Abstract
BACKGROUND:
Chronic kidney disease (CKD) is a major public health concern that affects 37 million adults in the United States. It is well known that CKD presents a large economic burden, especially in the Medicare population. However, studies of the economic burden of CKD in younger populations are scarce. In particular, there is a gap in understanding how the presence of type 2 diabetes mellitus (T2DM) affects the burden of CKD in commercially insured populations.
OBJECTIVES:
To describe the economic and health care resource utilization (HCRU) burden of CKD within 3 patient groups (T2DM only, CKD only, and CKD and T2DM) aged 45-64 years overall and by Kidney Disease Improving Global Outcomes (KDIGO) CKD estimated glomerular filtration rate–based stage categories.
METHODS:
A descriptive, observational retrospective cohort study was conducted using administrative medical and pharmacy claims integrated with laboratory results data available in the HealthCore Integrated Research Database from January 1, 2017, to December 31, 2019. Three mutually exclusive groups of commercially insured patients aged 45-64 years were identified: T2DM only, CKD only, and CKD and T2DM. All-cause and disease-specific HCRU and costs in total, by medical and pharmacy benefits and across all places of service, were described for each of these groups 12 months after index date. For the CKD only and CKD and T2DM groups, costs were also described by KDIGO CKD stage.
RESULTS:
The CKD and T2DM group (n = 13,052) had numerically higher 12-month post-index all-cause and CKD/T2DM-related HCRU across all places of service. Mean 12-month all-cause costs for this group were $35,649, whereas costs for the CKD only group (n = 7,876) were $25,010 and costs for the T2DM only group (n = 120,364) were $16,121. Costs also tended to increase as CKD stage increased, with the greatest increases beginning at KDIGO stage 3b and higher. Mean 12-month all-cause costs for the CKD and T2DM group ranged from $29,993 to $41,222 for stages 1 to 3a and from $46,796 to $119,944 for stages 3b to 5.
CONCLUSIONS:
Commercially insured patients aged 45-64 years with CKD, especially those who also have T2DM, present a substantial burden in terms of elevated HCRU and costs. Costs tend to increase across KDIGO CKD stages and increase most rapidly at stage 3b and later. Therefore, there is an opportunity to reduce the burden of CKD in this population by investing in interventions to prevent or delay CKD disease progression.
Plain language summary
This study describes health care use and costs across 3 groups. The 3 groups were patients with type 2 diabetes mellitus (T2DM), patients with chronic kidney disease (CKD), and patients with both. All patients were aged 45-64 years. Health care use and costs were highest among those with both and increased in later stages of CKD.
Implications for managed care pharmacy
These contemporary costs associated with CKD with and without T2DM provide an economic foundation to assist in the evaluation of new therapies entering the market for the treatment of individuals with CKD and T2DM.
Chronic kidney disease (CKD) is a major public health concern. Defined as abnormalities of kidney structure or function that are present for more than 3 months, CKD can be classified into stages with worsening prognoses based on estimated glomerular filtration rate (eGFR).1 Most recent estimates suggest approximately 1 in 7 (37 million) adults in the United States have CKD (excluding those with end-stage renal disease [ESRD] when treatment options become limited).2 CKD is expected to become even more common as the US population ages and the prevalence of risk factors for the disease, such as hypertension and obesity, increases among all age groups.3,4
Because CKD is most prevalent among people aged 65 years or older,2 much of the research on the economic burden of CKD has focused on the Medicare population. Costs from these patients (excluding those with ESRD) accounted for more than 22% ($81 billion) of Medicare fee-for-service spending in 2018. Additionally, increasing CKD stage and common comorbidities among these patients, such as diabetes and heart failure, contribute considerably to their cost of care.3,5
For commercial payers, the documented burden of CKD is more limited. Research has shown that the increase in all-cause costs due to increasing CKD stage among patients younger than 65 years is similar to the observed increase among patients aged 65 years and older.6 However, there remains a gap in understanding how comorbidities affect the burden of CKD in commercially insured patients. Given that diabetes is a leading cause of CKD and an estimated 44% of patients with type 2 diabetes mellitus (T2DM) have CKD,7 it is particularly important to understand how costs may differ for patients who have both CKD and T2DM in a commercially insured population.
To fill this gap, the current study was designed to describe the economic and health care resource utilization (HCRU) burden of CKD within 3 patient groups (T2DM only, CKD only, and both CKD and T2DM) aged 45-64 years with commercial health insurance. For patients with CKD only and CKD and T2DM, the cost burden was also described by Kidney Disease Improving Global Outcomes (KDIGO) CKD eGFR-based stage categories. To our knowledge, our article will be among the first to describe this burden in a commercially insured population. Findings will provide valuable baseline information regarding the potential for cost savings for commercial payers by preventing CKD progression.
Methods
STUDY DESIGN AND DATA SOURCE
An observational, descriptive retrospective cohort study was conducted using administrative medical and pharmacy claims submitted to the HealthCore Integrated Research Database (HIRD) from January 1, 2017, to December 31, 2019 (study period). The study period was extended back to January 1, 2006, in defining the study cohorts. The HIRD includes adjudicated administrative claims for 14 commercial and Medicare Advantage insurance plans, which covered more than 70 million enrollees from all US census regions at the time of this study. Health plan enrollment information is available in the HIRD, including the start and end date of members’ eligibility segments as well as demographic information. A subset of members (approximately 40%) have available laboratory results data. Individual-level race data were not fully available in the HIRD.
The management of all data and study materials conformed with the Health Insurance Portability and Accountability Act (HIPAA) rules. A limited dataset, which excluded patient-identifying information, was used for all analyses, as defined by the HIPAA Privacy Rule.
A study design diagram is presented in Supplementary Figure 1 (570.1KB, pdf) , available in online article.
SAMPLE SELECTION
Three mutually exclusive groups were identified: patients with T2DM without CKD (T2DM only), patients with CKD without T2DM (CKD only), and patients with both CKD and T2DM (CKD and T2DM) from January 1, 2018, to December 31, 2018 (patient identification period). The date when patients first met criteria for T2DM or CKD during the patient identification period was defined as their index date.
All patients were required to be aged 18 years or older as of the index date and have at least 12 months of continuous health plan enrollment prior to and following the index date. The patient cohort was then restricted to those aged 45-64 years with commercial insurance. The 12 months prior to and not including the index date was referred to as the baseline period, and the 12 months including and following the index date was referred to as the post-index period. Patients were excluded if they met any of the following criteria: claim indicating dialysis (including both hemodialysis and peritoneal dialysis), ESRD, or kidney transplant during the baseline period; claim indicating pregnancy during the study period; claim indicating any cancer (excluding basal cell carcinoma) during the study period; or enrolled in a Medicare plan on the index date. Additional group-specific criteria are reviewed below.
Patients in the T2DM only group were additionally required to have either (1) at least 2 claims with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes for T2DM on distinct service dates or (2) at least 1 fill for antidiabetic medication and at least 1 claim for T2DM during the patient identification period. Patients were excluded from this group if they had a diagnosis for type 1 diabetes mellitus (T1DM), which was defined as having at least 2 claims with ICD-10-CM codes for T1DM on distinct service dates, having at least 1 claim for insulin or insulin pump, and having no fill for noninsulin diabetes medications except metformin during the patient identification period. Patients were also excluded from this group if they had any claims for CKD prior to the index date (using all available data dating back to January 1, 2006).
Patients in the CKD only group were additionally required to have at least 2 claims with ICD-10-CM codes for CKD on distinct service dates during the patient identification period. Patients were excluded from this group if they had claims with International Classification of Diseases, Ninth Revision, Clinical Modification or ICD-10-CM codes for T2DM or ICD-10-CM codes for T1DM any time prior to the index date (using all available data dating back to January 1, 2006).
Patients in the CKD and T2DM group were additionally required to either (1) have at least 2 claims with ICD-10-CM codes specific for CKD and T2DM or (2) meet criteria for either T2DM or CKD during the patient identification period and have at least 1 claim for the other any time prior to the index date (using all available data dating back to January 1, 2006).
Patients in the CKD only and CKD and T2DM group with available eGFR values during the baseline or post-index periods were assigned to a KDIGO CKD stage category per the 2012 guidelines.8 Because only a small fraction of the patients had results for a urinary albumin creatinine ratio, only eGFR was used to place the patients into the KDIGO CKD categories. eGFR values were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) equation. The CKD-Epi equation is based on serum creatinine value, age, sex, and race. This study assumed White race because race information was not available in the data and prior studies in this dataset that have captured race have shown that the vast majority (≥ 90%) of members in the HIRD population are White.9-11 If multiple eGFR values were present, the lowest eGFR was used for assignment purposes. Based on KDIGO guidelines, patients were assigned to the following CKD stages based on their corresponding eGFR values (stage 1: eGFR ≥90, stage 2: eGFR 60-89, stage 3a: eGFR 45-59, stage 3b: eGFR 30-44, stage 4: eGFR 15-29, and stage 5: eGFR <15).
All codes described for patient selection are listed in Supplementary Table 1 (570.1KB, pdf) .
OUTCOME MEASURES
Outcome measures included post-index all-cause and CKD/T2DM-related resource utilization and costs for the 12-month period following the index date. CKD/T2DM-related medical costs were defined as costs for medical claims with ICD-10-CM codes for CKD or T2DM either separately for the appropriate T2DM or CKD only groups or both for the CKD/T2DM group. In addition, medical claims with ICD-10-CM codes for CKD and T2DM were included in the related costs for the CKD/T2DM group. CKD/T2DM-related medical costs in the inpatient and emergency department settings additionally required these codes to be in the primary position. CKD/T2DM-related prescription costs were defined as costs for pharmacy claims with National Drug Code numbers for antidiabetics, antihypertensives, or intravenous/oral iron or Healthcare Common Procedure Coding System codes for erythropoietin-stimulating agents. Resource utilization and cost measures were described both overall and according to place of service (ie, inpatient, emergency department, outpatient, skilled nursing facility, and pharmacy). Costs were reported as a sum of health plan paid and patient paid costs, in which health plan paid costs included coordination of benefit, and patient paid costs included all coinsurance, deductible, and copayment. Costs were adjusted to 2019 US dollars using the medical care component of the Consumer Price Index from the Bureau of Labor Statistics.12
STATISTICAL ANALYSIS
Descriptive statistics were reported for demographic characteristics, baseline clinical characteristics, and post-index all-cause and CKD/T2DM-related HCRU and costs in each of the 3 groups (T2DM only, CKD only, and CKD and T2DM). For the CKD only and CKD and T2DM groups, descriptive statistics for health care costs were also stratified by KDIGO CKD stage. For continuous variables, mean and SD were reported. For categorical variables, frequency and percentages were reported. Given the descriptive nature of our research questions and study design, no inferential statistics were generated. Descriptive statistics were qualitatively compared across the 3 groups. Analyses were completed using software from Instant Health Data (Panalgo) and SAS Enterprise Guide 8.3 (SAS Institute Inc.).
Results
PATIENT POPULATION
Figure 1 displays the study population attrition in detail.
FIGURE 1.
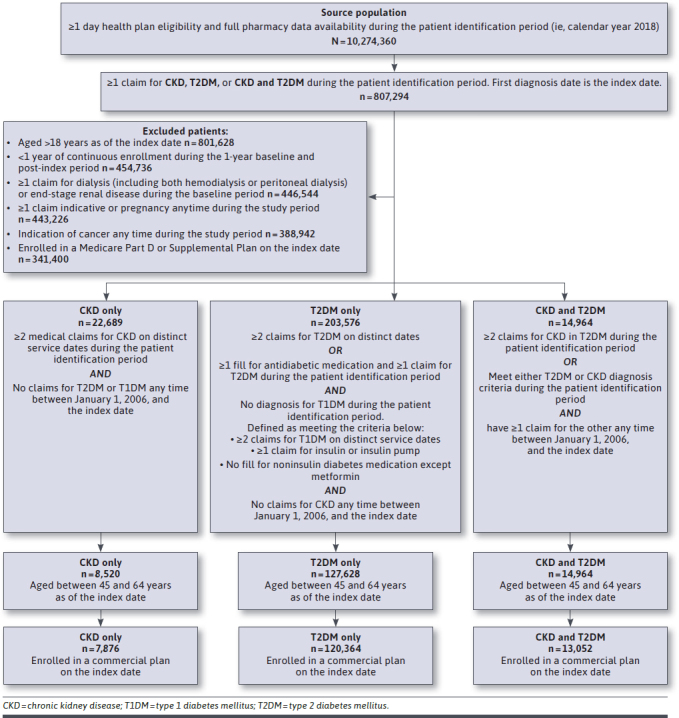
Flowchart of Patient Selection
We identified 10.3 million members with at least 1 day of health plan enrollment from the HIRD between January 1, 2018, and December 31, 2018. After applying inclusion and exclusion criteria, we identified 3 mutually exclusive groups: T2DM only (n = 203,576), CKD only (n = 22,689), and CKD and T2DM (n = 38,587). From these groups, we identified commercially insured members aged 45-64 years as of their index date: 120,364 members with T2DM only, 7,876 members with CKD only, and 13,052 members with CKD and T2DM.
BASELINE PATIENT CHARACTERISTICS
Table 1 describes baseline demographic and clinical characteristics for members in each of the 3 groups.
TABLE 1.
Demographic and Baseline Clinical Characteristics
| T2DM only (n = 120,364) | CKD only (n = 7,876) | CKD and T2DM (n = 13,052) | ||||
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age, mean (SD), years | 55.6 | 5.3 | 56.1 | 5.4 | 56.7 | 5.2 |
| Sex, n (%) | ||||||
| Female | 53,570 | 44.5 | 3,403 | 43.2 | 5,179 | 39.7 |
| Male | 66,794 | 55.5 | 4,473 | 56.8 | 7,873 | 60.3 |
| Plan type, n (%) | ||||||
| HMO | 31,090 | 25.8 | 2,112 | 26.8 | 3,422 | 26.2 |
| PPO | 70,052 | 58.2 | 4,358 | 55.3 | 7,594 | 58.2 |
| CDHP | 19,222 | 16.0 | 1,406 | 17.9 | 2,034 | 15.6 |
| Other | 0 | 0.0 | 0 | 0.0 | ≤ 10 | a |
| Coverage type, n (%) | ||||||
| Commercial | 120,364 | 100.0 | 7,876 | 100.0 | 13,052 | 100.0 |
| Medicare advantage | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Residence region, n (%) | ||||||
| Northeast | 15,938 | 13.2 | 1,007 | 12.8 | 1,613 | 12.4 |
| Midwest | 31,919 | 26.5 | 1,983 | 25.2 | 3,427 | 26.3 |
| South | 50,520 | 42.0 | 3,157 | 40.1 | 5,378 | 41.2 |
| West | 19,941 | 16.6 | 1,576 | 20.0 | 2,412 | 18.5 |
| Missing | 2,046 | 1.7 | 153 | 1.9 | 222 | 1.7 |
| Baseline clinical characteristics | ||||||
| Quan Enhanced-Charlson Comorbidity Index, n (%) | ||||||
| 0 | 83,401 | 69.3 | 2,685 | 34.1 | 2,873 | 22.0 |
| 1 | 22,639 | 18.8 | 3,387 | 43.0 | 4,288 | 32.9 |
| 2 | 9,312 | 7.7 | 913 | 11.6 | 3,304 | 25.3 |
| 3+ | 5,012 | 4.2 | 891 | 11.3 | 2,587 | 19.8 |
| Select comorbid conditions, n (%) | ||||||
| Coronary artery disease | 10,482 | 8.7 | 687 | 8.7 | 2,183 | 16.7 |
| Heart failure | 3,307 | 2.7 | 499 | 6.3 | 1,267 | 9.7 |
| Hypertension | 80,047 | 66.5 | 5,568 | 70.7 | 10,894 | 83.5 |
| Peripheral vascular disease | 5,679 | 4.7 | 513 | 6.5 | 1,553 | 11.9 |
| Depression | 13,461 | 11.2 | 1,073 | 13.6 | 1,797 | 13.8 |
| Dyslipidemia | 78,455 | 65.2 | 4,248 | 53.9 | 10,070 | 77.2 |
| Obesity | 40,857 | 33.9 | 2,142 | 27.2 | 5,520 | 42.3 |
| Diabetic neuropathy | 9,969 | 8.3 | 0 | 0.0 | 2,532 | 19.4 |
| Chronic obstructive pulmonary disease | 7,209 | 6.0 | 576 | 7.3 | 1,091 | 8.4 |
| Sleep apnea | 17,771 | 14.8 | 1,013 | 12.9 | 2,760 | 21.1 |
CDHP = consumer driven health products; CKD = chronic kidney disease; HMO = health maintenance organization; PPO = provider preferred organization; T2D = type 2 diabetes mellitus.
a Because of privacy restrictions, counts less than 11 are not reported.
The age distribution was similar across the 3 groups, with the mean age around 56 years for each group. The T2DM only and CKD only groups had similar sex compositions (44.5% female among the T2DM only group and 43.2% female among the CKD only group), but the proportion of female patients was slightly lower among the CKD and T2DM group (39.7%). In all 3 groups, the majority of the patients resided in the Midwest and South regions of the United States (26.5% and 42.0% among the T2DM only group, 25.2% and 40.1% among the CKD only group, and 26.3% and 41.2% among the CKD and T2DM group, respectively).
The CKD and T2DM group had the highest crude baseline comorbidity burden, followed by the CKD only group and the T2DM only group (Quan Enhanced–Charlson Comorbidity Index13 ≥3: 19.8%, 11.3%, and 4.2%). Among all 3 groups, the most prevalent comorbid conditions were hypertension (66.5%, 70.7%, and 83.5% among T2DM only, CKD only, and CKD and T2DM groups, respectively), dyslipidemia (65.2%, 53.9%, 77.2%), and obesity (33.9%, 27.2%, 42.3%). Cardiovascular conditions such as coronary artery and peripheral vascular disease were approximately twice as prevalent in the CKD and T2DM group compared with the other 2 groups.
12-MONTH POST-INDEX HCRU
The CKD and T2DM group had the highest crude post-index all-cause and CKD/T2DM-related HCRU across all places of service, followed by the CKD only group and the T2DM only group. Specifically, for all-cause utilization, the CKD and T2DM group had the highest proportion of patients with at least 1 inpatient hospitalization (19.4% vs 13.3% vs 8.5%), proportion of patients with at least 1 emergency department visit (24.3% vs 19.0% vs 18.6%), mean (SD) number of outpatient encounters per patient (32.1 [32.3] vs 27.0 [27.6] vs 19.9 [18.8]), and mean number of prescription fills per patient (32.2 [20.3] vs 21.9 [16.9] vs 24.1 [16.6]). The CKD and T2DM group similarly had the highest CKD/T2DM-related utilization (proportion of patients with at least 1 inpatient hospitalization: 7.9% vs 2.7% vs 1.7%, proportion of patients with at least 1 emergency department visit: 3.1% vs 0.8% vs 1.3%, and mean number of outpatient encounters per patient: 12.7 [13.9] vs 6.2 [6.8] vs 7.0 [5.9]). Full results for post-index HCRU are presented in Supplementary Table 2 (570.1KB, pdf) .
12-MONTH POST-INDEX HEALTH CARE COSTS
Figure 2 displays mean all-cause and CKD/T2DM-related crude health care costs per patient by place of service during the post-index period for members in each of the 3 groups. For all-cause costs, the T2DM only group had the lowest mean 12-month total costs ($16,121). Relative to this group, crude costs in the CKD only group were 55% higher ($25,010), and costs in the CKD and T2DM group were more than twice as high ($35,649). For CKD/T2DM-related costs, the mean total 12-month costs were relatively similar in the T2DM only and CKD only groups ($6,388 vs $5,086), and costs in the CKD and T2DM group were about 2-3 times as high as the costs in these groups ($16,078). For both all-cause and CKD/T2DM-related costs, inpatient costs constituted the greatest proportion of mean total costs for the CKD only and CKD and T2DM groups (all-cause: $3,652, $10,016, and $13,492; CKD/T2DM-related: $867, $2,616, and $5,965 [T2DM only, CKD only, and CKD and T2DM, respectively]). Prescription costs constituted the greatest proportion of mean total costs for the T2DM only group (all-cause: $6,134, $5,120, and $10,330; CKD/T2DM-related: $3,351, $378, and $5,355 [T2DM only, CKD only, and CKD and T2DM, respectively]).
FIGURE 2.
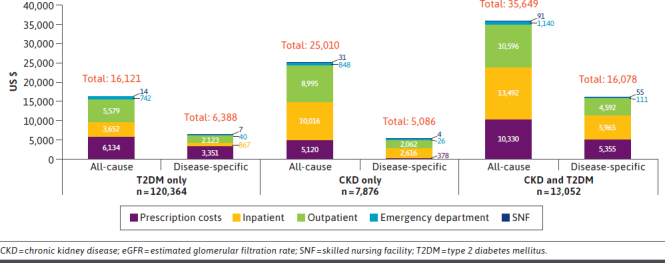
Mean 12-Month Post-Index Total Health Care Spending by Place of Service
Total cost trends were similar when stratified by KDIGO CKD stage (Figures 3 and 4). For a given CKD stage, both all-cause and CKD/T2DM-related crude costs for the CKD and T2DM group tended to be greater than costs for the CKD only group. Costs also tended to increase as CKD stage increased, with increases beginning at KDIGO stage 3b and higher. For example, mean CKD/T2DM-related costs for the CKD and T2DM group were lowest at stage 1 ($13,193) and only slightly increased at stage 2 ($16,982) and stage 3a ($17,452). Compared with these earlier stages, costs were substantially higher at stage 3b ($25,234), stage 4 ($27,023), and stage 5 ($59,121). At each CKD stage in the CKD only and CKD and T2DM groups, all-cause and CKD/T2DM-related medical costs were much higher than pharmacy costs.
FIGURE 3.
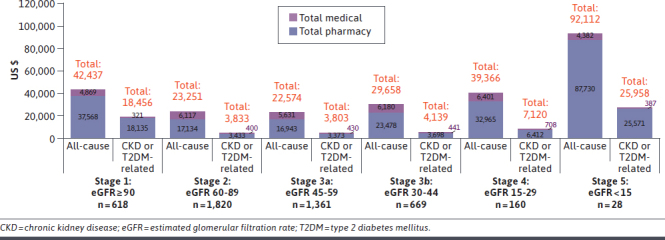
Mean 12-Month Post-Index Total Medical and Pharmacy Spending by CKD Stage: CKD Only
FIGURE 4.
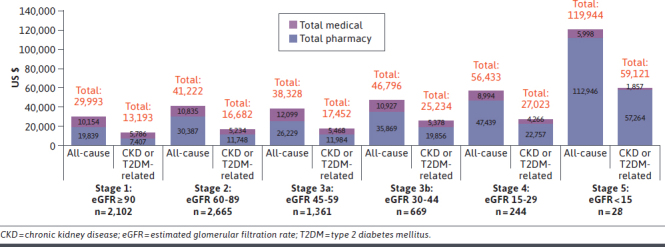
Mean 12-Month Post-Index Total Medical and Pharmacy Spending by CKD Stage: CKD and T2DM
Discussion
Although it is well established that T2DM is a leading cause of CKD,7,14,15 contemporary data describing the HCRU and cost burden of commercially insured patients with both T2DM and CKD are scarce. In this analysis, we described demographic and clinical characteristics, as well as quantified HCRU and costs, for commercially insured patients aged 45-64 years who had T2DM only, CKD only, and both CKD and T2DM in the real-world setting. For those who had CKD with and without T2DM, we also examined these outcomes by KDIGO CKD stage.
Overall, patients with CKD and T2DM presented with the greatest crude utilization and cost burden. Compared with patients with T2DM only ($16,121), patients with CKD only had increased utilization and costs ($25,010), whereas patients with CKD and T2DM had markedly higher utilization across all places of service and more than double the costs ($35,649). This indicates that CKD, especially when combined with T2DM, may impose a notable utilization and cost burden in a commercially insured population. These findings complement prior research that has been typically performed in populations of older patients. For example, Folkerts et al documented average annual costs of $24,029 for patients with T2DM with newly diagnosed CKD from 2008 to 2017.16 Although adults of all ages were included in this study, the average patient was approximately aged 71 years and Medicare Advantage patients comprised 73% of the population. Reduced costs in the Folkerts population, compared with our study, may be explained by their use of a newly diagnosed population; a much greater percentage of Medicare Advantage patients, who typically have lower contracted rates with health care providers compared with employer-based commercially insured individuals; and costs calculated from less contemporary years.
Moreover, our findings add to prior studies by showing how T2DM, a common comorbidity and leading cause of CKD, may increase the burden of CKD in a commercially insured US population, as reflected in the costs observed in patients with both CKD and T2DM and with CKD only. This is consistent with findings from a study of US patients of all ages with CKD by Damien et al, which found that those who had CKD and T2DM incurred 39% higher costs than those who had CKD without T2DM, although we observed a much higher increase.15 This could be attributed to the diversity of payer types, including Medicaid and those uninsured, which were not included in our cohort, and their use of multivariable modeling.
Among patients with CKD only and CKD and T2DM, the cost burden increased in later stages of CKD. Specifically, costs began to increase in patients with KDIGO CKD stage 3b and continued to increase through stage 5. At each stage, medical costs were significantly higher than pharmacy costs. This suggests that therapeutic interventions that avert or delay patients’ progression to higher KDIGO CKD stages may have the opportunity to reduce overall health care costs.
These findings supplement prior studies that have documented the increasing utilization and cost burden across CKD stages in adults of all ages and in adults older than 65 years.2,3,5,6 In the Folkerts et al study, the authors documented elevated utilization and costs in patients at stages 4 and 5 CKD compared with those at earlier stages in the mostly Medicare Advantage population. Golestaneh et al stratified their results by those of a commercially insured age (aged <65 years) and a Medicare age (age ≥65 years) with CKD. In the commercial age cohort, overall mean annualized costs (adjusted to 2016 US dollars) were estimated to be $16,770 at stage 2, $26,842-$43,547 at stages 3a and 3b, $76,969 at stages 4 and 5, and up to $121,948 for those with ESRD without dialysis. For the Medicare age cohort, estimated overall mean annualized costs for patients aged 65 years and older with CKD were about $15,000 at stage 2, increased to about $21,000-$27,000 at stages 3a and 3b, approximately doubled to $46,000 at stages 4 and 5, and topped out at $87,339 for those with ESRD without dialysis.6 A study of costs attributable to CKD among Medicare patients with CKD stages 1 through 4 performed by Honeycutt et al similarly found that costs were lowest at earlier stages and increased most drastically at stage 4.3
Our study complements the findings from all of these studies by describing similar trends in a commercially insured US population with CKD with and without diabetes. A notable difference based on our study’s findings, however, is that the substantial increase in costs associated with later stages of CKD appeared to begin as early as stage 3b, rather than stage 4, as these prior studies of older patients (Medicare age ≥ 65 years) have suggested. This aligns with the findings of Golestaneh et al, as well as other researchers that have quantified the burden of CKD in younger populations. For example, comparing overall mean annualized costs for patients aged younger than 65 years with CKD stage 3a with those with CKD stage 3b, a Canadian study by Manns et al found that costs nearly tripled,17 and the 2017 study by Golestaneh et al found an approximately 60% increase in costs in the United States.6
LIMITATIONS
There are several limitations of this study. First, this study is based on claims provided by major managed care health plans across the United States. The majority of individuals are members of private commercial health insurance programs, and thus the results can only be generalized to approximately 67% of the US population that is covered by such insurance.18
Second, about 54% of the CKD only and CKD and T2DM groups had at least 1 eGFR laboratory value available. Because only this subset of members could be included in the analysis stratified by KDIGO CKD eGFR-based stage, selection bias could influence the findings from this analysis.
Third, this study required a defined length of follow-up (ie, 1 year baseline and post-index) to estimate HCRU and costs. This requirement may have resulted in the selection of patients who were not at the end of their disease. Therefore, patients included in the study may have been in a better state of health and had lower HCRU and costs compared with other patients who did not survive long enough to be included in the study. CKD/T2DM-related resource utilization and costs may also be underestimated because of our attribution methodology, as suggested by prior research.19,20
Fourth, the first diagnosis for the condition of interest in this study was identified in the calendar year 2018. Because no washout period was applied, a patient may have had the condition of interest prior to 2018. Consequently, members included in the study may be heterogeneous in that the actual length of the period in which the condition of interest was present is subject to high variability. However, we did this purposefully, as we felt that this would best represent a health plan population with these conditions at a given point in time.
Fifth, our study was descriptive in nature and, as such, no inferential statistics, multivariable modeling, or matching techniques were performed. Limiting our cohort to those aged 45-64 years did balance age across the groups, which in our opinion allows for some high-level, qualitative comparisons, as described.
Finally, because individual race data were not available, the eGFR calculation using the CKD-Epi equation could not account for this factor. This potentially underestimates the eGFR for study patients of Black race. Generalizing the results of the study beyond White individuals is challenging because of their high representation in the underlying population.
Conclusions
To our knowledge, this real-world study was among the first to describe the HCRU and cost burden of CKD with and without T2DM in a commercially insured population. Individuals with CKD and T2DM had substantial burden in terms of HCRU and costs. Additionally, costs began to increase at KDIGO CKD stage 3b and continued increasing in later stages, as quantified by the magnitude described in the study. Therefore, there is an opportunity to reduce the burden of CKD in this population by investing in interventions to prevent or delay CKD disease progression.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1-115. doi:10.1016/j.kint.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Chronic kidney disease in the United States, 2021. Centers for Disease Control and Prevention. Accessed September 16, 2021. https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html [Google Scholar]
- 3.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478-83. doi:10.1681/ASN.2012040392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. . Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038-47. doi:10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System. Healthcare expenditures for persons with CKD. 2020. Annual report web site. Accessed September 16, 2021. https://adr.usrds.org/2020/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd [Google Scholar]
- 6.Golestaneh L, Alvarez PJ, Reaven NL, et al. . All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23(10 Suppl):S163-72. [PubMed] [Google Scholar]
- 7.Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: An updated national estimate of prevalence based on kidney disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. doi:10.1186/1756-0500-7-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group K. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter. 2013;3(1):1-150. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson JJ, Cepeda MS, Zhang J, et al. . The association between doctor and pharmacy shopping and self-reported misuse and abuse of prescription opioids: A survey study. J Pain Res. 2020;13:689-701. doi:10.2147/JPR.S232409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalirai S, Ivanova JI, Perez-Nieves M, et al. . Basal insulin initiation and maintenance in adults with type 2 diabetes mellitus in the United States. Diabetes Metab Syndr Obes. 2020;13:1023-33. doi:10.2147/DMSO.S237948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson JJ, Dembek C, Caldwell-Tarr A, Conto RM, Paullin M, Kerwin EM. Observational real-world study to assess clinical characteristics and device satisfaction in patients with COPD treated with glycopyrrolate/eFlow CS. Int J Chron Obstruct Pulmon Dis. 2020;15:1713-27. doi:10.2147/COPD.S248760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Bureau of Labor Statistics. Consumer price index. Accessed September 21, 2021. https://data.bls.gov/cgi-bin/surveymost?su
- 13.Quan H, Li B, Couris CM, et al. . Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-82. doi:10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 14.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-45. doi:10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. Chronic kidney disease: Common – serious – costly. Centers for Disease Control and Prevention. Accessed October 6, 2021. https://www.cdc.gov/kidneydisease/prevention-risk/CKD-common-serious-costly.html
- 16.Folkerts K, Petruski-Ivleva N, Kelly A, et al. . Annual health care resource utilization and cost among type 2 diabetes patients with newly recognized chronic kidney disease within a large U.S. administrative claims database. J Manag Care Spec Pharm. 2020;26(12):1506-16. doi:10.18553/jmcp.2020.26.12.1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manns B, Hemmelgarn B, Tonelli M, et al. . The cost of care for people with chronic kidney disease. Can J Kidney Health Dis. 2019;6:2054358119835521. doi:10.1177/2054358119835521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keisler-Starkey K, Bunch, L.N. Health Insurance Coverage in the United States: 2020. US Census Bureau; 2021. Accessed September 15, 2022. https://www.census.gov/library/publications/2021/demo/p60-274.html [Google Scholar]
- 19.Tunceli O, Wade R, Gu T, Bouchard JR, Aagren M, Luo W. Cost of diabetes: Comparison of disease-attributable and matched cohort cost estimation methods. Curr Med Res Opin. 2010;26(8):1827-34. doi:10.1185/03007995.2010.488544 [DOI] [PubMed] [Google Scholar]
- 20.Willey VJ, Kong S, Wu B, et al. . Estimating the real-world cost of diabetes mellitus in the United States during an 8-year period using 2 cost methodologies. Am Health Drug Benefits. 2018;11(6):310-8. [PMC free article] [PubMed] [Google Scholar]


