Abstract
BACKGROUND:
Few surveys about biosimilars have been conducted among US patients.
OBJECTIVE:
To evaluate attitudes about biosimilars among patients with rheumatoid arthritis (RA), psoriasis and/or psoriatic arthritis (PsO/A), and/or inflammatory bowel disease (IBD).
METHODS:
WebMD, LLC fielded a 16-item online survey to members of the US Dynata consumer panel meeting these criteria: aged 18 years or older; self-reported specialist diagnosis of RA, PsO/A, or IBD of at least 1 year; and not currently receiving an infliximab biosimilar. A quota of 500 was set, stratified by region and condition. The survey was exempt by the institutional review board, exploratory, and not registered.
RESULTS:
Overall, 44% (n = 221) of patients were on a biologic; 56% (n = 279) were not on a biologic (40% [n = 199] were biologic naive and 16% [n = 80] used biologics in the past). Among all patients, 66% were unaware of biosimilars and 24% were aware (10% unsure). After being shown the US Food and Drug Administration definition of a biosimilar, main concerns were side effects (59%), long-term safety (50%), and not knowing a lot (46%). Among current users, 43% would switch to a biosimilar and 26% would not (32% unsure). Of those unwilling to switch, 51% were concerned about side effects, 42% about financial support, and 40% about efficacy. When those not on a biologic were asked if their doctor prescribed an original anti–tumor necrosis factor α but their insurance required its biosimilar, 49% would switch and 8% would not (43% unsure). 51% of patients surveyed thought pharmacist-level substitution of an interchangeable biosimilar was acceptable with notification. Survey findings were consistent among the RA, PsO/A, and IBD subgroups.
CONCLUSIONS:
Although two-thirds of patients surveyed were unaware of biosimilars, the majority were potentially receptive to biosimilar treatment after being provided with the definition of a biosimilar. Patients expressed a desire to know more about biosimilars in general, how they compare with original biologics, their benefits, and cost.
Plain language summary
A 16-question online survey asked 500 adults with rheumatoid arthritis, psoriasis, psoriatic arthritis, and/or inflammatory bowel disease about biosimilars. Overall, 66% did not know what a biosimilar was and 24% did (10% unsure). Patients identified side effects, long-term safety, and lack of knowledge as top concerns. Around 40% of patients would switch to a biosimilar (30-40% unsure). Finally, 7 in 10 patients wanted more general information on biosimilars and 5 in 10 on the relationship between biosimilars and original biologics.
Implications for managed care pharmacy
Managed care pharmacists will need to understand how to address patient concerns about biosimilars vs reference biologics in terms of side effects, long-term safety, and general knowledge about biosimilar products. Patients prefer that their pharmacist tell them and their physician about substitution with an interchangeable biologic, suggesting that systems will need to be implemented to facilitate patient and provider notification.
Biologic medicines, such as anti–tumor necrosis factor α (TNFα) antibodies, are widely used for a broad range of immune-mediated inflammatory conditions, including rheumatoid arthritis (RA), psoriasis (PsO), psoriatic arthritis (PsA), and inflammatory bowel disease (IBD) (Crohn’s disease or ulcerative colitis).1 Since biologics were introduced in the United States in the 1990s, they have significantly improved outcomes in many patients, including work productivity and quality of life.1 To improve patient access to biologics and potentially lower costs by driving competition,2,3 biosimilar agents were introduced in the United States approximately 7 years ago. The US Food and Drug Administration (FDA) defines a biosimilar as a “biological product that is highly similar to and has no clinically meaningful differences in terms of safety, purity, and potency from the original FDA-approved reference product.”4 As of August 2022, a total of 39 biosimilars have been approved by the FDA, 13 of which are TNFα blockers.5 Interchangeable biologic products are biosimilars that meet additional FDA requirements and can be substituted at the pharmacy for the reference biologic without the intervention of the health care provider, in accordance with state pharmacy laws.6 Of note, Cyltezo (adalimumab-adbm, Boehringer Ingelheim Pharmaceuticals Inc.), a biosimilar referencing Humira (adalimumab, AbbVie), was approved by the FDA as an interchangeable product in October 2021, making it the first interchangeable monoclonal antibody.7
With more biosimilar products becoming available, it will be important for patients to be informed about biosimilars. Although there are numerous reports from Europe and other parts of the world surveying patients,8-13 few surveys have been conducted among US patients to assess their knowledge and attitudes toward biosimilars.14-16 This survey evaluated understanding of biosimilars, attitudes about switching to a biosimilar, and educational opportunities to understanding among US patients with RA, PsO/PsA, and/or IBD not currently receiving an infliximab biosimilar, which was the only TNFα blocker available in the United States at the time the survey was conducted. A graphical Plain Language Summary of this work is provided in Supplementary Exhibit 1 (680.3KB, pdf) , available in online article.
Methods
PATIENTS
Survey participants were identified from the Dynata consumer research panel and had to meet the following criteria: US resident, aged 18 years or older (≥21 years in Alabama, Mississippi, Nebraska, and Puerto Rico); self-reported specialist diagnosis of RA, PsO/PsA, or IBD for at least 1 year; not currently be receiving an infliximab biosimilar (Inflectra [infliximab-dyyb], Pfizer Inc; Renflexis [infliximab-abda], Organon; or Avsola [infliximab-axxq], Amgen, Inc.); and providing informed consent.
Patients with multiple conditions answered about 1 condition only; those with RA + PsO/PsA reported about RA; those with RA + IBD reported about IBD, and those with PsO/PsA + IBD reported about PsO/PsA. The survey screening questions asked patients if they were currently receiving or had ever received a biologic, including an anti-TNFα medicine; results were stratified by current biologic users and those not currently on a biologic (ie, biologicnaive or past biologic user).
SURVEY PROCESS
The survey was based on a questionnaire from previous work by one of the authors (Dr Peyrin-Biroulet),10 modified by the physician and patient authors for a US patient audience, and reviewed for plain language. The survey was initially piloted by sending it to 25 patients (December 3, 2020). The purpose of this pilot study was to assess the questions for clarity, understanding, and interpretation. After assessing the survey data, minor changes were made. For example, a definition of biologics was added for clarification, rheumatoid arthritis and juvenile idiopathic arthritis/juvenile rheumatoid arthritis were combined into one category, and the order of survey questions was revised for clarity. For the full launch, the survey was sent to 18,000 people who were members of the Dynata consumer panel, with members initially identified from 3 sources: integrated (web intercept/API), loyalty (invitation only), and open enrollment. A quota of 500 was set and stratified based on US geographic region (25% each for Northeast, South, Midwest, and West) and medical condition (33% each for RA, PsO/PsA, and IBD); once the quota was reached, no further surveys were administered for that region or condition.
QUESTIONNAIRE AND DATA ANALYSIS
The questionnaire consisted of 16 items (see Supplementary Materials (680.3KB, pdf) ). Patients were asked about their use of biologics, including TNFα blockers. The survey screening included a definition of a biologic medicine, with brand and generic names of 27 medicines provided as examples (see Supplementary Materials (680.3KB, pdf) ). The survey also explained that an anti-TNFα medicine is a type of biologic.
Patients were also asked if they had ever heard of biosimilars and if so, where; after this question, they were shown the FDA definition of a biosimilar.17 Subsequent questions assessed attitudes in relation to initiating or switching to a biosimilar and interchangeability. The survey also asked where participants get their information about biosimilars and what information they think they need. Survey questions were multiple-choice format and most used a 5-point Likert scale for responses. The survey was conducted from December 2020 to January 2021 and was fielded by WebMD, LLC. Responses to each survey item were summarized and analyzed descriptively.
ETHICS APPROVAL AND INFORMED CONSENT
Participants were informed about the intentions of the research and how their personal information and responses would be used, and their confidentiality protected; informed consent was obtained by having the participant check a box at the beginning of the survey. This survey received an exemption from institutional review board oversight by Advarra.
Results
PATIENT DEMOGRAPHICS AND BIOLOGIC USE
A total of 2,013 patients responded to the survey: 226 did not meet screening criteria and 1,287 responded after the quota for region or disease was reached; 500 patients completed the Web-based survey (Supplementary Table 1 (680.3KB, pdf) ). Per quota, one-third of patients were classed as having RA, PsO/A or IBD, respectively. The mean age of respondents was 48.6 years (Supplementary Table 2 (680.3KB, pdf) ). Patients with IBD were slightly older (mean, 50.1 years), than those with RA (mean, 48.9 years) or PsO/A (46.6 years). More than half of all patients were female (61%), with more females in the RA (63%) and IBD (67%) groups than in the PsO/A group (53%). More patients with IBD had been diagnosed more than 10 years previously (42%) compared with those who had PsO/A (35%) or RA (25%). Conversely, more patients with RA had been diagnosed less than 5 years ago (48%) compared with those who had PsO/A (38%) or IBD (34%).
Overall, 44% (n = 221) of patients were currently receiving a biologic and 56% (n = 279) were not currently on a biologic (ie, 40% [n = 199] were biologic naive and 16% [n = 80] used biologics in the past). More patients with RA (50%) and PsO/A (47%) were currently receiving biologics than patients with IBD (36%) (Supplementary Table 2 (680.3KB, pdf) ). Of note, 50% (n = 111/221) of current users said they received copay support. Most patients receiving biologics were on an anti-TNFα (68%, n = 150/221). Among those on an anti-TNFα, more patients with PsO/A than with other conditions used adalimumab (PsO/A 72% [n = 34/47], IBD 50% [n = 18/36], RA 46% [n = 31/67]), more patients with RA used etanercept (RA 37% [n = 25/67], PsO/A 17% [n = 8/47], IBD 16.7% [n = 6/36]), and more patients with IBD used infliximab (IBD 19% [n = 7/36], PsO/A, 6% [n = 3/47], RA 5% [n = 3/31]).
BIOSIMILAR KNOWLEDGE AND ATTITUDES
Overall, 66% (n = 329/500) of respondents had not heard of a biosimilar, 24% (n = 119/500) had, and 10% (n = 52/500) were unsure. Results were similar among those with RA (62% not heard, 26% have heard, 12% unsure), PsO/A (67%, 24%, 9%), and IBD (68%, 22%, 10%). Current biologic users (38%) were more likely to have heard of biosimilars than those not currently using biologics (12%). These results for those currently vs not currently receiving biologics also were consistent among groups with RA (36% vs 16%), PsO/A (38% vs 11%) and IBD (42% vs 10%).
Patients were shown the FDA definition of biosimilars before answering additional questions.2 The main concerns regarding biosimilars overall across immune-mediated inflammatory conditions were side effects (59%), long-term safety (50%), and not knowing “a lot about this kind of medicine” (46%); results were similar among those with RA, PsO/A, and IBD (Supplementary Figure 1 (680.3KB, pdf) ). Patients not currently using biologics were more concerned about not knowing a lot about these medicines and their side effects than those currently using biologics (Supplementary Figure 1 (680.3KB, pdf) ).
SWITCHING AND INTERCHANGEABILITY
Among current biologic users, 43% (n = 94/221) would accept a switch to a biosimilar of their current biologic medicine (question did not specify by whom), 32% (n = 70/221) were unsure, and 26% (n = 57/221) did not want to switch. These results were similar across RA and PsO/A subgroups of biologic users; however, a larger percentage of patients with IBD were unsure about switching (Figure 1). Among current biologic users who did not want to accept a switch, 51% (n = 29/57) were concerned about side effects, 42% (n = 24/57) about financial support, and 40% (n = 23/57) about the biosimilar not treating their condition as well. Among those unwilling to accept a switch, 53% (n = 30/57) would ask their doctor’s office to prevent the switch and 26% (n = 15/57) would ask their insurance to do the same.
FIGURE 1.
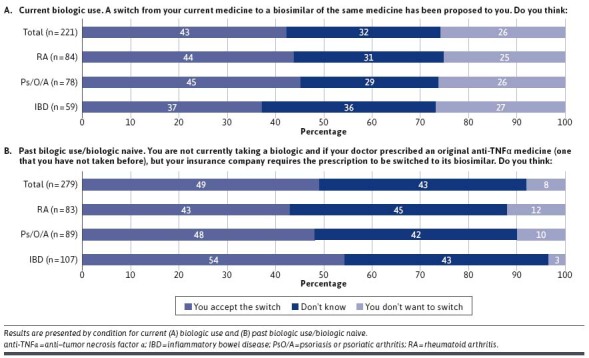
Patient Attitudes Toward Switching
When patients not currently taking a biologic were asked if their doctor prescribed an original anti-TNFα medicine, but their insurance company required a biosimilar of that medicine, 49% (n = 137/279) would accept the switch, 43% (n = 120/279) were unsure, and 8% (n = 22/279) did not want to switch. Again, findings were similar across RA and PsO/A subgroups; however, a larger percentage of patients with IBD were unsure about switching (Figure 1). Among all patients who were not currently receiving a biologic, when asked their preference if their doctor prescribed a biologic, 10% (n = 27/279) would prefer the original, 13% (n = 37/279) would take the biosimilar if it was available, 33% (n = 92/279) had no preference, and 44% (n = 123/279) responded that it would depend on cost.
Although no biosimilars were designated as interchangeable at the time the survey was conducted, patients were open to that possibility if the pharmacist substitution was communicated to themselves or their doctor, or both, by the pharmacist (Figure 2).
FIGURE 2.
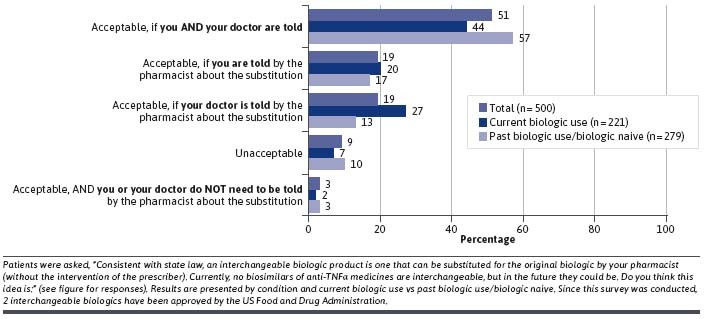
Patient Attitudes About Interchangeable Biologics
INFORMATION
Most respondents obtained their health care information from a health care provider (83%), a health website (46%), or an Internet search (46%) (Supplementary Table 3 (680.3KB, pdf) ). When asked what information respondents felt they needed to help them learn about biosimilars, responses included general information about biosimilars (71%), on the potential benefits of biosimilars (55%), the relationship between biosimilars and original biologics (53%), and biosimilar cost (48%). Most respondents preferred print (62%) vs face-to-face (37%), videos (35%), or infographics (22%).
Discussion
This article describes the attitudes toward biosimilars in US patients with self-reported RA, PsO/A, or IBD, including those who were currently receiving biologics and those who were not currently receiving biologics. Results indicate that many patients with RA, PsO/A, and IBD have knowledge gaps surrounding biosimilars. Approximately a quarter of patients had heard of biosimilars regardless of their underlying immune-mediated disease, with lower recognition among those who had never used a biologic or had used one in the past. This finding is to be expected, as patients who are naive to biologics may be at an earlier disease stage and not yet candidates for biologic therapy, including biosimilars.
After being shown a definition of biosimilars, patients expressed concerns regarding side effects, long-term safety, and “not knowing a lot about this kind of medicine.” A slightly larger proportion of patients with IBD were concerned about side effects than those with RA or PsO/A; this may be because overall biologic use was lower in the IBD group. Unsurprisingly, across all immune-mediated conditions, those who had never used or were not currently on a biologic were more concerned about “not knowing a lot about this kind of medicine” than current users of biologics. Patients expressed a desire to learn more about biosimilars in general, how they compare with original biologics, their benefits, and cost.
In general, patients across all immune-mediated diseases were receptive to receiving biosimilar treatment after being shown the FDA definition of biosimilars. About a quarter of current biologic users would not accept a switch to a biosimilar of their current biologic medicine, with around a third unsure if they would accept. These findings demonstrate better acceptance of biosimilars than reported in a 2019 survey on nonmedical switching conducted among patients with an autoimmune disease currently receiving a biologic, in which 85% of patients did not want “to switch to a biosimilar if the one I’m currently taking is helping my disease,” 85% were concerned that the biosimilar would not treat their disease as well, and 83% were concerned that switching may cause more side effects.16 This may relate to differences in the survey populations and question format. Whereas the nonmedical switch survey asked for agreement with statements that included qualifiers (ie, “helping my disease”), the present survey sequentially asked if patients would be accepting of a switch, and if not, why and what actions they would take to prevent the switch.
Of note in the present survey, approximately 50% of patients not currently using a biologic would accept a switch to a biosimilar if it were required by their insurance company, with only 8% of these patients not wanting to switch (43% were unsure). This finding suggests that patients initiating a new therapy may be more open to taking a biosimilar than patients already receiving a biologic. Similarly, results from a 2019 survey showed that US rheumatologists were hesitant to switch from a reference product to a biosimilar for a patient doing well on the reference product but were accepting of biosimilar treatment initiation of treatment in biologic-naive individuals.18
The present survey showed that among patients currently receiving and not currently receiving a biologic, biosimilar cost was considered part of the decision-making process for almost half of all respondents. This is consistent with findings from the 2019 nonmedical switching survey of patients receiving biologic therapy.16 Given the high cost of biologics and biosimilars, cost remains an important issue, with majority of respondents caring about insurance coverage and copay assistance programs.
Our survey was conducted prior to the FDA approval of the first interchangeable anti-TNFα blocker, Cyltezo, (adalimumab-adbm, Boehringer Ingelheim Pharmaceuticals, Inc.) referencing Humira (adalimumab, AbbVie Inc.). However, we did include a question about substitution in our survey. Most patients would be willing to accept pharmacist substitution of a biosimilar of its reference product, but the majority (51%) wanted the substitution communicated to themselves and their doctor, with 19% wanting to be informed themselves and 19% wanting their doctor to be informed. As of this writing, only 4 states have laws that prohibit a pharmacist substituting an interchangeable biologic without prior prescriber approval; however, the majority of states do require prescriber notification of such a substitution.19
LIMITATIONS
Limitations of this survey include a relatively small sample size. Additionally, the sample was drawn from the Dynata consumer panel and may not have been representative of US patients with immune-mediated conditions. Patients self-identified their specialist-diagnosed condition. Furthermore, the descriptive nature of the study may limit comparisons among groups with different immune-mediated conditions.
Conclusions
More than half of patients who completed the survey had not heard of biosimilars. The majority of patients were receptive to biosimilar treatment and 44% would consider cost as part of the decision-making process. The main concerns associated with biosimilars identified by patients included side effects, long-term safety, and how well their condition would be treated compared with an original biologic. These findings indicate that patients would benefit from educational programs to help them understand biosimilars and the increased treatment options they provide.
ACKNOWLEDGMENTS
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all content and editorial decisions, were involved at all stages of article development, and have approved the final version of the review that reflects the authors’ interpretation and conclusions. The authors received no direct compensation related to the development of the article. Writing support was provided by Linda Merkel, PhD, and Marissa Buttaro, MPH, of Elevate Scientific Solutions, which was contracted and compensated by BIPI.
REFERENCES
- 1.Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological therapies in immune-mediated inflammatory diseases: Can biosimilars reduce access inequities? Front Pharmacol. 2019;10:279. doi:10.3389/fphar.2019.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Biosimilars. Accessed July 18, 2022. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
- 3.Kvien TK, Patel K, Strand V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum. 2022;52:151939. doi:10.1016/j.semarthrit.2021.11.009 [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Guidances (drugs): Biosimilars. Accessed November 28, 2019. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
- 5.US Food and Drug Administration. Biosimilar product information: FDA-approved biosimilar products. Accessed January 25, 2022. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
- 6.US Food and Drug Administration. Interchangeable biological products. Accessed December 3, 2021. https://www.fda.gov/media/151094/download#:~:text=www.fda.gov,depending%20on%20state%20pharmacy%20laws
- 7.US Food and Drug Administration. FDA approves Cyltezo, the first interchangeable biosimilar to Humira. 2021. Accessed December 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-cyltezo-first-interchangeable-biosimilar-humira
- 8.Aladul MI, Fitzpatrick RW, Chapman SR. Patients’ understanding and attitudes towards infliximab and etanercept biosimilars: Result of a UK web-based survey. BioDrugs. 2017;31(5):439-46. doi:10.1007/s40259-017-0238-1 [DOI] [PubMed] [Google Scholar]
- 9.Frantzen L, Cohen JD, Trope S, et al. Patients’ information and perspectives on biosimilars in rheumatology: A French nation-wide survey. Joint Bone Spine. 2019;86(4):491-6. doi:10.1016/j.jbspin.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Ighani A, Wang JY, Manolson MF. An evaluation of psoriasis patient perceptions and understanding of biosimilars: A Canadian survey comparing biologic and nonbiologic users. J Cutan Med Surg. 2018;22(3):365-7. doi:10.1177/1203475417746337 [DOI] [PubMed] [Google Scholar]
- 11.Peyrin-Biroulet L, Lonnfors S, Avedano L, Danese S. Changes in inflammatory bowel disease patients’ perspectives on biosimilars: A follow-up survey. United European Gastroenterol J. 2019;7(10):1345-52. doi:10.1177/2050640619883704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyrin-Biroulet L, Lonnfors S, Roblin X, Danese S, Avedano L. Patient perspectives on biosimilars: A survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. J Crohns Colitis. 2017;11(1):128-33. doi:10.1093/ecco-jcc/jjw138 [DOI] [PubMed] [Google Scholar]
- 13.Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519-30. doi:10.2147/PPA.S129333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismailov RM, Khasanova ZD, Gascon P. Knowledge and awareness of biosimilars among oncology patients in Colorado, USA. Future Oncol. 2019;15(22):2577-84. doi:10.2217/fon-2019-0194 [DOI] [PubMed] [Google Scholar]
- 15.Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: An international cross-sectional survey. Patient Prefer Adherence. 2016;10:937-48. doi:10.2147/PPA.S104891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teeple A, Ginsburg S, Howard L, et al. Patient attitudes about non-medical switching to biosimilars: Results from an online patient survey in the United States. Curr Med Res Opin. 2019;35(4):603-9. doi:10.1080/03007995.2018.1560221 [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Biosimilar and interchangeable products. Accessed February 1, 2022. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologicsmore-treatment-choices#:~:text=An%20interchangeable%20biosimilar%20product%20is,biosimilar%20and%20interchangeable%20biosimilar%20medications
- 18.Gibofsky A, McCabe D.. US rheumatologists’ beliefs and knowledge about biosimilars: A survey. Rheumatology (Oxford). 2021;60(2):896-901. doi:10.1093/rheumatology/keaa502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CardinalHealth. State laws for biosimilar interchangeability. Accessed December 10, 2021. https://www.cardinalhealth.com/en/product-solutions/pharmaceutical-products/biosimilars/state-regulations-for-biosimilar.html


