Abstract
Background/aim
Lymphovascular invasion (LVI) is considered a high-risk factor for recurrence in early-stage breast cancer, hence examination of LVI in pathological samples is an absolute recommendation. We aim to investigate predictive factors of LVI in pre-neoadjuvant chemotherapy (NAC) patients with estrogen receptor positive (ER+) and human epidermal growth factor receptor 2 negative (HER2−) molecular subtypes of breast cancer.
Materials and methods
One hundred and thirty-four patients treated with NAC were included in this study who were ER+/HER2−. The clinical characteristics of the patients, the data obtained from the core needle biopsy before NAC and the LVI status in the pathology that examined after breast surgery were collected. Univariate and multivariate analysis were performed using the logistic regression model.
Results
An examination of the association between LVI and clinical-pathological patient characteristics showed that advanced age (>40 years old) (p = 0.021), ductal histology (p = 0.039), and presence of axillary lymph node metastasis (p = 0.005) were predictors of LVI. Independent predictors of LVI in a multivariate logistic model included advanced age (p = 0.037), and the presence of axillary lymph node metastasis prior to NAC (p = 0.006). The median RFS (Recurrence-free survival) time was 22.8 months for all patients. RFS was shorter in patients with LVI (log-rank p = 0.037).
Conclusion
Independent predictors of LVI are advanced age and lymph node positivity at the time of diagnosis. Our study is the first study that evaluates pre-NAC predictive factors of LVI in ER+/HER2− breast cancer patients treated with NAC.
Keywords: Breast cancer, lymphovascular invasion, predictive, chemotherapy
1. Introduction
Neoadjuvant chemotherapy (NAC) is one of the recommended treatments in patients with locally advanced breast cancer and inflammatory breast cancer. It has been well-established that the chance of breast-conserving surgery (BCS) and pathological complete response is increased following shrinkage of tumor with neoadjuvant therapy [1,2]. Responses given to neoadjuvant therapy vary depending on the phenotype therefore, the prognosis of breast cancer also varies depending on the response. Nowadays, molecular subtyping has become more important, patient subgroups with the poorest response to chemotherapy are known to Luminal A like tumors (ER +, HER2−) [3,4]. NAC may provide earlier identification of chemo-sensitivity of ER+, HER2− and other molecular subtypes of breast cancer [5].
Patient age, T and N status at staging, molecular subgroup, histological grade, hormone receptor status, lymphovascular invasion (LVI), and pathological complete response (pCR) following NAC are considered as well-established prognostic factors in breast cancer [6–10]. LVI has been identified as a prognostic factor in operated breast cancer patients regardless of axillary involvement, although the mechanisms of such association have not been fully elucidated [9,11]. Therefore, in major oncology guidelines, LVI is considered a high-risk factor for recurrence in early-stage breast cancer, hence examination of LVI in pathological samples is an absolute recommendation [1,12]. Despite this, studies examining LVI following NAC are scarce in number, and to the best of our knowledge, no previous studies have looked at the relationship between LVI and pre-NAC clinico-pathological characteristics of the patients. Previous studies detected LVI by examining the samples that were obtained from surgery. Additionally, analysis of histopathological factors associated with LVI has been performed with the same specimen. This analyzing method could avoid the detection of the true predictive factors of LVI since presurgical NAC treatment could affect the Ki-67 index and receptor expression. The objective of this study was to explore pretreatment factors predicting LVI status in patients undergoing NAC due to nonmetastatic luminal breast cancer who were ER +/HER2−, which are known to be associated with poorer response to chemotherapy as compared to other molecular subtypes.
2. Materials and methods Patients
Breast cancer patients undergoing NAC prior to surgery at Tekirdağ Namık Kemal University Hospital between 1 January 2016 and December 2021 were retrospectively examined upon receiving approval from the relevant ethics committee. Magnetic resonance imaging (MRI), abdominal ultrasound and chest X-ray were utilized to determine distant metastases, contralateral breast lesions, and disease stage. Positron emission tomography-computed tomography (PET-CT) was taken in patients with suspected metastasis. Exclusion criteria included subjects under 18 years of age, male subjects, HER2 receptor positivity and/or estrogen receptor (ER) negativity, use of different neoadjuvant treatments, and presence of findings suggesting metastasis.
2.1. Treatment
Indications for neoadjuvant therapy were as follows: clinically node positive, pathologically confirmed nodal metastases on lymph node biopsy, and primary tumor size ≥5 cm. All cases were discussed at the Institutional Multidisciplinary Tumor Board. Patients receiving 4 cycles of cyclophosphamide (600 mg/m2) + epirubicin (90 mg/m2) followed by either docetaxel (75 mg/m2) every 3 weeks for 4 cycles or weekly paclitaxel (80 mg/m2) for 12 cycles were included. All patients had undergone surgery following completion of NAC. All patients were treated with hormone therapy after surgery and adjuvant radiotherapy was given to eligible patients in collaboration with a radiation oncologist.
2.2. Pathology
Histological subtyping, SMA expression, CK7 expression, P63 expression, E-cadherin expression levels, Ki-67 and grading were based on biopsy samples obtained prior to NAC. Based on the American Society of Clinical Oncology/College of American Pathologists guidelines, patients whose ER and PgR (progesterone receptor) levels were higher than 1% were considered positive [13]. Patients were considered HER2 positive and thus excluded from the study, if HER2 immunohistochemical (IHC) analyses showed a score of +3, or a score of +2 with positive fluorescence in situ hybridization (FISH) analysis. ER expression was divided into two groups according to ASCO/CAP guidelines: ER > 10% (non-low expression) and ER ≤ 10% (low expression) [14]. Ki-67 expression was divided into two groups: Ki-67 ≥ 14% (high expression) and Ki-67 < 14% (low expression). This cut-off was determined according to Gallen International Expert Consensus [15]. The same cut-off was used for molecular subtyping. Axillary lymph node positivity was verified histopathologically.
2.3. Definition of LVI
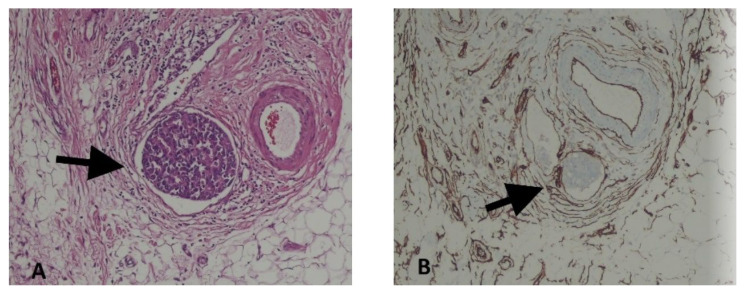
LVI positivity was defined as the presence of tumor cells within an endothelium-lined space (lymphatics or blood vessels) as demonstrated by hematoxylin-eosin staining and IHC on surgical slides after NAC. For inconclusive cases, a specific marker (CD34) was utilized (Figure 1A, 1B).
Figure 1.
Lymphovascular invasion (black arrow) seen in hematoxylin-eosin (A) and CD34 (B) stained sections of breast tumors from the same case; ×400.
2.4. Statistical analysis
The Fisher exact test and the Mantel–Haenszel chi-square test for trend were used to assess the association between categorical or ordinal variables and the presence of LVI. Univariate and multivariate analyses were performed using the logistic regression model. Odds ratio (OR) was reported with the corresponding 95% confidence intervals (95% CI) and p < 0.05 was considered statistically significant. Statistical analyzes were performed using SPSS Statistic software 24 (SPSS Inc., Chicago, III). To predict LVI, binary logistic regression using the “Forward:LR” method was used for multivariate analyses. Times of recurrence-free survival (RFS) was calculated according to the Kaplan-Meier method from the date of surgery to the occurrence of local recurrence or distant metastasis.
2.5. Ethical approval
The study was approved by the Tekirdağ Namık Kemal University ethics committee in accordance with the Helsinki declaration.
3. Results
A total of 134 patients were included in the study. The median age was 50 years (range: 38–79 years). Sixty-three (47.0%) patients had LVI positivity. In the classification made according to molecular status, 25 (18.7%) patients were Luminal A and 109 (81.3%) patients were Luminal B(Her2−). Table 1 shows the association between LVI status and clinicopathological patient characteristics.
Table 1.
Patient distribution according to clinicopathological characteristics and LVI status.
| Clinicopathological characteristics | Total (n, %) | LVI (−) n = 71 |
LVI (+) n = 63 |
|---|---|---|---|
| Age | |||
| <40 (Young adult) | 29 (21.7%) | 21 (72.4%) | 8 (27.6%) |
| ≥40 | 105 (78.3%) | 50 (47.6%) | 55 (52.4%) |
| Molecular subtype | |||
| Luminal A | 25 (18.7%) | 9 (36.0%) | 16 (64.0%) |
| Luminal B(HER2−) | 109 (81.3%) | 63 (57.8%) | 46 (42.2%) |
| Histologic type | |||
| Ductal | 109 (81.3%) | 53 (48.6) | 56 (51.4%) |
| Others | 25 (18.7%) | 18 (72.0%) | 7 (28.0%) |
| ER status | |||
| <10% | 10 (7.5%) | 7 (70.0%) | 3 (30.0%) |
| ≥10% | 124 (92.5%) | 64 (51.6%) | 60 (48.4%) |
| PgR status | |||
| Negative | 19 (14.2%) | 14 (73.7%) | 5 (26.3%) |
| Positive | 115 (85.8%) | 57 (49.6%) | 58 (50.4%) |
| Ki-67 | |||
| <14% | 33 (24.6%) | 14 (42.4%) | 19 (57.6%) |
| ≥14% | 101 (75.4%) | 57 (56.4%) | 44 (43.6%) |
| Menopausal status | |||
| Premenopausal | 61 (45.5%) | 37 (60.9%) | 24 (39.1%) |
| Postmenopausal | 73 (54.5%) | 34 (46.6%) | 39 (53.4%) |
| Grade | |||
| Grade 1 | 14 (10.4%) | 8 (57.1%) | 6 (42.9%) |
| Grade 2 | 81 (60.5%) | 41 (50.6%) | 40 (49.4%) |
| Grade3 | 39 (29.1%) | 22 (56.4%) | 17 (43.6%) |
| Pre-NAC tumor size | |||
| <2cm | 28 (20.9%) | 14 (50.0%) | 14 (50.0%) |
| ≥2cm | 106 (79.1%) | 57 (53.8%) | 49 (46.2%) |
| Pre-NAC lymph node status | |||
| Negative | 18 (13.4%) | 16 (88.9%) | 2 (11.1%) |
| Positive | 116 (86.6%) | 55 (47.4%) | 61 (52.6%) |
| CK-7 expression | |||
| Negative | 20 (15.0%) | 11 (55.0%) | 9 (45.0%) |
| Positive | 114 (85.0) | 60 (52.6%) | 54 (47.4%) |
| p63 expression | |||
| Negative | 111 (82.8%) | 55 (49.5%) | 56 (50.5%) |
| Positive | 23 (17.2%) | 16 (69.6%) | 7 (30.4%) |
| E-cadherin expression | |||
| Negative | 24 (17.9%) | 16 (66.7%) | 8 (33.3%) |
| Positive | 110 (82.1%) | 55 (50.0%) | 55 (50.0%) |
| SMA expression | |||
| Negative | 107 (79.9%) | 53 (49.5%) | 54 (50.5%) |
| Positive | 27 (20.1%) | 18 (66.7%) | 9 (33.3%) |
LVI, Lymphovascular invasion; ER, Estrogen receptor; PgR, Progesterone receptor; NAC, Neoadjuvant chemotherapy; HER-2, Human epidermal growth factor receptor 2; CK-7, Cytokeratin 7; SMA, Smooth muscle actin.
A regression analysis was performed to assess the association of clinicopathological data with LVI. Age over 40 years, ductal tumor histology, and presence of axillary lymph node metastasis prior to neoadjuvant chemotherapy were identified as predictors of LVI in univariate analysis (p = 0.021, p = 0.039, and p = 0.005, respectively). When a multivariate logistic model was applied, independent predictors of LVI were identified as advanced age (OR 2.69; 95% CI, 1.06–6.83; p = 0.037), and presence of axillary lymph node metastasis prior to NAC (OR 8.37; 95% CI, 1.82–38.52; p = 0.006). The results are shown in Table 2.
Table 2.
Univariate and multivariate analyses of factors for LVI in ER-positive/HER2-negative patients with neoadjuvant chemotherapy.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | Category | OR (95% CI) | p | OR (95% CI) | pf |
| Age | <40/≥40 | 2.89(1.17–7.10) | 0.021 | 2.69(1.06–6.83) | 0.037 |
| Molecular subtype | LuminalA/B(HER2−) | 0.43(0.17–1.05) | 0.064 | ||
| Histologic type | Ductal/others | 0.37(0.14–0.95) | 0.039 | ||
| ER status | <10%/≥10% | 2.19(0.54–8.85) | 0.272 | ||
| PgR status | Negative/positive | 2.85(0.96–8.43) | 0.058 | ||
| Ki-67 | <14%/≥14% | 0.57(0.26–1.26) | 0.164 | ||
| Menopausal status | Pre/post | 1.77(0.89–3.52) | 0.105 | ||
| Grade | 1/2/3 | 0.94(0.53–1.65) | 0.828 | ||
| Pre-NAC T size | <2cm/≥2cm | 0.86(0.37–1.98) | 0.722 | ||
| Pre-NAC N status | Negative/positive | 8.87(1.95–40.34) | 0.005 | 8.37(1.82–38.52) | 0.006 |
| Ck-7 expression | Negative/positive | 1.10(0.42–2.86) | 0.845 | ||
| p63 expression | Negative/positive | 0.43(0.16–1.13) | 0.086 | ||
| E-cadherin expression | Negative/positive | 2.00(0.79–5.06) | 0.143 | ||
| SMA expression | Negative/positive | 0.49(0.20–1.19) | 0.115 | ||
Significant values are indicated in bold. Pf: Forward: LR method LVI, lymphovascular invasion; HER-2, Human epidermal growth factor receptor 2; ER, Estrogen receptor; PgR, Progesterone receptor, NAC, Neoadjuvant chemotherapy; CK-7, Cytokeratin 7; SMA, Smooth muscle actin.
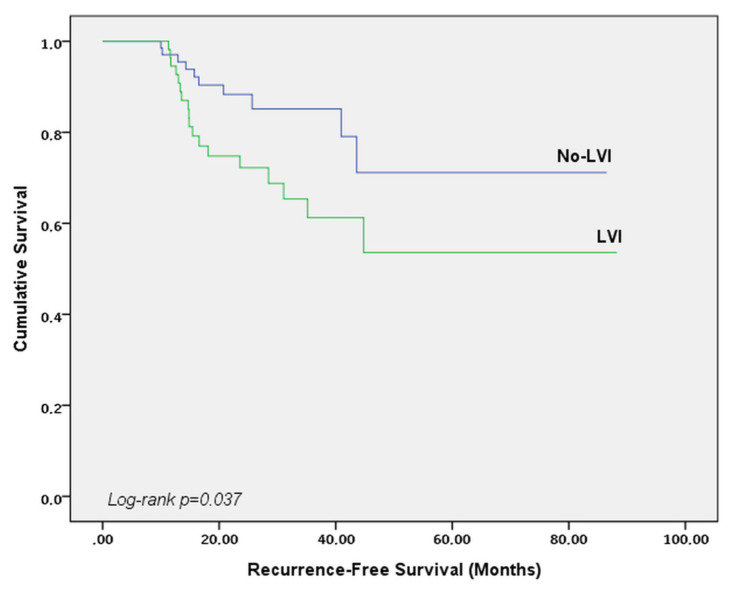
The median follow-up period of the patients after breast surgery was 43.7 months. Twenty-eight (20.9%) patients had recurrence (local or distant metastasis) during the follow-up period. The median RFS (mRFS) in all patients was 22.8 months (95% CI: 24.2–30.4). mRFS was 19.5 months in LVI positive patients, 23.3 months in LVI negative patients (log-rank p = 0.037) (Figure 2).
Figure 2.
Kaplan Meier survival curve by LVI status for recurrence-free survival (RFS).
4. Discussion
The response rate to NAC in ER +/HER2− luminal breast cancer subtypes is only one third of that in hormone negative molecular subtypes, and therefore these patients are generally considered chemotherapy-resistant [16–18]. In ER +/HER2− tumors showing inadequate response to chemotherapy, the survival benefit even in the presence of pathological complete response (pCR) is controversial [16,19]. In recent years, there has been an increase in the number of studies demonstrating a prognostic role for LVI in ER +/HER2− groups [9,20–24]. LVI is considered to represent an important step in tumor progression and metastasis [11,25].
In the literature, there are limited studies on the factors that predict LVI in breast cancer. Previous studies detected LVI by examining the samples that were obtained from surgery. Additionally, analysis of histopathological factors that are associated with LVI has been performed with the same specimen. Our study was designed differently and analyzed potential predictive histopathological factors of LVI based on data from a reexamination of patients’ pre-NAC biopsies. According to our results advanced age, ductal histology, and presence of lymph node metastasis were predictors of LVI. Among all the predictors, advanced age and lymph node metastasis emerged as independent predictors.
Zhao et al. evaluated the patients that were not treated with NAC before breast surgery and found that younger patients had a higher frequency of LVI than older patients [26]. However, Rakha et al. reported that LVI was frequently seen in advanced ages [11]. Ryu et al. included the patients that were treated with NAC before surgery and did not find relation between age and LVI [27]. There is no consensus on this issue in previous studies. In our study, we found that advanced age is an independent predictive factor of LVI. LVI was 2.69 times more common in patients over 40 years of age than patients under 40 years of age.
Axillary lymph node metastasis was reported to be associated with LVI [27–31]. However, previous studies designed differently and examined the relationship between pathological N status and LVI. In our study, pre-NAC axillary positivity was an independent predictor of LVI. In studies of Muhammed et al. and David et al., the presence of LVI was reported to be a significant predictor for histological invasion in regional lymph nodes [32,33]. In Ran et al.’s study as well as a meta-analysis by Zhang et al. involving 2920 patients, LVI and axillary lymph node metastases were found to be interrelated sequential steps that ultimately result in metastasis. It is unknown whether LVI is a reason for lymph node metastasis or a result of lymph node metastasis. However, it can be suggested that axillary lymph node metastasis and LVI status, which are correlated and considered to be developing via similar mechanisms, could predict each other as a result of the involvement of similar pathways. Axillary lymph node metastasis is an important component of the TNM staging system and is considered a prognostic marker for breast cancer and is strongly associated with LVI. This association between axillary lymph node metastasis and LVI supports the clinical significance of LVI in ER+/HER2 negative breast cancer patients [34–36].
In our study, we did not identify an independent association with menopause, histological type, hormone receptor levels, Ki-67, tumor size, and disease grade, which were reported to be associated with LVI in previous studies [28,29,32,37–42]. The lack of such association may be related to the fact that previous studies generally included patients with all molecular subtypes of breast cancer, and used different cut-off values for Ki-67 and hormone receptors. Alternatively, it may be related to the fact that our study utilized a different design with the analysis of pre-NAC factors which might have been altered due to NAC and lost their significance. Accordingly, alterations in Ki-67, PgR, and ER have been shown to occur in association with chemotherapy [43–46].
One limitation of our study was the use of IHC for molecular subtyping, which allows genotype-based breast cancer subtyping only, and this might lead to a certain degree of misclassification of tumors. In our study, patients with rare subtypes of breast cancer were limited in number, and more recent analytic techniques involving genomic testing were not utilized [47,48]. Additionally, core needle biopsy has a limited representation of all breast cancer tissue due to the small tissue volume. As far as we know, our study is the first that analyzes pre-NAC variables. Furthermore, CK-7, SMA, and p68 expression in breast cancer patients were analyzed for the first time in terms of LVI. Our findings are also significant due to the examination of pre-NAC variables specifically in a subgroup of ER +/HER2− subjects. Further prospective and more comprehensive studies with larger sample sizes or meta-analyses are required to reach firmer conclusions regarding LVI-related factors.
In conclusion, this study showed that advanced age and pre-NAC N status are independent predictive factors of LVI. We need further studies to examine the predictive factors of LVI, which is considered to be a prognostic marker for survival, particularly in ER+/HER2− molecular subtype of breast cancer with poor response to chemotherapy. Identification of predictors may pave the way for further studies in terms of disease monitoring and potential therapeutic target therapy.
Acknowledgment/Disclaimers/Conflict of interest
The funding was supported by the authors themselves without the involvement of grants, research scholarships, or any other funding sources.
The authors do not have any commercial or other association that might pose a conflict of interest.
Footnotes
Informed consent
The study was approved by the Tekirdağ Namık Kemal University ethics committee (30.03.2021; 2021.77.03.17) in accordance with the Helsinki declaration.
References
- 1. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 2. Bossuyt V, Provenzano E, Symmans WF, Boughey JC, Coles C, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Annals of Oncology. 2015;26(7):1280–1291. doi: 10.1093/annonc/mdv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. Journal of Clinical Oncology. 2005;23(1):41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 4. Bollet MA, Savignoni A, Pierga JY, Lae M, Fourchotte V, et al. High rates of breast conservation for large ductal and lobular invasive carcinomas combining multimodality strategies. British Journal of Cancer. 2008;98(4):734–741. doi: 10.1038/sj.bjc.6604229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sachelarie I, Grossbard ML, Chadha M, Feldman S, Ghesani M, et al. Primary systemic therapy of breast cancer. The Oncologist. 2006;11(6):574–589. doi: 10.1634/theoncologist.11-6-574. [DOI] [PubMed] [Google Scholar]
- 6. Tsuji W, Teramukai S, Ueno M, Toi M, Inamoto T. Prognostic factors for survival after first recurrence in breast cancer: a retrospective analysis of 252 recurrent cases at a single institution. Breast Cancer. 2014;21(1):86–95. doi: 10.1007/s12282-012-0358-x. [DOI] [PubMed] [Google Scholar]
- 7. Love RR, Duc NB, Dinh NV, Quy TT, Xin Y, Havighurst TC. Young age as an adverse prognostic factor in premenopausal women with operable breast cancer. Clinical Breast Cancer. 2002;2(4):294–298. doi: 10.3816/cbc.2002.n.005. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz AM, Henson DE, Chen D, Rajamarthandan S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER Program. Archives of Pathology and Laboratory Medicine. 2014;138(8):1048–1052. doi: 10.5858/arpa.2013-0435-OA. [DOI] [PubMed] [Google Scholar]
- 9. Lai HW, Kuo SJ, Chen LS, Chi CW, Chen ST, et al. Prognostic significance of triple negative breast cancer at tumor size 1 cm and smaller. European Journal of Surgical Oncology. 2011;37(1):18–24. doi: 10.1016/j.ejso.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 10. Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Europen Journal of Cancer. 2006;42(3):357–362. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 11. Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118(15):3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 12. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, et al. NCCN Guidelines Insights: Breast Cancer, Version 4.2021. Journal of the National Comprehensive Cancer Network. 2021;19(5):484–493. doi: 10.6004/jnccn.2021.0023. [DOI] [PubMed] [Google Scholar]
- 13. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Archives of Pathology and Laboratory Medicine. 2010;134(6):907–922. doi: 10.5858/134.7.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. Journal of Clinical Oncology. 2020;38(12):1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 15. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of Oncology. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Eostrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Bristish Journal of Cancer. 2004;91(12):2012–2017. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan MC, Al Mushawah F, Gao F, Aft RL, Gillanders WE, et al. Predictors of complete pathological response after neoadjuvant systemic therapy for breast cancer. The American Journal of Surgery. 2009;198(4):520–525. doi: 10.1016/j.amjsurg.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the Gepar Trio study. Breast Cancer Research and Treatment. 2010;124(1):133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 19. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clinical Cancer Research. 2020;26(12):2838–2848. doi: 10.1158/1078-0432.CCR-19-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mutai R, Goldvaser H, Shochat T, Peretz I, Sulkes A, et al. Prognostic value of the detection of lymphovascular invasion in hormone receptor-positive early breast cancer in the era of molecular profiling. Oncology. 2019;96(1):14–24. doi: 10.1159/000492429. [DOI] [PubMed] [Google Scholar]
- 21. Makower D, Lin J, Xue X, Sparano JA. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. npj Breast Cancer. 2021;7:20. doi: 10.1038/s41523-021-00231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurebayashi J, Kanomata N, Shimo T, Yamashita T, Aogi K, et al. Marked lymphovascular invasion, progesterone receptor negativity, and high Ki67 labeling index predict poor outcome in breast cancer patients treated with endocrine therapy alone. Breast Cancer. 2014;21(2):214–222. doi: 10.1007/s12282-012-0380-z. [DOI] [PubMed] [Google Scholar]
- 23. Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biology and Medicine. 2016;13(4):496–504. doi: 10.20892/j.issn.2095-3941.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao GS, Hsu HM, Chu CH, Hong ZJ, Fu CY, et al. Prognostic role of lymphovascular invasion and lymph node status among breast cancer subtypes. Journal of Medical Sciences. 2018;38:54–61. doi: 10.4103/jmedsci.jmedsci_105_17. [DOI] [Google Scholar]
- 25. Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, et al. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. Journal of Breast Cancer. 2011;14(3):198–203. doi: 10.4048/jbc.2011.14.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao Y, Yang N, Wang X, Huang Y, Zhou X, et al. Potential roles of lymphovascular space invasion based on tumor characteristics provide important prognostic information in T1 tumors with ER and HER2 positive breast cancer. Clinical and Translational Oncology. 2020;22(12):2275–2285. doi: 10.1007/s12094-020-02369-9. [DOI] [PubMed] [Google Scholar]
- 27. Ryu YJ, Kang SJ, Cho JS, Yoon JH, Park MH. Lymphovascular invasion can be better than pathologic complete response to predict prognosis in breast cancer treated with neoadjuvant chemotherapy. Medicine (Baltimore) 2018;97(30):e11647. doi: 10.1097/MD.0000000000011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He KW, Sun JJ, Liu ZB, Zhuo PY, Ma QH, et al. Prognostic significance of lymphatic vessel invasion diagnosed by D2-40 in Chinese invasive breast cancers. Medicine (Baltimore) 2017;96(44):e8490. doi: 10.1097/MD.0000000000008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lauria R, Perrone F, Carlomagno C, De Laurentiis M, Morabito A, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer. 1995;76(10):1772–1778. doi: 10.1002/1097-0142(19951115)76:10<1772::aid-cncr2820761014>3.0.co;2-o. doi. [DOI] [PubMed] [Google Scholar]