Abstract
As high-cost and innovative therapies continue to enter the market, health care decision makers (HCDMs) are expressing a need for early information on a product’s clinical and economic impacts. Preapproval information exchange (PIE) fulfills these data needs by allowing manufacturers to share drug information with HCDMs prior to US Food and Drug Administration approval. With recent regulatory milestones, such as the Pre-approval Information Exchange Act of 2022, HCDMs look to leverage PIE to forecast budgets and inform reimbursement decisions. However, a lack of stakeholder alignment has challenged the evolving applications of PIE. In addition, manufacturers are still seeking regulatory clarity regarding best practices, and many are developing their own policies for content dissemination. Varying practices have led to heterogeneity of preapproval communications across manufacturers, which may not fully align with HCDM needs and interests. However, recently collected survey data from FormularyDecisions, which focused on HCDM perceptions of both PIE webinars and other formats of PIE (eg, PIE decks and dossiers), indicate that HCDMs have strong preferences regarding the timing and content shared in preapproval engagements. Additionally, product indication and clinical trial information are highly valued, and although desired by HCDMs in other studies, in FormularyDecisions survey data, exact pricing data do not currently appear to be a critical component of PIE. Preapproval communications are expected less than 1 year before anticipated product approval, and PIE webinars, specifically, should prioritize therapeutic areas and products anticipated to have a significant impact on organizational budgets. Although HCDMs prefer nonmanufacturer representatives for PIE webinars and virtual presentations, health outcome liaisons or medical science liaisons are ideal among manufacturer representatives for in-person preapproval engagements. The expectations of HCDMs should be considered as manufacturers establish PIE practices to ensure the exchange of quality and relevant information.
Plain language summary
Preapproval information exchange (PIE) is the delivery of drug information from manufacturers to health care decision makers (HCDMs) before US Food and Drug Administration (FDA) approval. PIE allows HCDMs to plan for future drug approvals and manufacturers to raise awareness about new products. Preapproval communications have increased in recent years and are highly valued by HCDMs. Highlighting what data HCDMs need to plan for new drug approvals can help manufacturers provide the most relevant information.
Implications for managed care pharmacy
PIE has become increasingly important for HCDMs and manufacturers, as it provides an opportunity for the bidirectional exchange of clinical and economic information of therapies prior to FDA approval. By characterizing the key HCDM perspectives and preferences of PIE, this research serves as a guidance for manufacturers to tailor their preapproval information to the needs of HCDMs. Precise selection of PIE topics by manufacturers enables HCDMs to accurately forecast budget impact and inform formulary access.
As more high-cost, novel therapies enter the market, health care decision makers (HCDMs) are citing an increasing need for early clinical and economic information of a product prior to US Food and Drug Administration (FDA) approval. This need for data is being fulfilled through preapproval information exchange (PIE), in which manufacturers deliver information to HCDMs on a product’s clinical and health care economic information to inform value-based formulary decisions. PIE is a bidirectional communication system that improves awareness of a product in development for an initial or expanded indication.1 The aim of this article is to outline the current landscape of PIE and make recommendations for best practices in designing preapproval information engagements based on our newly conducted research in 2021, as well as previous research. The first part of this article will review the evolution of PIE use among manufacturers and HCDMs based on published literature. To identify key literature, a systematic literature review was conducted using PubMed, Academy of Managed Care Pharmacy (AMCP) conference abstract archives, and International Society for Pharmacoeconomics and Outcomes Research conference abstract archives (Supplementary Exhibit 1 (479KB, pdf) , available in online article). The second part of this article will describe the methodology and results of a recent FormularyDecisions survey we conducted regarding PIE webinars and make recommendations for future preapproval information communications.
The Evolution of Manufacturer Preapproval Information Dissemination
Engaging with HCDMs before FDA approval allows manufacturers to cultivate relationships, share valuable information about their products, and streamline the formulary review process.2 Historically, manufacturers have been hesitant to engage in PIE because of unclear regulatory guidance and the perceived risk of legal liability, as well as the disruption of planned regulatory filings.2 However, the FDA guidance document and newer proposed legislation, such as the Preapproval Information Exchange Act of 2022, are facilitating increased frequency and quality of proactive communication with HCDMs.3-7 Although in-person engagements were historically common, other means of communication, including web-based formats, have further facilitated the exchange of information.7
With growing attention to PIE, manufacturers are establishing their own guidelines for preapproval information dissemination.2,7 According to a survey conducted in 2020, from 2018 to 2020, the number of manufacturers with a process or guidance in place to approve preapproval materials has nearly doubled.8 Although manufacturers are developing methods to equip HCDMs with essential preapproval information, challenges to current practices remain. In the same study, 70% of manufacturers reported barriers to providing PIE, including confidentiality concerns, lack of resources to develop materials, internal education needs, and lack of alignment across various departments within the organization on the need for PIE.8 Pricing also appears to be a sensitive topic for manufacturers because of uncertainties surrounding product value and the competitive landscape until final FDA approval.2 HCDMs rated product pricing information as “very difficult” or “extremely difficult” to obtain from manufacturers at higher rates in 2020 compared with 2018 (50% vs 0%, respectively).8 Although trends show that manufacturers are attempting to meet the needs of HCDMs in their approaches to PIE, strategies will likely remain heterogenous until further guidance or regulatory clarity provides best practices for sharing preapproval content.1,8
USE OF PIE AMONG HCDMs
As products continue to enter the market at an accelerating rate, HCDMs are tasked with predicting their value and potential impacts on organizational budgets and policies. Many difficulties in planning for new product assessments arise from the absence of information about a product’s value prior to FDA approval.9 Additionally, therapies with breakthrough designations further impede this process when a therapy’s approval precedes the availability of clinical and economic data.9 To mitigate these current challenges, HCDMs have begun leveraging preapproval information to evaluate pipeline products and new indications for existing products. HCDMs use preapproval information to forecast budget impacts, make informed formulary decisions, and develop policies that facilitate patient access to novel therapies.1,5,10 Preapproval information also allows HCDMs to anticipate patient-access barriers and establish strategies like value-based contracts to expedite the availability of new interventions.1
HCDMs have begun to establish what they view as best practices for PIE as they continue to leverage these communications for internal efforts. HCDMs appear to have a strong preference for stakeholder engagement with medical science liaisons and health outcomes liaisons among manufacturer representatives for peer-to-peer communications.1,3-5,11 They also prefer these engagements well in advance of FDA approval but in line with the organization’s timeline for product reviews.5,8,11 In a survey conducted by Begovic and colleagues, 95% of respondents stated that they would prefer to receive PIE less than 1 year prior to a product’s anticipated approval.6
Among data communicated, clinical and FDA guideline information appears to be highly valued in the assessment of pipeline products.12 In the same survey by Begovic and colleagues, respondents considered indication sought, clinical trial results, anticipated timeline for FDA approval, and product pricing as very or extremely important prior to product approval.6 HCDMs also noted information gaps in current preapproval communications and expressed a desire for epidemiological and disease-specific characteristics, clinical and economic outcomes, and financial information.1 Desired financial information includes disclosure of the projected price range and wholesale acquisition cost. Although HCDMs are articulating preferred standards and infrastructures for PIE, they are aware of the need for a legislative “safe harbor” for manufacturers to further facilitate the exchange of relevant information.1,13
HCDM PERCEPTIONS OF PIE: RESULTS FROM A 2021 SURVEY
Although in-person engagements are historically common, other means of communication, including webinars, have facilitated PIE. Limited evidence exists evaluating perceptions of PIE webinars among HCDMs and the importance of PIE in key therapeutic areas and/or investigational products. The objective of the FormularyDecisions survey was to assess the current use of preapproval information among HCDMs and describe the perspectives of interest to help guide best practices for PIE webinar content development. To gather HCDM perspectives on PIE webinars, web-based surveys were fielded to a market research panel of 31 FormularyDecisions users. FormularyDecisions is a secure third-party platform facilitating the exchange of information between life sciences manufacturers and HCDMs to help support the product evaluation process.
Survey questions were asked in the context of AMCP PIE webinars except for 2 questions, which asked about the experience and use of PIE and types of preapproval information valuable to HCDMs in the context of all formats of PIE (ie, PIE decks, webinars, and dossiers). Survey questions were developed by the FormularyDecisions team and reviewed by several stakeholders, including the Xcenda Market Research Team, the AmerisourceBergen Office of Compliance, and AMCP to ensure validity. Survey questions were also tested internally by the Xcenda Market Research Team prior to fielding.
Participation in the survey was voluntary and a modest honorarium was paid by Xcenda to participants who completed the survey. Survey participants were HCDM users of FormularyDecisions from public and private payer organizations (eg, third-party payers, health plan sponsors, and state Medicaid programs), formulary committees (eg, Pharmacy and Therapeutics Committees), drug information centers, technology assessment committees, pharmacy benefit managers, third-party administrators, and other multidisciplinary entities (eg, integrated delivery networks, hospitals, and health systems) that review scientific and/or technology assessments on behalf of health care organizations to inform coverage and reimbursement decisions on a population basis regarding the formulary management of drug and device. A total of 23 out of 31 advisors (74%) participated in the survey and represented pharmacy benefit managers (n = 10), health plans (n = 7), academic centers (n = 3), integrated delivery networks (n = 1), independent practices (n = 1), and self-funded employers (n = 1).
Since the FDA issued guidance in 2018, more manufacturers are participating in preapproval engagements, thus improving awareness and use in patient-access decisions. In the FormularyDecisions survey, for all types of PIE (eg, PIE decks, webinars, and dossiers), 83% of respondents indicated experience with preapproval information, whereas 74% affirmed its use in formulary decision-making (Figure 1). In line with increased use, inclusion of key preapproval information topics by manufacturers ensures a successful engagement that can lead to further bidirectional communication or future formulary access for plan members. The FDA guidance highlights key points of interest to share in preapproval communications, such as approval timeline, clinical data, pricing information, and marketing strategies.14 In the FormularyDecisions survey, across all formats of PIE (eg, PIE decks, webinars, and dossiers), respondents highly valued the inclusion of product indication and factual presentation of the clinical trial design and results in preapproval content (Supplementary Exhibit 2 (479KB, pdf) ). This finding resonates with previous literature, as a survey by Begovic and colleagues also found that 82% and 84% of payers value product indication and clinical trial results, respectively, in preapproval communications.4
FIGURE 1.

Importance and Frequency of Use of PIE Among Health Care Decision Makers (N=23)
Product pricing has been an important topic in recent years with the emergence of new, high-cost therapies. This importance has translated to an increased need by HCDMs for pricing information prior to product approval. Although respondents in the FormularyDecisions survey did not rank product pricing as an important component of PIE, several other surveys indicated pricing as important.4,6,15 This importance alludes to HCDMs’ desires to focus on product value and potentially initiate pricing conversations early to minimize future budget impact or implement alternative payment models.
PIE webinars should emphasize disease areas of interest for payers. In the FormularyDecisions survey, respondents were interested in areas such as diabetes (n = 7; 30%), oncology (n = 7; 30%), and atopic dermatitis (n = 5; 22%) for PIE webinars (Supplementary Exhibit 3 (479KB, pdf) ). Not only do these diseases have a high budget impact for payers, but they also signal an increasing emphasis in targeting new areas that are chronic and high growth and/or have increased prevalence.16-18 Similarly, for PIE webinars, products with a novel mechanism of action and high population uptake were also considered to be valuable in the FormularyDecisions survey, with respondents citing products such as gene therapies (n = 5), targeted immunomodulators (n = 2), and biosimilars (n = 2) as target areas (Supplementary Exhibit 4 (479KB, pdf) ). Respondents also mentioned several specific products such as teplizumab (n = 2), donanemab (n = 1), and Semglee (n = 1) as therapies of interest for PIE webinars.
Survey respondents in the FormularyDecisions survey were also asked their preference on the speaker type for PIE webinars. Most respondents (96%) were interested in a clinician expert or specialist in the field as a speaker, followed by a peer/other HCDM (61%), health economist (61%), and manufacturer representative (13%). This wide variation might be due to a perceived lack of bias from a clinical expert as opposed to a manufacturer representative.
RECOMMENDATIONS FOR STAKEHOLDER ALIGNMENT ON PIE
Although the use of PIE has increased among stakeholders, there are still discrepancies between what manufacturers are providing and what HCDMs are asking for. A gap between available and needed preapproval information was noted by 45% of HCDMs in a 2018 survey.4 Furthermore, although product pricing information was indicated as valuable by HCDMs in previous research, only a minority of manufacturers approved of proactively sharing product pricing in a study published in 2019.3 As such, limitations in stakeholder alignment and regulatory shortcomings continue to challenge the evolving applications of PIE.
HCDM priorities should be considered as manufacturers establish internal policies and processes for PIE. Regulatory entities should also acknowledge current trends when establishing future legislation. Figure 2 highlights the drivers, barriers, and use of preapproval information for key stakeholders involved in PIE. Additionally, it also provides recommendations for improving preapproval engagements based on findings from the systematic literature review and our recent FormularyDecisions survey.
FIGURE 2.
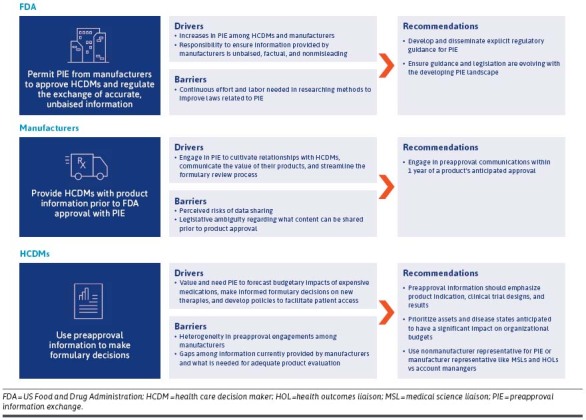
The Use of PIE Among Stakeholders and Recommendations for Future Preapproval Dissemination
Conclusions
The results of our FormularyDecisions research further support known HCDM perceptions of PIE and address gaps regarding HCDM familiarity and the preferred content of PIE. Based on our survey, for all formats of PIE (eg, PIE decks, webinars, and dossiers), product indication and clinical trial information are highly valued and should be standard evidence included. The perceived value of preapproval information varies among products; therefore, PIE webinars should prioritize therapeutic areas and assets anticipated to have a significant impact on budgets because of disease prevalence, forecasted market share, and/or product cost. HCDMs’ desire for nonmanufacturer representatives to conduct PIE webinars highlights the need for data that are perceived to be unbiased. Although nonmanufacturer representatives are preferred for PIE webinars, health outcomes liaisons or medical science liaisons are preferred among manufacturer representatives for in-person engagements. Although desired by HCDMs, our data showed that pricing information does not currently appear to be a critical component of preapproval information provided by manufacturers.
Alignment of manufacturer practices for PIE with HCDM expectations will facilitate the exchange of quality and relevant information. Future research will further explore the perceptions of PIE webinars with a larger audience and perform qualitative research of real-world examples of PIE to better understand how sharing preapproval content translates into changes in product awareness and formulary decisions.
ACKNOWLEDGMENTS
The authors would like to acknowledge Jasmine Knight, PharmD, MS, for her assistance in reviewing this article.
REFERENCES
- 1.Brixner D, Woodward TC, Seifter N, et al. Preapproval information exchange: Perspectives of US population health decision makers on preferences for early engagement with investigational therapies. J Manag Care Spec Pharm. 2019;25(2):164-73. doi:10.18553/jmcp.2019.25.2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niyazov A, Lenci D. Communicating healthcare economic and pre-approval information with healthcare decisionmakers: Opportunities following the 21st Century Cures Act and FDA guidance. Front Public Health. 2018;6:304. doi:10.3389/fpubh.2018.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitez B, Duhig A, Jackson J. PNS91 comparing payer and manufacturer experiences with HCEI and PIE after passage of the 21st Century Cures Act. Value Health. 2019;22 Supplement 2:S301. doi:10.1016/j.jval.2019.04.1450 [Google Scholar]
- 4.Begovic E, Duhig A, Jackson J, et al. The impact of legislative changes and regulatory guidance on proactive dissemination of healthcare economic information: Payers’ experiences pre and post product approval. Value Health. 2018;21:Supplement 1:S92. [Google Scholar]
- 5.Clark J, Psyhojos M, Benitez B, Duhig A, Mody L, Jackson J. Trends in payer experiences, attitudes, and perceptions of pre-approval information exchange. J Manag Care Spec Pharm. 2019;25(10):S2-95. [Google Scholar]
- 6.Begovic E, Duhig A, Jackson J, et al. Payers’ perceptions of the importance and usefulness of preapproval information exchange of healthcare economic information prior to product approval. Poster presented at: AMCP Managed Care and Specialty Pharmacy Annual Meeting; April 24-26, 2018; Boston, MA. [Google Scholar]
- 7.Duhig A, Jackson J, Kaufman S, Hughes J, Sarnes M, Carden M. Manufacturers’ experiences regarding proactive dissemination of healthcare economic information following legislative and regulatory guidance changes. Poster presented at: Academy of Managed Care Pharmacy Nexus; October 22-25, 2018; Orlando, FL. [Google Scholar]
- 8.Clark J, Hughes J, Gorey C, Duhig A, Jackson J. Trends in manufacturer perceptions and practices regarding preapproval information. Poster presented at: Academy of Managed Care Pharmacy Nexus Virtual; October 29-23, 2020. [Google Scholar]
- 9.Fendt PR, Ung B, Vogenberg FR. The value of pre-FDA approval healthcare economic information exchange between payers and drug manufacturers. Am Health Drug Benefits. 2017;10(8):424-6. [PMC free article] [PubMed] [Google Scholar]
- 10.Gladman J, Sampsel E, Drummond M, Fazio L. How do payers utilize the AMCP eDossier System for pre-approval information and could it qualify as a safe harbor? Poster presented at: AMCP Managed Care & Specialty Pharmacy Annual Meeting; March 27-30, 2017; Denver, CO. [Google Scholar]
- 11.Clark J, Hughes J, Gorey C, Choi M, Duhig A, Jackson J. Trends in payer perceptions and preferences regarding pre-approval information. Value Health. 2020;23:Supplement 1:S294. [Google Scholar]
- 12.Sampsel E, Gladman J, Carleton M, Mak E. Comparing US payers and hospital providers: the current trend for preapproval information requests to support formulary decisions. Poster presented at: Academy of Managed Care Pharmacy Nexus 2017; October 16-19, 2017; Dallas, TX. [Google Scholar]
- 13.AMCP Partnership Forum. Enabling the exchange of clinical and economic information pre-FDA approval. J Manag Care Spec Pharm. 2017;23(1):105-12. doi:10.18553/jmcp.2016.16366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Drug and device manufacturer communications with payors, formulary committees, and similar entities - questions and answers. Accessed February 10, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-and-device-manufacturer-communications-payors-formulary-committees-and-similar-entities
- 15.Benitez B, Duhig A, Jackson J. PNS137 are medical directors’ and pharmacy directors’ attitudes different towards proactive HCEI and PIE? Value Health. 2019;22:Supplement 2:S308-9. doi:10.1016/j.jval.2019.04.1494 [Google Scholar]
- 16.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6-12. doi:10.1089/pop.2015.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Cancer statistics. Cancer.gov. Accessed February 27, 2022. https://www.cancer.gov/about-cancer/understanding/statistics#:~:text=Cancer%20is%20among%20the%20leading,related%20deaths%20to%2016.4%20million
- 18.Nutten S. Atopic dermatitis: Global epidemiology and risk factors. Ann Nutr Metab. 2015;66 Suppl 1:8-16. doi:10.1159/000370220 [DOI] [PubMed] [Google Scholar]


