidal anti-inflammatory drugs, antimalarials, immunosuppressants, and biologics), disease severity, flares, and OCS ADD. This approach included fitting a logistic regression model for the probability of observing a positive cost and fitting a generalized linear regression model, with a γ distribution and log link, among patients who incurred health care costs during the observation period. Nonparametric bootstrap procedures with 999 replications were applied to determine 95% CIs and P values. Sensitivity analyses for the association between OCS use and all-cause health care costs were carried out using alternative definitions for OCS use: ADD (days supply) instead of ADD (observation period) and a 7.5 mg/day instead of 5 mg/day OCS dose threshold.
HCRU comparisons between the exposure categories of the prevalent OCS use cohort and the no OCS use cohort used adjusted incidence rate ratios (IRRs), estimated using a generalized linear regression model with a negative binomial distribution to account for overdispersion.
Adjusted odds ratios (ORs) were estimated using a multivariate logistic regression model to compare the odds of developing an OCS-related AE between the prevalent OCS use cohort and the no OCS use cohort.
ADD analyses were stratified by age group (5-17 years and ≥18 years) and Wilcoxon rank sum tests used for statistical comparisons between age groups.
Results
BASELINE DEMOGRAPHICS AND DISEASE CHARACTERISTICS
Of the 399,000 patients with at least 1 SLE claim identified, 16,209 were included in the prevalent OCS use cohort and 21,517 in the no OCS use cohort (Supplementary Figure 1 (477.3KB, pdf) ).
During the observation period, 33.3%, 27.7%, and 39.0% of patients in the prevalent OCS cohort had 0, 1, and 2 6-month periods of OCS use greater than 5 mg/day, respectively.
Most patients were female (86.4%-89.1%). Compared with the prevalent OCS use cohort, patients in the no OCS use cohort were older, with a mean (SD) age of 49.4 (13.9) years vs 46.7 (13.7) years, 45.6 (13.9) years, and 45.8 (13.6) years for the prevalent OCS use cohort with 0, 1, and 2 periods of OCS use greater than 5 mg/day, respectively. The no OCS use cohort had lower mean (SD) Quan-Charlson comorbidity index scores, with 0.9 (1.1) vs 1.5 (1.1), 1.6 (1.2), and 1.7 (1.3) for the prevalent OCS use cohort with 0, 1, and 2 periods of OCS use greater than 5 mg/day, respectively. The no OCS use cohort had fewer concomitant medications dispensed vs the prevalent OCS use cohort with 0, 1, and 2 periods of OCS use greater than 5 mg/day (antimalarials: 28.6% vs 66.2%, 64.1%, and 60.8%; nonsteroidal anti-inflammatory drugs: 14.2% vs 21.8%, 23.8%, and 25.3%; and immunosuppressants/biologics: 6.2% vs 46.2%, 52.9%, and 52.4%; all P < 0.01; Table 1).
Fewer patients in the no OCS use cohort were classified as having moderate or severe SLE during the baseline period vs patients with 0, 1, and 2 periods of prevalent OCS use greater than 5 mg/day (20.0% vs 68.0%, 82.7%, and 92.5% respectively; all P < 0.01). Fewer patients in the no OCS use cohort also experienced at least 1 flare during the baseline period vs the prevalent OCS use cohort with 0, 1, and 2 periods of OCS use greater than 5 mg/day (46.6% vs 70.9%, 74.2%, and 75.5% respectively; all P < 0.01) and across all flare severities (mild: 11.8% vs 20.2%, 20.9%, and 22.3%; moderate: 38.8% vs 60.8%, 64.6%, and 63.6%; and severe: 1.7% vs 4.2%, 7.0%, and 9.7%; all P < 0.01; Table 1).
Baseline all-cause health care costs in the no OCS use cohort were lower than for patients with 0, 1, and 2 periods of prevalent OCS use greater than 5 mg/day ($7,520 vs $13,668, $18,992, and $22,761, respectively; all P < 0.01), driven by total medical costs in both cohorts (Table 1).
CLINICAL AND ECONOMIC OUTCOMES DURING THE 12-MONTH OBSERVATION PERIOD
Disease Severity and Flares. During the observation period, 25.8% of patients in the no OCS use cohort were classified as having moderate or severe SLE, vs most patients in the prevalent OCS use cohort with 0, 1, and 2 periods of OCS use greater than 5 mg/day (74.0%, 92.3%, and 97.5% respectively, all P < 0.01). Fewer patients in the no OCS use cohort experienced at least 1 flare in the observation period (60.8%) vs patients with 0, 1, and 2 periods of prevalent OCS use >5 mg/day (89.2%, 94.1%, and 94.9% respectively; all P < 0.01) and had a lower flare severity (Supplementary Table 2 (477.3KB, pdf) ).
Health Care Costs. Unadjusted all-cause mean (SD) health care costs for all prevalent OCS use cohort exposure categories were higher than those in the no OCS use cohort: no OCS use cohort, $12,109 (22,821); 0 periods of OCS use, $23,265 (36,526); 1 period of OCS use, $34,799 (54,405); and 2 periods of OCS use, $45,486 (65,374; Figure 2).
FIGURE 2.
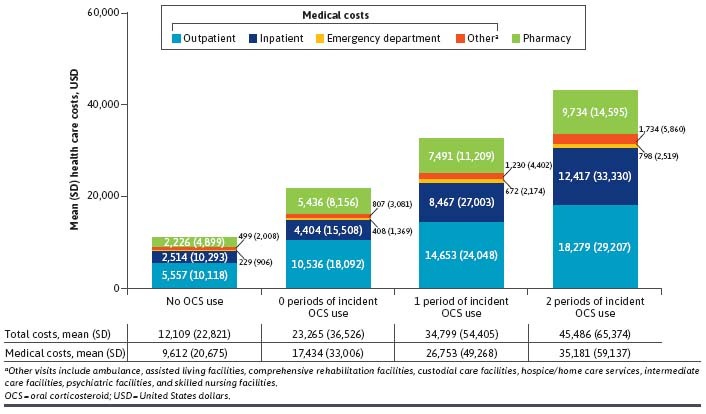
Health Care Cost Differences Between the Prevalent OCS Use (>5 mg/day) Exposure Categories and the No OCS Use Cohort (N = 37,725)
Baseline covariate-adjusted all-cause costs were significantly higher in all prevalent OCS use exposure categories than for the no OCS use cohort. Adjusted cost differences (95% CI) vs the no OCS use cohort were $5,439 ($4,537-$6,371), $11,830 ($10,321-$13,282), and $17,856 ($16,368-$19,498) for patients with 0, 1, and 2 periods of prevalent OCS use, respectively (all P < 0.01, except “other” costs for patients with 0 periods of prevalent OCS use [P = 0.02]). Costs were driven by inpatient stays and outpatient visits (Figure 3).
FIGURE 3.
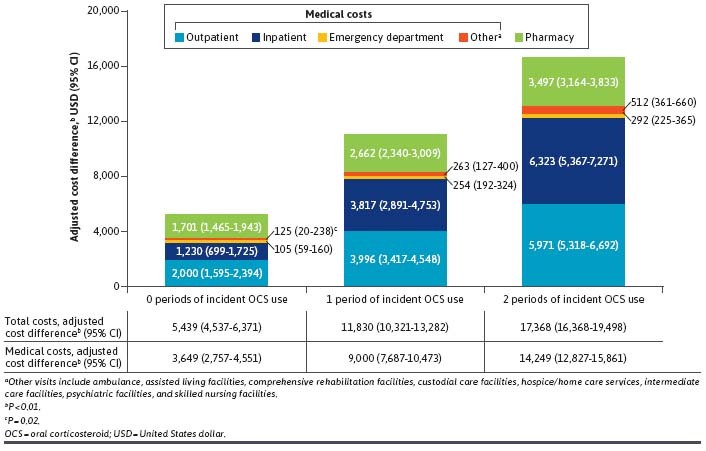
Adjusted Health Care Cost Differences Between the Prevalent OCS Use (>5 mg/day) Exposure Categories and the No OCS Use Cohort (N = 37,725)
Similar results were observed when using alternative definitions for OCS use. A higher threshold of greater than 7.5 mg/day resulted in larger adjusted cost differences (95% CI) vs the no OCS use cohort of $7,033 ($6,187-$7,907), $16,231 ($14,438-$18,184), and $23,020 ($20,632-$25,305) for 0, 1, and 2 periods of prevalent OCS use, respectively (all P < 0.01; Supplementary Table 3 (477.3KB, pdf) ). Defining ADD by days supply resulted in similar adjusted cost differences (95% CI) vs the no OCS use cohort of $5,347 ($4,425-$6,342), $10,179 ($8,845-$11,480), and $17,488 ($16,011-$19,030) for 0, 1, and 2 periods of prevalent OCS use, respectively (all P < 0.01).
HCRU. Over the observation period, most patients in both cohorts had at least 1 health care visit (prevalent OCS use cohort: ≥99.6%; no OCS use cohort: 93.0%; Table 2). After adjusting for baseline covariates, incidence rates per patient per year were significantly higher for each of the prevalent OCS use exposure categories vs the no OCS use cohort for all-cause, outpatient, inpatient stays, and emergency room visits. Adjusted IRRs (95% CI) vs the no OCS use cohort across any HCRU visit were 1.20 (1.16-1.23), 1.33 (1.29-1.38), 1.47 (1.41-1.52) for exposure categories with 0, 1, and 2 periods of prevalent OCS use greater than 5 mg/day, respectively (all P < 0.01; Table 2). A similar trend was observed for all types of HCRU visits, with the largest IRR observed for inpatients stays among patients with 2 periods of prevalent OCS use greater than 5 mg/day (adjusted IRR [95% CI] = 1.28 [1.16-1.42], 1.85 [1.67-2.05], and 2.45 [2.22-2.71] for 0, 1, and 2 periods of prevalent OCS use, respectively [all P < 0.01]).
TABLE 2.
Incidence Rates of HCRU for the Prevalent OCS Use Cohort Exposure Categories and the No OCS Use Cohort, Over the 12-Month Observation Period (N = 37,725)
| All-cause HCRU, n (%) | Incidence rate per person-year | Adjusted IRR (95% CI) vs the no OCS use cohort | P value | |
|---|---|---|---|---|
| No OCS use (n = 21,516) | ||||
| Any visits | 20,013 (93.0) | 20.57 | — | — |
| Outpatient | 19,898 (92.5) | 16.63 | — | — |
| Inpatient | 2,109 (9.8) | 0.15 | — | — |
| Emergency department | 3,703 (17.2) | 0.35 | — | — |
| Othera | 4,714 (21.9) | 3.46 | — | — |
| 0 periods of OCS use (n = 5,390) | ||||
| Any visits | 5,372 (99.7) | 29.59 | 1.20 (1.16-1.23) | <0.01 |
| Outpatient | 5,362 (99.5) | 24.52 | 1.24 (1.21-1.28) | <0.01 |
| Inpatient | 764 (14.2) | 0.22 | 1.28 (1.16-1.42) | <0.01 |
| Emergency department | 1,283 (23.8) | 0.53 | 1.37 (1.25-1.50) | <0.01 |
| Othera | 1,396 (25.9) | 4.31 | 0.97 (0.86-1.09) | 0.63 |
| 1 period of OCS use (n = 4,491) | ||||
| Any visits | 4,473 (99.6) | 36.88 | 1.33 (1.29-1.38) | <0.01 |
| Outpatient | 4,468 (99.5) | 29.19 | 1.36 (1.31-1.41) | <0.01 |
| Inpatient | 956 (21.3) | 0.38 | 1.85 (1.67-2.05) | <0.01 |
| Emergency department | 1,286 (28.6) | 0.77 | 1.67 (1.51-1.85) | <0.01 |
| Othera | 1,444 (32.2) | 6.54 | 1.14 (0.99-1.32) | 0.06 |
| 2 periods of OCS use (n = 6,328) | ||||
| Any visits | 6,319 (99.9) | 42.55 | 1.47 (1.41-1.52) | <0.01 |
| Outpatient | 6,313 (99.8) | 32.82 | 1.49 (1.44-1.54) | <0.01 |
| Inpatient | 1,798 (28.4) | 0.52 | 2.45 (2.22-2.71) | <0.01 |
| Emergency room | 1,948 (30.8) | 0.92 | 1.83 (1.66-2.03) | <0.01 |
| Othera | 2,291 (36.2) | 8.29 | 1.34 (1.17-1.54) | <0.01 |
a Including ambulance, assisted living facilities, comprehensive rehabilitation facilities, custodial care facilities, hospice/home care services, intermediate care facilities, psychiatric facilities, and skilled nursing facilities.
HCRU = health care resource utilization; IRR = incidence rate ratio; OCS = oral corticosteroid.
ODDS OF EXPERIENCING OCS-RELATED AES
Most patients in the prevalent OCS use cohort (85.7%-91.9%) and the no OCS use cohort (75.3%) experienced at least 1 OCS-related AE during the observation period (Supplementary Table 4 (477.3KB, pdf) ). The proportion experiencing OCS-related AEs increased with increasing periods of prevalent OCS use greater than 5 mg/day. The odds of experiencing any OCS-related AE were significantly higher in each of the prevalent OCS use exposure categories compared with the no OCS use cohort (OR [95% CI] = 1.39 [1.25-1.55], 1.80 [1.57-2.06], and 2.32 [2.02-2.68]; all P < 0.01 for patients with 0, 1, and 2 periods of OCS use >5 mg/day, respectively).
Patients with 2 periods of prevalent OCS use greater than 5 mg/day were significantly more likely to develop bone and muscle, immune system–related, central nervous system, metabolic and endocrine, cardiovascular, gastrointestinal, ophthalmologic, and hematologic/oncologic AEs compared with patients with no OCS use (all P < 0.01). The greatest increases in the odds of experiencing an AE were in the hematologic/oncologic (OR [95% CI] = 1.32 [1.06-1.64], 1.78 [1.43-2.22], and 2.53 [2.05-3.11]) and immune system–related (OR [95% CI] = 1.40 [1.29-1.52], 1.65 [1.51-1.81], and 2.02 [1.85-2.21]) categories for patients with 0, 1, and 2 periods of prevalent OCS use greater than 5 mg/day, respectively, vs the no OCS use cohort (all P < 0.01; Supplementary Table 4 (477.3KB, pdf) ).
OCS TREATMENT PATTERNS
Cumulative OCS Dose. The cumulative dose of OCS per 6-month period increased with the number of periods of prevalent OCS use greater than 5 mg/day. Patients with 0, 1, and 2 periods of prevalent OCS use had cumulative mean (SD) doses of 464.9 (241.7), 1,257.6 (5,689.9), and 6,323.5 (314,259.4) mg/6-month period, respectively.
ADD. The mean (SD) ADD (observation period) of OCS increased with the number of periods of prevalent OCS use greater than 5 mg/day (2.5 [1.3] mg/day, 6.9 [31.1] mg/day, and 34.6 [1,717.3] mg/day, for patients with 0, 1, and 2 periods of prevalent OCS use, respectively; Supplementary Figure 2 (477.3KB, pdf) ). A similar trend was observed using ADD (days supply; Supplementary Figure 2 (477.3KB, pdf) ).
Patients in the aged younger than 18 years group had a significantly higher mean (SD) ADD (observation period) than patients in the aged 5-17 years group (0 periods of prevalent OCS use: 2.6 [1.3] mg/day vs 2.2 [1.3] mg/day; 1 period of prevalent OCS use: 6.9 [31.7] mg/day vs 6.8 [3.4] mg/day; 2 periods of prevalent OCS use: 35.2 [1,744.4] mg/day vs 14.7 [12.6] mg/day; all P < 0.01).
The number of OCS claims per patient per 6-month period increased with the number of periods of prevalent OCS use greater than 5 mg/day. Mean (SD) OCS claims per patient were 3.4 (1.7), 4.1 (1.8), and 5.2 (2.1) for patients with 0, 1, and 2 periods of prevalent OCS use, respectively.
Discussion
Findings from this large population-based cohort revealed that, compared with patients with no OCS use, patients with prevalent (≥6 months) OCS use had significantly higher health care costs, HCRU, and odds of experiencing OCS-related AEs in the following 12 months of observation. These differences increased with increasing periods of prevalent OCS use, with the greatest costs, HCRU, and odds of experiencing OCS-related AEs observed for patients with 2 periods of OCS use greater than 5 mg/day. Outpatient and inpatient costs accounted for approximately 40%-46% and 19%-28% of costs in patients with prevalent and no OCS use, respectively. These findings remained robust following adjustment for baseline covariates, including disease severity, concomitant medication, and flares, as well as in the sensitivity analyses using alternative definitions for OCS use.
The substantial medical costs and HCRU incurred by patients with OCS use has previously been characterized.15-17 Two claims-based analyses in the United States demonstrated an association between increasing corticosteroid dose with increasing HCRU and health care costs;15,16 low doses of corticosteroid (≤5 or ≤7.5 mg/day) incurred 1.4 times the costs of patients with no OCS use, whereas higher doses (>15 mg/day and >20 mg/day) incurred 2.8 times and 3.6 times the costs of patients with no OCS use. Similar to the present study, inpatient, emergency department, and outpatient visits were greater in patients with corticosteroid use than those without corticosteroid use, with costs largely driven by hospitalizations.15,16
Two recent studies conducted using the IQVIA Real-World Data Adjucated Claims – US, IQVIA, Inc, database showed that patients with established OCS use (≥12 months of continuous OCS use) or newly initiated OCS use experience a significantly greater clinical and economic burden than patients with no OCS use; adjusted cost differences (95% CI) following 2 periods of OCS use greater than 5 mg/day were $30,119 ($26,492-$33,774) and $28,985 ($25,546-$32,885), respectively (all P < 0.01).18,28 Although these results cannot be directly compared because of differences in study cohorts, the current study extends these findings by demonstrating that patients with prevalent OCS use similarly incur substantially greater health care costs than patients with no OCS use, with an adjusted cost difference (95% CI) following 2 periods of OCS use greater than 5 mg/day of $17,856 ($16,368-$19,498).
The odds of experiencing an OCS-related AE were greater in patients with prevalent OCS use vs in patients with no OCS use across most organ domain–level categories explored. This may partly explain the increased costs and HCRU observed in the current study, as corticosteroid-related AEs contribute substantially to annual health care costs. One claims-based analysis reported annual costs for managing chronic corticosteroid-related AEs to be around $2,400-$9,800, with type 2 diabetes and hypertension incurring the highest costs ($9,764 and $8,773, respectively).14
This study further demonstrates that the odds of experiencing an OCS-related AE increase with the number of periods of prevalent OCS use greater than 5 mg/day. A recent 12-year longitudinal study similarly found this relationship to be associated with corticosteroid dose and intensity,29 particularly for osteonecrosis and osteoporosis, with such damage being detected as early as 1 month following high-dose corticosteroid initiation.29-31 This is consistent with the current study, as even patients with only 1 period of prevalent OCS use had significantly higher odds of experiencing OCS-related bone and muscle AEs than patients with no OCS use.
A key goal for SLE treatment outlined by the British Society for Rheumatology and the European Alliance of Associations for Rheumatology is to prevent flares at the lowest possible dose of corticosteroids.4,5 Despite this, in the current study 66.7% of patients with OCS use had at least 1 period of prevalent OCS use greater than 5 mg/day during the observation period, with a mean ADD of 6.9 and 34.6 mg/day for patients with 1 and 2 periods of prevalent OCS use greater than 5 mg/day, respectively. This is consistent with previous data demonstrating that 50.5% of all adult patients with SLE received OCS, of whom 23% were prescribed greater than 7.5-15 mg/day and almost 60% were prescribed at least 15 mg/day of OCS.8 These results highlight the continued reliance on moderate-to-high–dose corticosteroids, despite clinical recommendations.
By investigating alternative definitions for ADD, prescription patterns among patients with SLE were further elucidated. Of note, calculated values for ADD (days supply) were higher than ADD (observation period), particularly for patients with 2 periods of prevalent OCS use greater than 5 mg/day. This may indicate patients receiving more frequent bursts of high-dose OCS rather than consistently high doses of OCS.
Strengths of this study include the identification of a large cohort of patients with SLE who had prevalent or no OCS use during a 13-year period. Associations between prevalent OCS use and health care costs and HCRU were strong and remained robust even after adjusting for baseline characteristics and using different definitions for OCS daily dose.
LIMITATIONS
Several limitations should be considered. First, the study utilized medical and pharmacy claims sourced from the IQVIA Real-World Data Adjudicated Claims – US, IQVIA, Inc, database, which was primarily developed for health service and payment purposes; therefore, confounders such as ethnicity, clinical biomarkers, and measures of disease activity (eg, the Systemic Lupus Erythematosus Disease Activity Index) were unavailable. This study attempted to control for confounding by indication bias to a feasible extent by adjusting for disease severity and frequency/severity of flares during the baseline period. Results indicate that increased OCS utilization, rather than OCS use due to more severe disease, is associated with increased HCRU, health care costs, and risk of OCS-related AEs. However, the possibility of residual confounding bias should be considered given the use of claims data, which are not intended for research purposes. Second, the cost and HCRU data were limited to medical services captured within the claims database and could not account for records or patient practices (eg, over the counter medications) not captured in the database. As such, patients with dual coverage or supplemental health insurance may not have their costs and HCRU accurately captured, and there may be missing data or misclassification in the calculation of drug dose and duration. Third, the study population was limited to patients with prevalent OCS use with prespecified continuous eligibility; therefore, the results may not be generalizable to patients without consistent health care access or patients who received care outside of a managed care population (eg, Medicare or Medicaid). Finally, as the study was observational, interpretation of a causal relationship between OCS use and the risk of OCS-related AEs, as well as determining whether the AEs were truly corticosteroid emergent, was not possible.
Conclusions
This study highlights that among patients with SLE with prevalent OCS use, the economic burden associated with OCS treatment is substantial and increases with increasing length of exposure. The use of effective and safe treatments that allow for a reduction of OCS dose and duration may lower rates of HCRU and AEs and improve the overall management of disease and reduce health care burden.
ACKNOWLEDGMENTS
Medical writing support and submission support was provided by Olivia Hill, MPharmacol, of Fishawack Indica, Ltd, UK, part of Fishawack Health, and was funded by GSK.
REFERENCES
- 1.Gyori N, Giannakou I, Chatzidionysiou K, Magder L, van Vollenhoven RF, Petri M. Disease activity patterns over time in patients with SLE: Analysis of the Hopkins Lupus Cohort. Lupus Sci Med. 2017;4(1):e000192. doi:10.1136/lupus-2016-000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica (Bucur). 2011;6(4):330-6. [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman MM, Moniruzzan M, Sayeed JB, et al. 239 Patterns of organ involvement in SLE and their outcome: A real life experience in a lupus clinic. Lupus Sci Med. 2019;6(Suppl 1). doi:10.1136/lupus-2019-lsm.239 [Google Scholar]
- 4.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019. update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736-45. doi:10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 5.Gordon C, Amissah-Arthur MB, Gayed M, et al. ; British Society for Rheumatology Standards; Audit and Guidelines Working Group. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford). 2018;57(1):e1-e45. doi:10.1093/rheumatology/kex286 [DOI] [PubMed] [Google Scholar]
- 6.Sciascia S, Mompean E, Radin M, Roccatello D, Cuadrado MJ. Rate of adverse effects of medium- to high-dose glucocorticoid therapy in systemic lupus erythematosus: A systematic review of randomized control trials. Clin Drug Investig. 2017;37(6):519-24. doi:10.1007/s40261-017-0518-z [DOI] [PubMed] [Google Scholar]
- 7.Thamer M, Hernan MA, Zhang Y, Cotter D, Petri M. Prednisone, lupus activity, and permanent organ damage. J Rheumatol. 2009;36(3):560-4. doi:10.3899/jrheum.080828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birt JA, Wu J, Griffing K, et al. Corticosteroid dosing and opioid use are high in patients with SLE and remain elevated after belimumab initiation: A retrospective claims database analysis. Lupus Sci Med. 2020;7(1):e000435. doi:10.1136/lupus-2020-000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond ER, Desta B, Near AM, Wang X, Jiang M. Frequency, severity and costs of flares increase with disease severity in newly diagnosed systemic lupus erythematosus: A real-world cohort study, United States, 2004-2015. Lupus Sci Med. 2021;8(1):e000504. doi:10.1136/lupus-2021-000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson JE, Fu Q, Rao S, Magder LS, Petri M. Quantifying the burden of steroid-related damage in SLE in the Hopkins Lupus Cohort. Lupus Sci Med. 2018;5(1):e000237. doi:10.1136/lupus-2017-000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladman DD, Urowitz MB, Rahman P, Ibanez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30(9):1955-9. [PubMed] [Google Scholar]
- 12.Al Sawah S, Zhang X, Zhu B, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med. 2015;2(1):e000066. doi:10.1136/lupus-2014-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I, Medina JA, Moran MA, Ruiz-Irastorza G. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2014;53(8):1470-6. doi:10.1093/rheumatology/keu148 [DOI] [PubMed] [Google Scholar]
- 14.Shah M, Chaudhari S, McLaughlin TP, et al. Cumulative burden of oral corticosteroid adverse effects and the economic implications of corticosteroid use in patients with systemic lupus erythematosus. Clin Ther. 2013;35(4):486-97. doi:10.1016/j.clinthera.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Chen SY, Choi CB, Li Q, et al. Glucocorticoid use in patients with systemic lupus erythematosus: Association between dose and health care utilization and costs. Arthritis Care Res (Hoboken). 2015;67(8):1086-94. doi:10.1002/acr.22574 [DOI] [PubMed] [Google Scholar]
- 16.Kabadi S, Yeaw J, Bacani AK, et al. Healthcare resource utilization and costs associated with long-term corticosteroid exposure in patients with systemic lupus erythematosus. Lupus. 2018;27(11):1799-809. doi:10.1177/0961203318790675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petri M, Bechtel B, Dennis G, et al. Burden of corticosteroid use in patients with systemic lupus erythematosus: Results from a Delphi panel. Lupus. 2014;23(10):1006-13. doi:10.1177/0961203314532699 [DOI] [PubMed] [Google Scholar]
- 18.Wang M, DerSarkissian M, Duh MS, et al. Clinical and economic burden associated with new oral corticosteroid users with systemic lupus erythematosus in the United States. J Manag Care Spec Pharm. 2021;27(4):S1-152. [Google Scholar]
- 19.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667-77. doi:10.3111/13696998.2013.778270 [DOI] [PubMed] [Google Scholar]
- 20.Narayanan S, Wilson K, Ogelsby A, Juneau P, Durden E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J Occup Environ Med. 2013;55(11):1262-70. doi:10.1097/JOM.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 21.Ruperto N, Hanrahan LM, Alarcon GS, et al. ; Lupus Foundation of America, Inc. International Flare Consensus Initiative. International consensus for a definition of disease flare in lupus. Lupus. 2011;20(5):453-62. doi:10.1177/0961203310388445 [DOI] [PubMed] [Google Scholar]
- 22.Murimi-Worstell IB, Lin DH, Kan H, et al. Healthcare utilization and costs of systemic lupus erythematosus by disease severity in the United States. J Rheumatol. 2021;48(3):385-93. doi:10.3899/jrheum.191187 [DOI] [PubMed] [Google Scholar]
- 23.Speyer CB, Li D, Guan H, et al. Comparison of an administrative algorithm for SLE disease severity to clinical SLE Disease Activity Index scores. Rheumatol Int. 2020;40(2):257-61. doi:10.1007/s00296-019-04477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon C, Sutcliffe N, Skan J, Stoll T, Isenberg DA. Definition and treatment of lupus flares measured by the BILAG index. Rheumatology (Oxford). 2003;42(11):1372-9. doi:10.1093/rheumatology/keg382 [DOI] [PubMed] [Google Scholar]
- 25.Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: A systematic literature review. Clin Ther. 2011;33(10):1413-32. doi:10.1016/j.clinthera.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 26.Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY. Methods for analyzing health care utilization and costs. Annu Rev Public Health. 1999;20:125-44. doi:10.1146/annurev.publhealth.20.1.125 [DOI] [PubMed] [Google Scholar]
- 27.Honeycutt AA, Segel JE, Hoerger TJ, Finkelstein EA. Comparing cost-of-illness estimates from alternative approaches: An application to diabetes. Health Serv Res. 2009;44(1):303-20. doi:10.1111/j.1475-6773.2008.00909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S, Wang M, DerSarkissian M, et al. PSY26 healthcare costs, resource utilization, and adverse events associated with long-term oral corticosteroid use in patients with systemic lupus erythematosus in the United States. Value Health. 2021;24(Supplement 1):S233. doi:10.1016/j.jval.2021.04.1164 [Google Scholar]
- 29.Chen HL, Shen LJ, Hsu PN, Shen CY, Hall SA, Hsiao FY. Cumulative burden of glucocorticoid-related adverse events in patients with systemic lupus erythematosus: Findings from a 12-year longitudinal study. J Rheumatol. 2018;45(1):83-9. doi:10.3899/jrheum.160214 [DOI] [PubMed] [Google Scholar]
- 30.Oinuma K, Harada Y, Nawata Y, et al. Osteonecrosis in patients with systemic lupus erythematosus develops very early after starting high dose corticosteroid treatment. Ann Rheum Dis. 2001;60(12):1145-8. doi:10.1136/ard.60.12.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-27. doi:10.1080/03007995.2018.1474090 [DOI] [PubMed] [Google Scholar]


