Abstract
BACKGROUND:
Lipoprotein(a) (Lp(a)) is an inherited, independent, and causal risk factor for atherosclerotic cardiovascular disease (ASCVD).
OBJECTIVE:
To assess the burden of elevated Lp(a) for patients with ASCVD in a real-world setting in the United States.
METHODS:
This retrospective cohort study assessed US patients with available Lp(a) measurement and established ASCVD using Optum’s Clinformatics Data Mart database (2007-2020). Index date was defined as the first diagnosis of an ASCVD event. Patient demographics, medications, health care resource utilization (HCRU), and occurrence of cardiovascular events were assessed for patients with elevated (≥150 nmol/L) vs normal (≥65 nmol/L) Lp(a) levels, within the first year of index date. HCRU was characterized by inpatient hospitalization, inpatient length of stay (LOS), outpatient visits, and emergency department (ED) visits. All comparative analyses of patients with elevated (≥150 nmol/L) vs normal (≥65 nmol/L) Lp(a) levels within the first year of index date were adjusted for age, sex, baseline statin use, and diabetes.
RESULTS:
8,372 patients with ASCVD and Lp(a) measurement in nmol/L were included in this study. Patient demographics and baseline clinical characteristics were similar among those with normal and elevated Lp(a). However, the proportion of patients receiving statins and β-blockers at baseline were significantly higher in the elevated vs normal Lp(a) group (54.76% vs 42.91%, P < 0.0001, and 30.92% vs 27.32%, P = 0.0183, respectively). At 1 year of follow-up, the rates per 100 person-years for ASCVD-related inpatient hospitalizations, outpatient hospitalizations, and ED visits were higher among patients with elevated Lp(a) compared with normal Lp(a) (13.33 vs 9.46, 89.08 vs 85.10, and 2.89 vs 2.29, respectively). The mean LOS per ASCVD-related hospitalization was 7.21 days in the elevated and 6.26 days in the normal Lp(a) group (P = 0.3462). During the 1-year post-index follow-up period, 15% of patients in the elevated Lp(a) group required revascularization compared with 10% of patients in the normal Lp(a) group (P = 0.0002). The odds of composite myocardial infarction, ischemic stroke, and revascularization occurrence of events within the first year of index was significantly higher in the elevated Lp(a) group compared with the normal Lp(a) group (1.46; 95% CI = 1.20-1.77; P < 0.05).
CONCLUSIONS:
HCRU within the first year of ASCVD diagnosis is substantial among patients with ASCVD and elevated Lp(a). Relatively higher rates of inpatient hospitalizations, increased LOS per hospitalization, and requirement of revascularization procedures within the first year of ASCVD index diagnosis were observed in patients with elevated Lp(a) compared with normal Lp(a) levels. Lp(a) testing in routine clinical practice could help in identification of high-risk patients with ASCVD and play an important role in the overall cardiovascular risk management, aiming to reduce the HCRU associated with ASCVD.
Plain language summary
One in five people worldwide have elevated lipoprotein(a) (Lp(a)), an inherited risk factor for atherosclerotic cardiovascular disease (ASCVD). This study shows the impact of elevated Lp(a) on increased ASCVD-related health care resource utilization in US patients with ASCVD.
Implications for managed care pharmacy
Elevated Lp(a) is an inherited, independent, and causal ASCVD risk factor, affecting 1 in 5 people worldwide. This study provides novel insights on the increased health care resource utilization associated with elevated Lp(a) in US patients with ASCVD. Wider knowledge and screening of Lp(a) may aid identification of high-risk patients and overall cardiovascular disease management, aiming to reduce the economic burden associated with ASCVD.
The past decade has seen an increase in interest in and knowledge of the relationship between lipoprotein(a) (Lp(a)) and atherosclerotic cardiovascular disease (ASCVD). Epidemiological, meta-analysis, genome-wide association, and Mendelian randomization studies1-4 have established Lp(a) as an inherited, independent, and causal ASCVD risk factor.2,5 Lp(a) levels are largely genetically determined and are not significantly modified by diet and exercise.1 Elevated Lp(a) levels are associated with greater cardiovascular (CV) risk, including atherosclerosis,6 and affect an estimated 1 in 5 people worldwide.2,7,8
The 2018 American College of Cardiology/American Heart Association,9 Canadian Cardiovascular Society,10 2019 National Lipid Association,11 and American Association of Clinical Endocrinologists and American College of Endocrinology12 suggest that Lp(a) values greater than or equal to 50 mg/dL or greater than or equal to 100 nmol/L10-12 or greater than or equal to 125 nmol/L9 are considered elevated and risk enhancing. Lp(a) testing is generally recommended in individuals with a history of premature ASCVD and in individuals with familial hypercholesterolemia, but many guidelines outside of the United States (eg, Canada, China, India, Poland, and France) are in the process of transitioning from recommending screening in at-risk individuals to screening in all adults.10 The European Society of Cardiology and European Atherosclerosis Society guidelines currently recommend that Lp(a) measurement should be considered at least once in each adult person’s lifetime to identify those with very high inherited Lp(a) levels greater than 180 mg/dL (> 430 nmol/L) who may have an elevated lifetime risk of ASCVD equivalent to the risk associated with heterozygous familial hypercholesterolemia.13 Despite the recommendations in guidelines and existing evidence on the association of elevated Lp(a) levels with increased ASCVD risk, Lp(a) measurement and profiling in patients has not been widely adopted in routine clinical practice.
ASCVD remains the leading cause of morbidity and mortality across the globe.14 In the United States, an estimated 15.4 million adults have coronary artery disease, approximately 8.5 million individuals suffer from peripheral artery disease (PAD), and 7 million individuals have had a stroke.15 ASCVD also has a substantial economic impact with US-specific expenditures of $126 billion in 2015, which is projected to increase by more than 2.5-fold to $309 billion in 2035.16 Although the national impact of rising costs related to ASCVD management has been actively studied, there is a lack of studies evaluating health care resource utilization (HCRU) among patients with ASCVD with elevated Lp(a) levels. Therefore, the objective of this study is to investigate the burden related to elevated Lp(a) on occurrence of CV events and ASCVD-related HCRU, quantified as inpatient hospitalizations, inpatient length of stay (LOS), outpatient visits, and emergency department (ED) visits, in a US cohort.
Methods
STUDY DESIGN
This is a noninterventional, retrospective cohort study of patients with ASCVD with an Lp(a) measurement in nmol/L, using the US Optum Clinformatics Data Mart (CDM) database. The study period was from January 1, 2007, to June 30, 2020. The identification period was from January 1, 2008, to June 30, 2019 (Supplementary Figure 1 (2.4MB, pdf) , available in online article).
The CDM database captures anonymized inpatient and outpatient medical claims, pharmacy claims, and laboratory results from enrollees of a large commercial and Medicare Advantage plan and is geographically diverse across all 50 states, including the District of Columbia. During the study period, there were 68,243,631 enrollees in the Optum CDM database, of whom 0.37% had an available Lp(a) measurement (nmol/L or mg/dL). Because no identifiable or protected health information was accessed during this study, institutional review board approval or waiver of authorization and informed consent were not required.
STUDY POPULATION
The study population included adult patients (aged ≥ 18 years) with an Lp(a) measurement in nmol/L and an established ASCVD diagnosis (defined by a prior myocardial infarction [MI], ischemic stroke [IS], transient ischemic attack, prior revascularization, or a diagnosis of PAD, unstable angina, or stable angina) during the identification period. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM codes were used to identify the ASCVD diagnosis and events (Supplementary Table 2 (2.4MB, pdf) ). Logical Observation Identifier Names and Code 43583-4 (nmol/L) was used to identify Lp(a) measurements. For each patient, the earliest Lp(a) measurement during the study period was considered. Assuming that Lp(a) level does not change significantly over time, the earliest measurement, which was the first measurement during study period regardless of enrollment criteria, was considered in this study.17 Lp(a) measurements with missing values and/or multiple Lp(a) measurements on the same day were excluded.
The index date was defined as the first diagnosis of ASCVD during the identification period, following 1 year of continuous enrollment. Patients were followed up to 1 year from the index date, until the end of enrollment, or the end of the study period, whichever occurred first. The ASCVD population was further stratified based on the Lp(a) thresholds: normal Lp(a) (< 65 nmol/L) or elevated Lp(a) (≥150 nmol/L).18-21
ASSESSMENTS AND OUTCOMES
Baseline patient characteristics were reported at index date. Patient characteristics included demographics, comorbidities, procedures, medications, and laboratory values. HCRU, procedures, and medications were reported at 1 year post-index. HCRU included the following variables: inpatient hospitalization, inpatient LOS, outpatient visits, and ED visits.
Inpatient hospitalizations were defined using inpatient encounters with valid admission dates and LOS from Inpatient Confinement tables. The number of hospitalizations per patient were counted based on the admission dates. When ED visits were followed by hospitalizations, in which the primary reason for ED visit and hospitalization were the same, the LOS and ED visit duration were summed for total LOS. ED visits were defined by “emergency room” encounters in Medical and Inpatient Confinement tables. As with inpatient visits, the primary diagnosis of the ED visit was used to define whether an ED visit was specifically for cardiovascular or for other causes. ICD-9 and ICD-10 codes were used to capture and classify the primary diagnosis for the hospitalization and ED visit. Intensive care unit in hospitalizations was identified using revenue code. For patients with 1 or more hospitalizations, the LOS was the sum of LOSs of all the hospitalizations within 1 year after the index date. Total LOS can be larger than 365 days for patients whose discharge date is 1 year after admission date. The LOS per hospitalization was defined as the total LOS (days in the 12-month follow-up period) divided by the total number of hospitalization visits (in the 12-month follow-up period). Cardiology visits were defined by outpatient encounters with cardiology-related ICD codes.
Outpatient visits were defined as those occurring in an outpatient setting and included office/clinic visits, ambulatory care, and procedure visits (labs and imaging), home visits, urgent care, nursing homes, and potentially other outpatient-related types in the medical tables. The primary diagnosis of the outpatient visit was used to define the cause of the outpatient visit. General practitioner visits or rehabilitation visits were identified using diagnosis-related group code, revenue code, the type of provider, or place of service in medical tables. Cardiology visits were defined by outpatient encounters with cardiology-related ICD codes including established CVD and ASCVD visits. Rehabilitation after an event was defined by rehabilitation visits with a diagnosis of MI, stroke, or PAD.
Annualized rates of patients with at least 1 MI, stroke (ischemic or hemorrhagic), or coronary revascularization event were reported and compared for normal and elevated Lp(a) groups. In addition, odds ratios (ORs) were reported to compare the occurrence of MI, ischemic stroke (fatal or nonfatal), and coronary revascularization events, between normal and elevated Lp(a) groups.
STATISTICAL METHODS
Continuous variables were summarized as either mean±SD or median (IQR), whereas all categorical variables were summarized as frequencies and percentages. Patient demographics were compared using t-tests for continuous variables and Fisher’s exact test for categorical variables. P values for categorical variables were calculated using Fisher’s exact tests, due to imbalance of groups, and for continuous variables using t-tests. The Mann-Whitney U-test was used to compare median statistics.
Inpatient hospitalizations, outpatient visits, ED visits, and rate of composite MI, stroke (ischemic or hemorrhagic), or coronary revascularization are presented as annualized rates per 100 person-years within the 1-year follow-up period. Annualized rate was calculated as the number of encounters divided by person-years in follow-up. Poisson regression was used with the Lp(a) group as the primary exposure, using log follow-up time as an offset. Variances were calculated with a robust sandwich estimator22; 95% CIs and P values comparing incidence rates for normal and elevated Lp(a) groups come directly from the regression model. Adjusted incidence rates for the normal and elevated Lp(a) groups are the least-squares means centered at the mean covariate values in our dataset. Covariate adjustment was done for age (as continuous), sex, baseline statin use, and baseline diabetes.
Logistic regression was used to calculate and compare ORs for occurrence of MI, IS (fatal or nonfatal), or coronary revascularization, between normal and elevated Lp(a), with the Lp(a) group as the primary exposure. Adjusted ORs are the exponentiated coefficient on the Lp(a) variable with P values coming directly from the regression model. Covariate adjustment was done for age (as continuous), sex, baseline statin use, and baseline diabetes. All analyses were performed using SAS statistical package, version 3.8.1 (SAS Institute).
Results
In total, 8,372 patients met our study inclusion criteria of ASCVD and had Lp(a) measurement in nmol/L (Supplementary Figure 2 (2.4MB, pdf) ). Of these, 7,633 patients had data available for the full 1-year follow-up period.
BASELINE CHARACTERISTICS
Demographics and clinical characteristics across normal Lp(a) (< 65 nmol/L) and elevated Lp(a) (≥ 150 nmol/L) groups are presented in Table 1. The baseline demographics and clinical characteristics were similar across normal and elevated Lp(a) groups in terms of age, health plan, index diagnosis, procedures, baseline medications, and comorbidities. However, the proportion of female patients appeared higher in those with elevated Lp(a) (56.52%) compared with patients with normal Lp(a) (49.50%).
TABLE 1.
Demographics and Clinical Characteristics of the ASCVD Population, Stratified by Normal and Elevated Lp(a) Concentration, at Baseline (Index)
| Characteristics | Normal Lp(a) (< 65 nmol/L) | Elevated Lp(a) (≥150 nmol/L) | P value |
|---|---|---|---|
| Total, n | 4,360 | 1,711 | |
| Age, mean (SD), years | 65.70 (12.12) | 65.24 (11.70) | 0.1967 |
| Sex, n (%) | |||
| Male | 2,202 (50.50) | 744 (43.48) | < 0.0001 |
| Female | 2,158 (49.50) | 967 (56.52) | |
| Health plan type, n (%) | |||
| Commercial | 1,547 (35.48) | 619 (36.18) | 0.5670 |
| Medicare | 2,811 (64.47) | 1,092 (63.82) | |
| Index ASCVD diagnosis, n (%) | |||
| MI, PAD, or IS | 3,107 (71.26) | 1,272 (74.34) | 0.0170 |
| MI | 943 (21.63) | 457 (26.71) | < 0.0001 |
| PAD | 1,999 (45.85) | 797 (46.58) | 0.6066 |
| IS | 818 (18.76) | 351 (20.51) | 0.1200 |
| TIA | 536 (12.29) | 174 (10.17) | 0.0210 |
| Unstable angina | 370 (8.49) | 161 (9.41) | 0.2666 |
| Stable angina | 998 (22.89) | 387 (22.62) | 0.8384 |
| Postrevascularization | 372 (8.53) | 180 (10.52) | 0.0172 |
| Index diagnosis not MI, PAD, or IS | 1,807 (41.44) | 660 (38.57) | 0.0421 |
| Procedures, n (%) | |||
| Dialysis | 44 (1.01) | 12 (0.70) | 0.2982 |
| Revascularization | 328 (7.52) | 154 (9.00) | 0.8914 |
| Baseline medications, n (%) | |||
| Statin | 1,871 (42.91) | 937 (54.76) | < 0.0001 |
| ACEIs/ARBs | 1,814 (41.61) | 729 (42.61) | 0.4878 |
| β-blockers | 1,191 (27.32) | 529 (30.92) | 0.0053 |
| Antiplatelets | 228 (5.23) | 108 (6.31) | 0.1047 |
| Fibrates | 216 (4.95) | 56 (3.27) | 0.0038 |
| Ezetimibe | 105 (2.41) | 73 (4.27) | 0.0002 |
| PCSK9i (alirocumab, evolocumab) | 2 (0.05) | 2 (0.12) | 0.3164 |
| None of the above treatments | 1,394 (31.97) | 445 (26.01) | < 0.0001 |
| Lp(a), median (Q1, Q3) | 23.00 (14.00, 39.00) | 221.00 (180.00, 291.00) | < 0.0001 |
| Laboratory values, mean (SD)a | |||
| LDL-C, mg/dL | 104.81 (37.39), n = 2,085 | 113.35 (41.23), n = 774 | < 0.0001 |
| HDL-C, mg/dL | 54.65 (18.25), n = 2,071 | 57.38 (17.70), n = 768 | 0.0004 |
| Total cholesterol, mg/dL | 186.64 (43.95), n = 2,088 | 197.24 (48.64), n = 781 | < 0.0001 |
| Triglycerides, mg/dL | 139.68 (88.30), n = 2,092 | 136.47 (114.59), n = 775 | 0.4806 |
| Hs-CRP, mg/L | 4.91 (14.63), n = 494 | 4.47 (6.26), n = 175 | 0.5859 |
| Baseline cardiovascular comorbidities, n (%)b | |||
| Hypertension | 3,165 (72.59) | 1,268 (74.11) | 0.2346 |
| Atrial fibrillation | 345 (7.91) | 119 (6.95) | 0.2171 |
| Cardiac amyloidosis | 5 (0.11) | 1 (0.06) | 1.0000 |
| Chronic kidney disease (stage III) | 372 (8.53) | 173 (10.11) | 0.0578 |
| Chronic kidney disease (stage IV-V) | 89 (2.04) | 30 (1.75) | 0.5371 |
| Heart failure | 334 (7.66) | 158 (9.23) | 0.0469 |
| Aortic valve stenosis | 232 (5.32) | 100 (5.84) | 0.4153 |
a This endpoint was identified in the baseline period (12 months before the index date, including the index date).
b This endpoint was identified any time before the index date, not including the index date.
ACEIs = angiotensin-converting enzyme inhibitors; ARBs = angiotensin II receptor blockers; ASCVD = atherosclerotic cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; hs-CRP = high-sensitivity C-reactive protein; IS = ischemic stroke; Lp(a) = lipoprotein(a); LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; PAD = peripheral artery disease; Q = quartile; TIA = transient ischemic attack.
The most common index diagnosis across normal and elevated Lp(a) groups was PAD (45.85% and 46.58%), MI (21.63% and 26.71%), and IS (18.76% and 20.51%), respectively. The leading medication prescribed at baseline across the normal and elevated Lp(a) groups was statins (42.91% and 54.76%), showing a significant difference in the elevated Lp(a) group (P < 0.0001). The most common comorbidity at baseline across the normal and elevated Lp(a) groups was hypertension (72.59% and 74.11%; P = 0.2346). Demographics and clinical characteristics across subgroups of patients with MI, PAD, and IS are presented in Supplementary Table 1 (2.4MB, pdf) .
RATE OF INPATIENT HOSPITALIZATIONS
At 1 year of follow-up, inpatient hospitalization rates were significantly higher in the elevated Lp(a) group than the normal Lp(a) group for ASCVD-specific hospitalizations (13.33 vs 9.46; P < 0.0001), IS hospitalizations (4.01 vs 2.17; P = 0.0019), and postrevascularization hospitalizations (5.34 vs 3.99; P = 0.0037) (Figure 1). A numerical trend of higher hospitalization rates was observed for all other assessed primary diagnoses for hospitalization.
FIGURE 1.
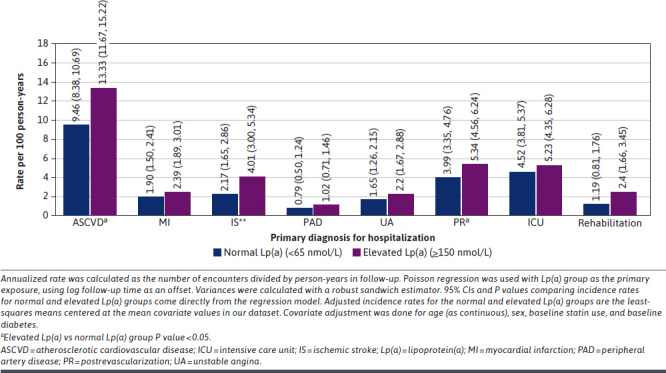
Rate of Inpatient Hospitalizations per 100 Person-Years for Patients With Normal and Elevated Lp(a), Within 1 Year of Follow-Up
Consistent trends were also shown in the subgroups of patients with MI, PAD, and IS index diagnoses (Supplementary Figures 3-5 (2.4MB, pdf) ). In the subgroup of patients with MI index diagnosis, inpatient hospitalization rates at 1 year of follow-up were significantly higher in the elevated Lp(a) than in the normal Lp(a) group for IS hospitalizations (3.20 vs 0.70; P = 0.0018) and postrevascularization hospitalizations (9.54 vs 6.36; P = 0.0108). In the subgroup of patient with IS index diagnosis, inpatient hospitalization rates were significantly higher in the elevated Lp(a) group than in the normal Lp(a) group for ASCVD-specific (28.35 vs 18.07; P = 0.0083) and IS (19.57 vs 10.74; P = 0.0041) hospitalizations. In the smallest subgroup of patients with PAD index diagnosis, the rates of inpatient hospitalizations were observed to be numerically higher in the elevated Lp(a) group than in the normal Lp(a) group.
INPATIENT LOS
In the 1 year of follow-up, the mean (SD) LOS per ASCVD-specific hospitalization was higher in the elevated Lp(a) group than in the normal Lp(a) group (7.21 [12.0] days vs 6.26 [8.51] days; P = 0.3462). Particularly, the mean (SD) LOS per MI-related hospitalizations was higher in the elevated Lp(a) group (11.26 [22.0] days) than in the normal Lp(a) group (6.53 [7.11] days; P = 0.2222); however, none of those result were statistically significant (P > 0.05).
In the subgroup of patients with MI, PAD, or IS as index diagnosis, there was no statistically significant difference in the mean LOS per ASCVD-related hospitalizations between elevated and normal Lp(a) groups.
RATE OF OUTPATIENT AND ED VISITS
Rates of outpatient and ED visits are presented in Supplementary Figures 6 and 7 (2.4MB, pdf) , respectively. Significantly higher rates of cardiology outpatient visits were reported in the elevated Lp(a) group compared with the normal Lp(a) group (468.10 vs 410.56; P = 0.0007). The rates of total outpatient and ED visits at 1 year of follow-up were numerically, but not statistically (P > 0.05), higher in the elevated Lp(a) than in the normal Lp(a) group for ASCVD-specific, MI-related, PAD-related, and IS-related visits.
PROCEDURES AND MEDICATIONS AT BASELINE AND AT 1 YEAR POST-INDEX
The proportion of patients who had a revascularization procedure at baseline was 7.52% in the normal Lp(a) and 9.0% in the elevated Lp(a) group (P = 0.8914). At 1 year post-index, 15% of patients in the elevated Lp(a) group underwent revascularization compared with 10% of patients in the normal Lp(a) group (P = 0.0002).
The most common medications prescribed at baseline were statins, β-blockers, and angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers (ACEIs/ARBs) (Figure 2). The proportion of patients receiving statins and β-blockers at baseline in the elevated Lp(a) group was significantly higher compared with the normal Lp(a) group (54.76% vs 42.91%, P < 0.0001, and 30.92% vs 27.32%, P = 0.0183, respectively). A significant difference was also observed in the proportion of patients receiving antiplatelet therapy and ezetimibe in elevated Lp(a) vs normal Lp(a) group (6.31% vs 5.23%, P < 0.0001, and 4.27 vs 2.41, P < 0.0001, respectively). However, there was no significant difference in the proportion of patients receiving ACEIs/ARBs in the elevated Lp(a) and normal Lp(a) groups: 42.61% and 41.61% (P = 0.7076), respectively.
FIGURE 2.
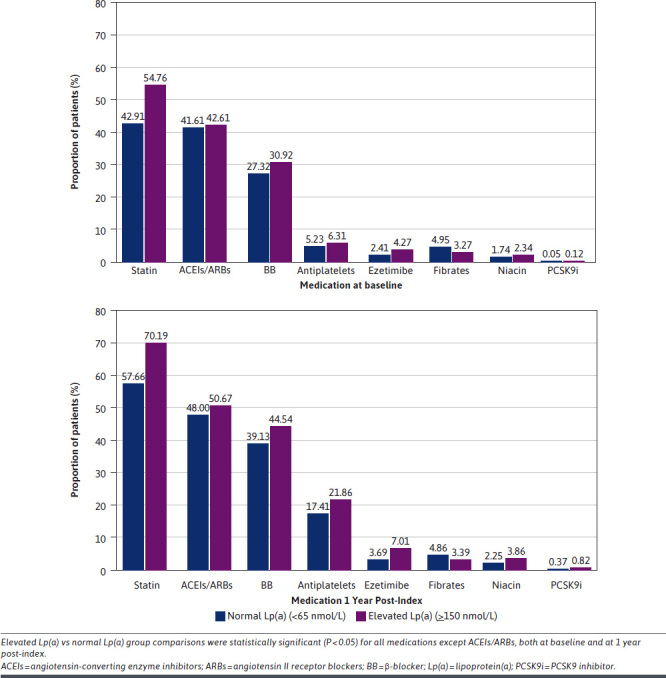
Proportion of Patients With Normal and Elevated Lp(a) Receiving Medications at Baseline and During the 1-Year Post-Index Period Across the Lp(a) Groups
Similar findings were observed at the 1-year post-index follow-up period comparing elevated and normal Lp(a) groups with significant results for statins (70.19% vs 57.66%; P < 0.0001), β-blockers (44.54% vs 39.13%; P = 0.0001), platelet therapy (21.86% vs 17.41%, P < 0.0001), and ezetimibe (7.01 vs 3.69; P < 0.0001), and findings were nonsignificant for ACEIs/ARBs (50.67% vs 48.0%; P = 0.0636).
Across both normal and elevated Lp(a) groups, a relative increase was observed in the proportion of patients receiving statins, ACEIs/ARBs, β-blockers, antiplatelet therapy, and ezetimibe during the 1-year post-index follow-up period compared with the proportion of patients receiving these medications at baseline (Figure 2). The proportion of patients receiving statins increased from 42.91% at baseline to 57.66% at 1-year post-index follow-up in the normal Lp(a) group and 54.76% to 70.19% in the elevated Lp(a) group. The proportion of patients receiving ACEIs/ARBs, β-blockers, antiplatelet therapy, and ezetimibe in the normal Lp(a) group at baseline was 41.61%, 27.32%, 5.23%, and 2.41%, respectively, which increased to 48.0%, 39.13%, 17.41%, and 3.69% at 1 year post-index. Similarly, in the elevated Lp(a) group, the proportion of patients receiving ACEIs/ARBs, β-blockers, antiplatelet therapy, and ezetimibe at baseline was 42.61%, 27.32%, 6.31% and 4.27%, respectively, which increased to 50.67%, 44.54%, 21.86% and 7.01% at 1 year post-index.
COMPOSITE MI, STROKE, AND REVASCULARIZATION RATES AND OCCURRENCE OF EVENTS
During the 1-year follow-up period, the rate of composite MI, stroke (ischemic or hemorrhagic), or revascularization was significantly higher in the elevated Lp(a) group than in the normal Lp(a) group (10.19 vs 7.88; P < 0.0001) (Figure 3). The rate of coronary revascularization was significantly higher in the elevated Lp(a) group than in the normal Lp(a) group (6.81 vs 5.40; P = 0.0003). Although the rates of MI and stroke (ischemic or hemorrhagic) were numerically higher in the elevated Lp(a) group than in the normal Lp(a) group, the difference was not significant (P > 0.05) (Figure 6).
FIGURE 3.
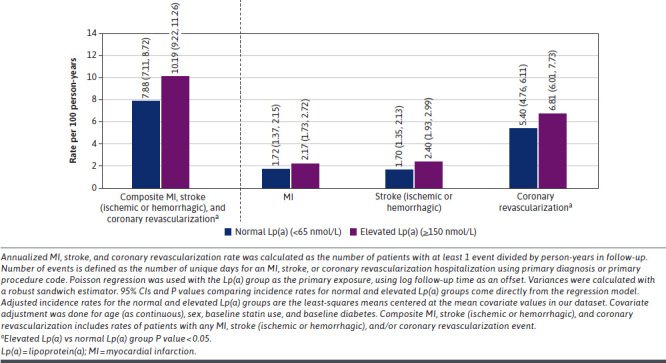
Rate of Composite MI, Stroke (Ischemic or Hemorrhagic), and Coronary Revascularization per 100 Person-Years for Patients With Normal and Elevated Lp(a) With 1-Year Follow-Up
Figure 4 presents odds ratios (ORs) (95% CIs) comparing the occurrence of composite MI, IS (fatal or nonfatal), and coronary revascularization events in the elevated vs normal Lp(a) groups. The OR (95% CI) for composite MI, IS, and coronary revascularization was 1.46 (1.20-1.77). This suggests an increased odds associated with elevated Lp(a) levels. Increased odds of coronary revascularization were also observed to be associated with elevated Lp(a), with an OR (95% CI) of 1.53 (1.23-1.91).
FIGURE 4.
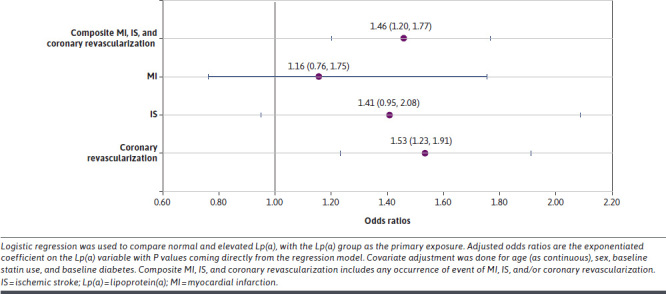
Forest Plot for Composite MI, Ischemic Stroke, and Coronary Revascularization Occurrence of Events Odds Ratios for Patients With Elevated (≥150 nmol/L) vs Normal Lp(a), With 1-Year Follow-Up
Discussion
This real-world study using US claims data provides the first insights on the increased health care resource use of patients with elevated Lp(a) compared with those with normal Lp(a). Within the first year of ASCVD diagnosis, elevated Lp(a) was associated with higher rates of inpatient hospitalizations and increased LOS per hospitalization and higher risk of cardiovascular events (MI, stroke, and coronary revascularization).
For MI-related hospitalization, it was observed that the mean LOS per hospitalization was almost double in the elevated Lp(a) group compared with the normal Lp(a) group. The doubled LOS, although not statistically significant, may suggest that patients with elevated Lp(a) experience a more severe course of disease, but future investigation is warranted. It was also observed that within the first year of ASCVD diagnosis, a significantly higher proportion of patients with elevated compared with normal Lp(a) levels required revascularization procedures. Indeed, the odds (OR = 1.53) of having coronary revascularization were significantly higher in the elevated Lp(a) group. The occurrence of composite MI, IS, and coronary revascularization was also found to be significantly higher with elevated Lp(a) (OR = 1.46). These results are in line with a recent multicenter, retrospective observational study assessing 765 patients with acute coronary syndrome from China, which reported significantly higher incidence of recurrent CV events and revascularization in the high-Lp(a) group (Lp(a) ≥ 30 mg/dL) than in the low-Lp(a) group (Lp(a) < 30 mg/dL) (P < 0.05).23 In another study among patients who underwent coronary artery bypass grafting, elevated Lp(a) levels were significantly associated with an increased rate of 1-year vein graft occlusions and long-term CV outcomes.24
In our study, we observed trends of increased HCRU among patients with elevated Lp(a). It is evident from the literature that despite treatment, patients with ASCVD remain at high risk for CV events, placing a significant and increasing burden on the US health care system.16,25,26 The United States spends an estimated $126 billion direct costs annually for patients with coronary artery disease,27 $21 billion for PAD,28 $19 billion for stroke,29 and $75 billion for acute coronary syndrome.30
The study by Zhao et al examined HCRU and total health care costs for patients with ASCVD in a commercially insured population in the United States.31 The total mean (SD) health care cost per patient during the first year of follow-up was estimated to be $8,699 ($25,655), with inpatient care representing the majority (53%; $4,587 [$22,486]) of ASCVD-related total costs, followed by outpatient care (31%; $2,727 [7,862]) and pharmacy costs (16%; $1,385 [$1,838]).31 An HCRU study among patients with ASCVD in Italy indicated that the rates of hospitalization for CV events are high and are associated with significant costs, with hospitalization accounting for most of the total costs.32 Given our observed increased rate and LOS of hospitalizations, and higher inpatient and outpatient resource utilization due to elevated Lp(a), this may suggest that elevated Lp(a) contributes substantially to the economic burden related to ASCVD, which was previously unrecognized.
A review conducted by Nicholson et al synthesized international cost estimates of CV events based on studies published during 2007-2012 and adjusted all costs for inflation to 2013 values.33 The coronary revascularization—coronary bypass graft surgery—cost in the United States averaged $57,577 (median $61,445), with a range of cost estimates from $17,731 to $124,221 in 11 studies. The percutaneous coronary intervention cost in the United States averaged $20,146 (median $19,429), with a range from $16,104 to $25,641 based on 6 studies. Mean percutaneous coronary intervention costs in the United States ($20,146) were nearly one-third of the mean coronary bypass graft surgery costs in US studies ($57,577).33 This review indicates that the worldwide cost burden of CVD remains significant. Although comparison of results is hampered by the differences in study design, several themes emerge. The findings from the literature suggest that in the United States and Europe, revascularization procedures and MI are CV events with the highest acute costs. The increased occurrence of coronary revascularization procedures and rates of MI-related hospitalizations and LOS observed among patients with ASCVD with elevated Lp(a) in this study therefore highlights important health care cost implications for the US population.
It was observed in our study that within 1 year of follow-up, a significantly higher proportion of patients received statins in the elevated Lp(a) than in the normal Lp(a) group. Direct Lp(a) testing in clinical practice would help in identifying patients with increased disease risk and introduce statin or other lipid-lowering therapies early. A future study with a longer follow-up period will help ascertain this relationship and even explore the potential association of Lp(a) testing with changes in other health behaviors.
These findings indicate that early identification of at-risk patients by Lp(a) testing can play an important role in reducing HCRU and easing the economic burden on the US health care system. However, based on the data for the current study, it was observed that less than 1% of patients with ASCVD had Lp(a) measurements, indicating a lack of Lp(a) screening and awareness in routine clinical practice.
LIMITATIONS
Although our study provides an understanding of routine practice in the United States, the results should be interpreted within the context of potential limitations. First, owing to the nature of the claims data, the diagnoses and data recorded may be subject to coding errors. In the Optum CDM database, approximately 30% of patients had laboratory data; however, for our ASCVD cohort, 68.5% had available laboratory data. Therefore, only a subset of patients who had an Lp(a) measurement in the real world may have been included in this study. Second, as Lp(a) is not tested routinely, the results may have had a selection bias, as it was uncertain who the patients receiving the test were and whether this was at random or for a specific cause. Third, only Lp(a) levels reported in nmol/L were included in this analysis, and the majority of Lp(a) measurements reported in nmol/L pertain to patients with Medicare, which is not representative of the entirety of the ASCVD population with an Lp(a) measurement in the United States. Measurements in nmol/L were the more contemporary measurements in the Optum database and were supported by current clinical guideline recommendations moving toward reporting Lp(a) thresholds in molar concentration units (nmol/L), reflecting actual particle numbers; this is a positive development given that Lp(a) exists as isoforms of varying molecular weights, making mass measurement a less accurate method.9,11,13,34-37 This represents an older population and may miss those with more premature stages of the disease. Fourth, the cost component was not evaluated in the current study. Lastly, there were inherent limitations associated with the retrospective study design and the secondary use of data, including missing data among others.
Conclusions
Our observed trends of higher rates of hospitalizations, increased LOS per hospitalization, requirements for coronary revascularization procedures, and significantly increased odds of CV events within the first year of index indicate substantial HCRU among patients with ASCVD with elevated Lp(a) (≥ 150 nmol/L). Such demand for health care resources within the first year of ASCVD diagnosis causes a substantial burden on individual patients with elevated Lp(a) as well as on the health care system. Implementation of Lp(a) testing in routine clinical practice could help in identification of high-risk patients with ASCVD and play an important role in the overall CV risk management, aiming to reduce the HCRU associated with ASCVD.
ACKNOWLEDGMENTS
The authors thank Japinder Kaur for providing medical writing assistance with this manuscript.
REFERENCES
- 1.Tsimikas S. A test in context: Lipoprotein(a): Diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692-711. doi:10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 2.Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71(2):177-192. doi:10.1016/j.jacc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess S, Ference BA, Staley JR, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: A Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627. doi:10.1001/jamacardio.2018.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamina C, Kronenberg F; Lp-GWAS-Consortium. Estimation of the required lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes: A Mendelian randomization analysis. JAMA Cardiol. 2019;4(6):575-579. doi:10.1001/jamacardio.2019.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK. Lipoprotein(a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 2019;71(2):99-112. doi:10.1016/j.ihj.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. doi:10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 7.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412-23. doi:10.1001/jama.2009.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339. doi:10.1001/jama.2009.801 [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021. Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129-1150. doi:10.1016/j.cjca.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 11.Wilson DP, Jacobson TA, Jones PH, et al. Use of lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374-392. doi:10.1016/j.jacl.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 12.Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Management of Dyslipidemia and Prevention of Cardiovascular Disease Algorithm - 2020 executive summary. Endocr Pract. 2020;26(10):1196-1224. doi:10.4158/CS-2020-0490 [DOI] [PubMed] [Google Scholar]
- 13.Mach F, Baigent C, Catapano AL, et al. 2019. ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi:10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 14.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi:10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation. 2014;129(3):e28-e292. doi:10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khera R, Valero-Elizondo J, Nasir K. Financial toxicity in atherosclerotic cardiovascular disease in the United States: Current state and future directions. J Am Heart Assoc. 2020;9(19):e017793. doi:10.1161/JAHA.120.017793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg F. Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc Drugs Ther. 2016;30(1):87-100. doi:10.1007/s10557-016-6648-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: A population-based study. Arterioscler Thromb Vasc Biol. 2020;40(1):255-266. doi:10.1161/ATVBAHA.119.312951 [DOI] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Wilson Tang WH, Scoffone H, et al. Lipoprotein(a) levels and long-term cardiovascular risk in the contemporary era of statin therapy. J Lipid Res. 2010;51(10):3055-3061. doi:10.1194/jlr.M008961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel AP, Wang M, Pirruccello JP, et al. Lp(a) (lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: New insights from a large national biobank. Arterioscler Thromb Vasc Biol. 2021;41(1):465-474. doi:10.1161/atvbaha.120.315291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UCSF Health. Lipoprotein-a. Accessed January 20, 2023. https://www.ucsfhealth.org/medical-tests/007262
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi:10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 23.Yang SQ, Liu HX, Yu XQ, et al. Elevated lipoprotein(a) levels as an independent predictor of long-term recurrent events in patients with acute coronary syndrome: An observational, retrospective cohort study. Coron Artery Dis. 2022;33(5):385-393. doi:10.1097/MCA.0000000000001134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezhov MV, Afanasieva OI, Il’ina LN, et al. Association of lipoprotein(a) level with short- and long-term outcomes after CABG: The role of lipoprotein apheresis. Atheroscler Suppl. 2017;30:187-192. doi:10.1016/j.atherosclerosissup.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 25.Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14(1):1-10. doi:10.1007/s11883-011-0219-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora S, Wenger NK, DeMicco DA, et al. Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy. Circulation. 2012;125:1979-1987. doi:10.1161/CIRCULATIONAHA.111.088591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124(9):827-33 e5. doi:10.1016/j.amjmed.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3(6):642-51. doi:10.1161/CIRCOUTCOMES.109.930735 [DOI] [PubMed] [Google Scholar]
- 29.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125(1):e2-e220. doi:10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turpie AG. Burden of disease: Medical and economic impact of acute coronary syndromes. Am J Manag Care. 2006;12(16 Suppl):S430-S434. [PubMed] [Google Scholar]
- 31.Zhao Z, Zhu Y, Fang Y, Ye W, McCollam P. Healthcare resource utilization and costs in working-age patients with high-risk atherosclerotic cardiovascular disease: Findings from a multi-employer claims database. J Med Econ. 2015;18(9):655-65. doi:10.3111/13696998.2015.1041966 [DOI] [PubMed] [Google Scholar]
- 32.Sciattella P, Maggioni AP, Arcangeli E, Sidelnikov E, Kahangire DA, Mennini FS. Healthcare resource utilization, cardiovascular event rate and use of lipid-lowering therapies in secondary prevention of ASCVD in hospitalized patients in Italy. Adv Ther. 2022;39(1):314-327. doi:10.1007/s12325-021-01960-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson G, Gandra SR, Halbert RJ, Richhariya A, Nordyke RJ. Patient-level costs of major cardiovascular conditions: A review of the international literature. Clinicoecon Outcomes Res. 2016;8:495-506. doi:10.2147/CEOR.S89331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes-Soffer G. Ginsberg HN, Berglund L, et al. Lipoprotein(a): A genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e48-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langlois MR, Chapman MJ, Cobbaert C, et al. Quantifying atherogenic lipoproteins: Current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A consensus statement from EAS and EFLM. Clin Chem. 2018;64(7):1006-1033. doi:10.1373/clinchem.2018.287037 [DOI] [PubMed] [Google Scholar]
- 37.Nordestgaard BG, Langlois MR, Langsted A, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: Consensus-based recommendations from EAS and EFLM. Atherosclerosis. 2020;294:46-61. doi:10.1016/j.atherosclerosis.2019.12.005 [DOI] [PubMed] [Google Scholar]


