Abstract
BACKGROUND:
Cancer diagnostic pathways are highly variable and not clearly established in the United States, which can lead to a diagnosis process that takes more time and exposes patients to invasive or unnecessary procedures, delays in treatment, worsening patient outcomes, and elevated health care resource utilization (HRU) and health care system costs.
OBJECTIVE:
To investigate current trends in time to diagnosis and diagnostic-related HRU preceding the patient’s cancer diagnosis across all cancer types in the United States.
METHODS:
A retrospective claims analysis was conducted on patients newly diagnosed with cancer identified from 2018-2019 using Optum’s de-identified Clinformatics Data Mart database, which includes Medicare Advantage and commercially insured members. Patients were identified using International Classification of Diseases, Tenth Revision codes and were required to have at least 2 outpatient visits at least 30 days apart or at least 1 inpatient cancer visit without prior cancer claims. The first diagnostic test was identified based on an algorithm of a 60-day gap between diagnostic tests prior to diagnosis. The index date was defined as the first diagnostic test date or an office visit less than 4 weeks prior to the first diagnostic test date. Patient characteristics, time to diagnosis, and HRU were descriptively analyzed for all patients and by cancer type.
RESULTS:
Among the 458,818 patients newly diagnosed with cancer included in this analysis, the mean age was 70.6 years, approximately half were female, and most were White people (65.0%) with Medicare Advantage coverage (74.0%). Patients with cancer had an overall mean (SD) time to diagnosis of 156.2 (164.9) days and 15.4% of patients waited longer than 180 days before a cancer diagnosis. High heterogeneity among cancer types was observed, with a mean time to diagnosis ranging from 121.6 days (bladder cancer) to 229.0 days (multiple myeloma). Imaging resource use during the diagnostic pathway was high for radiology (60.7%), computerized tomography (50.8%), magnetic resonance imaging (48.6%), and ultrasound (42.6%). A total of 69.3% of patients had endoscopy without biopsy, 36.5% had endoscopy with biopsy, 62.5% had other biopsies, and most patients did general urine and serum tests (91.3%) and nongenetic cancer-specific laboratory tests (84.3%). Resource use was highly varied by cancer type but tended to increase with a longer time to diagnosis.
CONCLUSIONS:
The proportion of patients experiencing a diagnostic process of longer than 180 days is clinically and economically meaningful. Diagnostic-related HRU was significant and highly variable, highlighting the inefficiencies in the cancer diagnostic process in the United States and the need for policies, guidelines, or medical interventions to streamline cancer diagnostic pathways to optimize patient outcomes and reduce health care system burden.
Plain language summary
Almost 16% of patients newly diagnosed with cancer waited longer than 180 days to be diagnosed. Doctors used health care resources like imaging equipment or laboratory tests while trying to diagnose cancer, and this resource use often increased along with a greater time taken to diagnose the patient. This suggests that improvements can be made to decrease the wait time for cancer diagnosis that may also decrease the number of resources needed and improve these patients’ health later.
Implications for managed care pharmacy
Given that 15.8% of patients newly diagnosed with cancer experienced a diagnostic process of longer than 180 days and had high health care resource use that tended to increase with greater time to diagnosis, addressing diagnostic process inefficiencies should be prioritized. Managed care pharmacy professionals can promote minimization of diagnostic inefficiencies and health care resource burden while improving patient outcomes through engaging in and supporting efforts to develop policies/guidelines and/or improve access to medical interventions to reduce time to diagnosis.
Cancer disease burden is substantial in the United States, with cancer representing one of the leading causes of death1 and cancer-related deaths estimated at 609,360 in the United States in 2022.2 Diagnosing and treating cancer at earlier stages to minimize the chance of progression and need for more intensive treatment may result in improved clinical outcomes and less costly cancer treatment and management.3
Many people are diagnosed with cancer after presenting with signs and symptoms to a primary care provider, such as a general practitioner or internist, before later referral to an oncologist, hematologist, and/or other care specialists. Estimates based on 2020 data suggest approximately 63%-82% of cancers in the United States are diagnosed after symptomatic presentation.3-6
Delays in diagnosis or misdiagnosis as a result of an undirected cancer diagnosis pathway may have detrimental impacts on patients. A published systematic literature review including 177 articles reporting on 209 international studies (47 in the United States) found the strongest associations between time to diagnosis and patient clinical outcomes, such as survival, among breast, colorectal, head and neck, and testicular cancers and melanoma.7 The researchers concluded it is “reasonable” to assume that efficient cancer diagnosis is “likely” to enable earlier-stage diagnosis and improved patient survival and quality of life.7-10
Undirected cancer diagnosis pathways can also lead to diagnostic errors. Diagnostic error among melanoma, lung, colorectal, breast, and prostate cancer has been reported ranging from 2.4% to 22.5%, with severity-weighted serious harm rates per diagnostic error ranging from 41.2% to 61.9% and serious misdiagnosis-related harm rates per incident cancer case ranging from 1.2% to 13.9%.11
Several non-US countries (United Kingdom, Denmark, Sweden, Canada, and Australia) have identified the importance and potential impact of efforts to achieve earlier-stage diagnosis for symptomatic cancers by establishing national guidelines, special programs, and pathways.12-18 Although the United States has cancer diagnostic pathway guidance outlined for some cancers (eg, the National Comprehensive Cancer Network diagnostic guidelines for breast,19 colorectal,20 lung,21 and prostate cancer22), these guidelines are not available for all cancers and there may be limited awareness and consistent use of these guidelines to oncologist and hematologists rather than the primary care providers who often are the first to be presented with initial signs and symptoms. Given the limited guidelines available and potential primary care provider accessibility and use issues, the cancer diagnostic route may be long and complicated, potentially resulting in diagnosis at a more advanced stage associated with worse clinical outcomes, increased economic burden, and compromised quality of life. One recent qualitative lung cancer study found that multiple diagnostic routes resulted in patient perceptions of delays, inefficiencies, and lack of coordination, which ultimately added to their distress.23
Although some single cancer studies have been conducted in the United States assessing the time to diagnosis in cancer,24-32 the heterogeneity in methodology has made it difficult to compare across cancer types as well as between studies of the same cancer type. Given the limited recent US data in characterizing cancer diagnoses pathways, this study investigated current trends in time to diagnosis and diagnostic-related health care resource utilization (HRU) preceding the patient’s cancer diagnosis across 20 cancer types in the United States.
Methods
Patients newly diagnosed with cancer were identified between January 1, 2018, and December 31, 2019, from Optum’s deidentified Clinformatics Data Mart database, which includes Medicare Advantage and commercially insured members. Patients with cancer were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes (Supplementary Table 1 (602.9KB, pdf) , available in online article). Patients were required to have at least 2 outpatient claims (at least 30 days apart to reduce misclassification) or 1 inpatient claim with an ICD-10-CM cancer diagnosis code of the same cancer type in any position.33 The first qualifying cancer diagnosis claim was defined as the cancer diagnosis date. Patients with cancer before the diagnosis date were excluded. Patients were also required to have at least 1 Current Procedural Terminology diagnostic test code potentially related to cancer diagnosis documented prior to diagnosis (Supplementary Table 2 (602.9KB, pdf) ) and at least 3 months of continuous enrollment prior to the index date (date of an office visit occurring within 4 weeks prior to the first diagnostic test date or the first diagnostic test date if no office visit was recorded). The first diagnostic test was identified by searching for a gap of more than 60 days between tests prior to the cancer diagnosis; the date of the test before the gap was defined as the first diagnostic test date (Supplementary Table 3 (602.9KB, pdf) ). Patients’ baseline clinical characteristics were examined during the 3 months prior to the index date (Figure 1).
FIGURE 1.
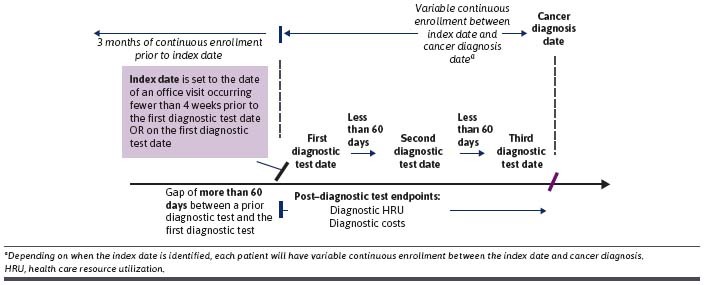
Study Design
Outcomes—time to diagnosis and diagnostic-related HRU (imaging, endoscopy, biopsy, and laboratory identified by Current Procedural Terminology codes; Supplementary Table 2 (602.9KB, pdf) )—were evaluated from the index date until the cancer diagnosis date (Figure 1). The time to diagnosis was reported in days and was presented as a continuous variable and by time to diagnosis categories (“0-30 days,” “> 30-90 days,” “> 90-180 days,” and “> 180 days”).
Patient characteristics, time to diagnosis, HRU, and HRU by time to diagnosis were descriptively analyzed for continuous (mean and SD) and categorical variables (number of observations and percentages in each category). Results were reported individually and overall for the 20 cancer types captured. More detailed results were also reported for 7 cancer types selected to reflect cancer types with diverse diagnostic pathways: bladder, breast, colorectal, lung, ovarian, pancreas, and prostate cancers. All statistical analyses were conducted using SAS 9.4 and SAS Studio 3.81.
Results
A total of 458,818 eligible members newly diagnosed with cancer from January 1, 2018, to December 31, 2019, were included in this analysis (Figure 2; Table 1). The mean age was 70.6 years, and most patients (76.6%) were aged 65 years or older. The population diagnosed with cancer was almost evenly split between men (50.4%) and women (49.6%) and was predominately White (65.0%) and resided in the South (53.7%) with Medicare Advantage insurance coverage (74.0%). Patients had a mean Charlson Comorbidity Index value of 2.1, and the 3 most common comorbidities were hypertension and other heart disease (41.8%), diabetes (16.1%), and psychiatric-related (12.9%). Breast (24.3%), lung (12.0%), and prostate (17.8%) were the 3 most common cancer types.
FIGURE 2.
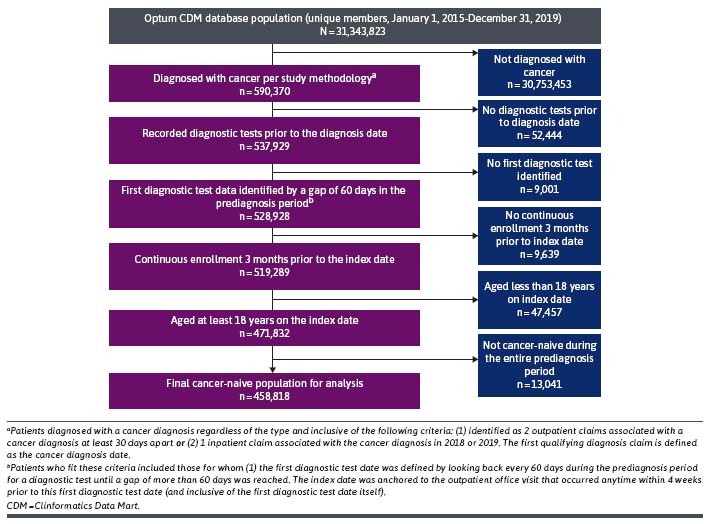
Population Attrition Flow Chart
TABLE 1.
Baseline Demographics and Clinical Characteristics, All Cancers and by Select Cancer Types
| Patients diagnosed with cancer | All cancers a N = 458,818 | Bladder b n = 39,949 | Breastn = 120,859 | Colorectaln = 22,649 | Lung c n = 59,801 | Ovariann = 4,334 | Pancreas n = 3,061 | Prostaten = 88,417 |
|---|---|---|---|---|---|---|---|---|
| Age, years | ||||||||
| Mean (SD) | 70.6 (1.1) | 74.5 (2.3) | 69.2 (2.8) | 65.6 (2.0) | 72.6 (2.2) | 65.7 (3.1) | 70.0 (6.2) | 72.7 (3.4) |
| Sex, % | ||||||||
| Male | 50.4 | 74.0 | 0.4 | 51.0 | 48.8 | 0.0 | 48.4 | 100.0 |
| Female | 49.6 | 26.0 | 99.6 | 49.0 | 51.2 | 100.0 | 51.6 | 0.0 |
| Race and ethnicity, % | ||||||||
| White | 65.0 | 71.8 | 77.8 | 69.4 | 79.7 | 71.3 | 68.4 | 69.1 |
| Black | 11.1 | 8.4 | 8.0 | 8.2 | 8.2 | 9.6 | 12.9 | 13.5 |
| Hispanic | 8.3 | 8.3 | 5.3 | 9.5 | 6.7 | 9.4 | 9.8 | 8.6 |
| Asian | 2.5 | 1.1 | 1.8 | 2.8 | 1.1 | 3.1 | 2.8 | 2.2 |
| Missing | 13.2 | 10.4 | 7.2 | 10.1 | 4.3 | 6.6 | 6.1 | 6.5 |
| Primary insurance, % | ||||||||
| Medicare Advantage | 74.0 | 81.3 | 75.8 | 67.3 | 84.5 | 59.9 | 66.6 | 56.8 |
| Commercial | 24.0 | 14.6 | 23.4 | 26.0 | 11.0 | 35.7 | 27.7 | 39.8 |
| Both | 2.0 | 4.1 | 0.8 | 6.8 | 4.5 | 4.4 | 5.7 | 3.4 |
| Charlson Comorbidity Indexd | ||||||||
| Mean (SD) | 2.1 (1.4) | 1.9 (1.1) | 1.7 (0.9) | 2.3 (0.8) | 2.5 (0.7) | 1.5 (1.2) | 2.2 (0.9) | 2.0 (1.3) |
a “All cancers” includes 20 cancer types.
b Bladder includes urothelial.
c Lung includes bronchus.
d Charlson Comorbidity Index score after adjusting for age.
TIME TO DIAGNOSIS
Across all patients newly diagnosed with cancer, the mean time to diagnosis was 156.2 days (SD, 164.9 days) and the median was 118 days (Q1 = 33.0 days; Q3 = 215.0 days) (Figure 3; Supplementary Table 4 (602.9KB, pdf) ). High heterogeneity in mean (median) time to diagnosis was exhibited by cancer type with ranges spanning 121.6 (102.0) days for bladder to 229.0 (194.0) days for multiple myeloma and among patients within the same type of cancer with large SDs close to or larger than the mean. A nontrivial proportion (15.8%) of patients with cancer experienced a time to diagnosis of greater than 180 days. Among the cancer types of interest, colorectal cancer (44.6%) represented the largest percentage, with greater than 180 days to diagnosis, and breast cancer (4.6%) represented the smallest.
FIGURE 3.
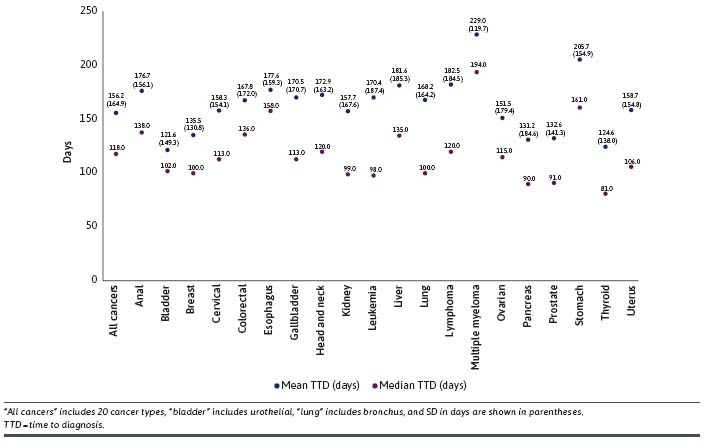
Mean and Median TTD from the First Diagnostic Test to Cancer Diagnosis, All Cancers and by Cancer Type
DIAGNOSTIC-RELATED HRU DURING THE DIAGNOSTIC PATHWAY
All Cancers. Among all patients newly diagnosed with cancer, imaging resource use was high, with the top 4 categories recorded for radiology (60.7%), computerized tomography (CT) (50.8%), magnetic resonance imaging (MRI) (48.6%), and ultrasound (42.6%). A total of 69.3% had endoscopy without biopsy and 36.5% had endoscopy with biopsy; more than half (62.5%) had other biopsies (not in conjunction with endoscopy); and there was high use in the laboratory category for general urine and serum tests (91.3%) and nongenetic cancer-specific laboratory tests (84.3%) (Table 2). Diagnostic-related resource use varied highly by cancer type as well as by the time to diagnosis (Supplementary Tables 5-11 (602.9KB, pdf) ).
TABLE 2.
Diagnostic-Related Health Care Resource Utilization During the Diagnostic Pathway, All Cancers and by Select Cancer Type
| Patients diagnosed with cancer | All cancers a N = 458,818 | Bladder b n = 39,949 | Breastn = 120,859 | Colorectaln = 22,649 | Lung c n = 59,801 | Ovariann = 4,334 | Pancreasn = 3,061 | Prostaten = 88,417 |
|---|---|---|---|---|---|---|---|---|
| Imaging,d n (%) | ||||||||
| CT | 233,261 (50.8) | 27,092 (67.8) | 34,298 (28.4) | 18,939 (83.6) | 41,287 (69.0) | 3,175 (73.3) | 1,999 (65.3) | 48,846 (55.3) |
| PET/PET-CT | 37,854 (8.3) | 1,111 (2.8) | 6,582 (5.5) | 4,488 (19.8) | 11,317 (18.9) | 758 (17.5) | 684 (22.4) | 3,112 (3.5) |
| MRI | 222,943 (48.6) | 4,675 (11.7) | 29,653 (24.5) | 20,487 (90.5) | 16,787 (28.1) | 1,151 (26.6) | 1,142 (37.3) | 25,051 (28.3) |
| Nuclear medicinee | 122,902 (26.8) | 3,705 (9.3) | 17,534 (14.5) | 3,169 (14.0) | 5,303 (8.9) | 561 (12.9) | 569 (18.6) | 24,766 (28.0) |
| Radiologyf | 278,386 (60.7) | 33,103 (82.9) | 95,147 (78.7) | 21,409 (94.5) | 18,081 (30.2) | 3,149 (72.7) | 1,861 (60.8) | 67,463 (76.3) |
| Ultrasound | 195,244 (42.6) | 35,361 (88.5) | 44,001 (36.4) | 0 (0.0) | 38,642 (64.6) | 542 (12.5) | 231 (7.6) | 15,882 (18.0) |
| Endoscopy, n (%) | ||||||||
| Endoscopy without biopsy | 317,866 (69.3) | 11,512 (28.8) | 0 (0.0) | 10,521 (46.5) | 32,643 (54.6) | 0 (0.0) | 93 (3.0) | 7,058 (8.0) |
| Endoscopy with biopsy | 167,340 (36.5) | 6,314 (15.8) | 55,794 (46.2) | 19,261 (85.0) | 6,950 (11.6) | 910 (21.0) | 1,471 (48.1) | 13,303 (15.1) |
| Other biopsies, n (%) | ||||||||
| 286, 538 (62.5) | 13,288 (33.3) | 71,218 (58.9) | 18,433 (81.4) | 23,157 (38.7) | 3,000 (69.2) | 1,620 (52.9) | 84,822 (95.9) | |
| Laboratory,d n (%) | ||||||||
| Genetic tests | 89,092 (19.4) | 944 (2.4) | 34,146 (28.3) | 3,114 (13.8) | 4,453 (7.5) | 1,529 (35.3) | 776 (25.4) | 2,983 (3.4) |
| General urine and serum tests | 418,890 (91.3) | 38,825 (97.2) | 120,218 (99.5) | 21,497 (94.9) | 55,883 (93.5) | 4,111 (94.9) | 2,530 (82.7) | 86,475 (97.8) |
| Nongenetic cancer-specific laboratory tests | 386,860 (84.3) | 32,282 (80.8) | 18,341 (15.2) | 19,113 (84.4) | 7,769 (13.0) | 3,723 (85.9) | 1,643 (53.7) | 70,865 (80.2) |
a “All cancers” includes 20 cancer types.
b Bladder includes urothelial.
c Lung includes bronchus.
d The number of imaging procedures or laboratory tests were counted as 1 if multiple claims for the same procedure or test were present on the same day.
e Nuclear medicine includes imaging (thyroid, parathyroid, adrenal, bone marrow, spleen, lymphatic and lymph nodes, liver, spleen, hepatobiliary system, salivary, gastric mucosa, gastric emptying, acute gastrointestinal blood loss, intestine, bone and/or joint, noncardiac vascular flow, myocardial perfusion, acute venous thrombosis, myocardial, cardiac blood pool, pulmonary ventilation, brain, cerebrospinal fluid flow, kidney, and testicular) and other diagnostic nuclear medicine procedures (full code list in the Supplementary Materials).
f Radiology includes radiological examinations, X-rays, angiograms, mammograms, tomosynthesis, lymphangiography, shuntogram, splenoportography, and venography.
CT = computerized tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
Bladder Cancer. In contrast with all cancers, among patients diagnosed with bladder cancer, the imaging categories with high use were ultrasound (88.5%), radiology (82.9%), and CT (67.8%). These patients had relatively low endoscopy without biopsy (28.8%) or other biopsies (33.3%); and laboratory use of nongenetic cancer-specific laboratory tests (80.8%) and general urine and serum tests (97.2%) remained high (Table 2). Most diagnostic-related HRU followed a general trend of an increase in use with greater time to the diagnosis of bladder cancer. The HRU with the largest absolute percentage increase between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” (“> 180 days” percentage minus “0-30 days” percentage) were biopsy (+ 45.3%); endoscopy without biopsy (+ 43.2%); and CT (+ 30.4%). Exceptions to the trend were for ultrasound (-3.3%) and general urine and serum tests (-3.6%), in which slight absolute percentage decreases were observed (Supplementary Table 5 (602.9KB, pdf) ). Large changes of 9.8, 4.3, and 4.0 times between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” (“> 180 days” percentage divided by “0-30 days” percentage) were found among positron emission tomography (PET)/PET-CT, genetic tests, and nuclear medicine categories, respectively.
Breast Cancer. For patients with breast cancer, the highest imaging resource use was for any radiology (78.7%). Endoscopy only occurred with biopsy (46.2%) and more than half had other biopsies (58.9%); almost all had laboratory use for general urine and serum tests (99.5%) (Table 2). Like bladder cancer, diagnostic-related HRU for patients with breast cancer tended to increase with a greater time to diagnosis. Some of the larger absolute percentage increases in HRU between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were for nuclear medicine (+ 50.3%), biopsy (+ 44.8%), CT (+ 44.7%), genetic tests (+ 38.1%), nongenetic cancer-specific laboratory tests (+ 37.7%), and MRI (+ 30.4%) (Supplementary Table 6 (602.9KB, pdf) ). The exception to the trend was for endoscopy with biopsy, for which there was a decrease of 16.2% in use between those diagnosed in “0-30 days” and those diagnosed in “> 180 days.” Large changes of 10.1, 6.3, and 4.4 times between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were found among PET/PET-CT, nuclear medicine, and nongenetic cancer-specific laboratory tests categories, respectively.
Colorectal Cancer. Among patients diagnosed with colorectal cancer, high imaging use was found for radiology (94.5%), MRI (90.5%), and CT (83.6%); endoscopy with biopsy (85.0%) and other biopsies (81.4%) were common; and the predominantly used laboratory resources were general urine and serum tests (94.9%) and nongenetic cancer-specific laboratory tests (84.4%) (Table 2). Patients with colorectal cancer had small absolute percentage increases of around 10% in diagnostic-related HRU for biopsy and nongenetic cancer-specific laboratory tests and 24.6% for CT between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” (Supplementary Table 7 (602.9KB, pdf) ). Other HRU categories had no change or small decreases in the absolute percentage between the 2 time to diagnosis cohorts except PET/PET-CT, nuclear medicine, and genetic tests (all ≥ 26.1%). Times changes between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were relatively constant.
Lung Cancer. Patients diagnosed with lung cancer had high CT (69.0%) imaging use. More than half had endoscopy without biopsy (54.6%) and a relatively small amount had endoscopy with biopsy (11.6%) or other biopsies (38.7%); there was high use in the laboratory category of general urine and serum tests (93.5%) (Table 2). The trend of increasing diagnostic-related HRU with a longer time to diagnosis was also found among patients with lung cancer. Absolute percentage increases in use were generally below 30.0%, except radiology (+ 49.0%) and PET/PET-CT (+ 40.2%) use, which increased more dramatically in those diagnosed in “0-30 days” compared with those diagnosed in “> 180 days” (Supplementary Table 8 (602.9KB, pdf) ). Large changes of more than 4 times between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were found among genetic tests, PET/PET-CT, and endoscopy with biopsy categories.
Ovarian Cancer. Patients with ovarian cancer had high imaging resource utilization for CT (73.3%) and radiology (72.7%), low use of endoscopy with biopsy (21.0%), no use of endoscopy without biopsy, high use of other biopsies (69.2%), and high laboratory use for general urine and serum test (94.9%) and nongenetic cancer-specific laboratory tests (85.9%) (Table 2). Patients diagnosed with ovarian cancer followed the trend of increasing diagnostic-related HRU with time to diagnosis. Absolute percentage increases in HRU use were less dramatic than in other cancer types between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” (ranged from + 5.5% for general urine and serum tests to + 21.4% for CT) (Supplementary Table 9 (602.9KB, pdf) ). Modest changes of 2.7 and 2.0 times between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were found among the PET/PET-CT and ultrasound categories, respectively.
Pancreatic Cancer. Patients with pancreatic cancer had high records of imaging resource use for CT (65.3%) and radiology (60.8%), almost half used endoscopy with biopsy (48.1%), a little more than half had recorded other biopsies use (52.9%), and laboratory imaging use was highest for general urine and serum tests (82.7%) (Table 2). The trend of increasing diagnostic-related HRU use with longer time to diagnosis continued among patients with pancreatic cancer, with large absolute percentage increases between those diagnosed in “0-30 days” and those in “> 180 days” in the following HRU categories: PET/PET-CT (+ 89.8%), nuclear medicine (+ 82.2%), nongenetic cancer-specific laboratory tests (+ 64.2%), genetic tests (+ 63.5%), endoscopy with biopsy (+ 62.7%), radiology (+ 59.2%), MRI (+ 59.2%), and CT (+ 49.2%) (Supplementary Table 10 (602.9KB, pdf) ). Large changes of 11.3, 10.3, 7.7, and 4.0 times between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were found among the nuclear medicine, PET/PET-CT, endoscopy without biopsy, and genetic tests categories, respectively.
Prostate Cancer. Among those diagnosed with prostate cancer, radiology (76.3%) represented the largest imaging resource use; few had an endoscopy with biopsy (15.1%) or endoscopy without biopsy (8.0%); almost all had other biopsies (95.9%) and a high laboratory resource use was observed for general urine and serum tests (97.8%) and nongenetic cancer-specific laboratory tests (80.2%) (Table 2). Patients with prostate cancer did not follow a clear trend in HRU with time to diagnosis. Absolute percentage differences in diagnostic-related HRU between those diagnosed in “0-30 days” compared with those diagnosed in “> 180 days” were relatively small (< +/-10%), the one exception being the large absolute percentage decrease in nongenetic cancer-specific laboratory tests (-49.7%) (Supplementary Table 11 (602.9KB, pdf) ). Times changes between those diagnosed in “0-30 days” and those diagnosed in “> 180 days” were relatively constant at values close to 1.0.
Discussion
By assessing the time to diagnosis and HRU during cancer diagnoses pathways using recent data from a large claims database, this study significantly helps to fill the evidence gap on recent US trends and patterns in cancer diagnoses across multiple cancer types. Overall, the results suggest high heterogeneity exists in the time to diagnosis by cancer type and within cancer types and that a clinically and economically meaningful proportion of patients experienced a time to diagnosis of more than 180 days. Few diagnostic guidelines on the recommended time to diagnosis are available, and they widely differ by cancer type and country. The few identified suggest that the maximum this interval ranges, from 14 days to 120 days, depends on symptom presentation and cancer type.34-36 Notably, the intervals recommended are maximums rather than optimal to allow earlier cancer staging and improved clinical outcomes. Thus, a time to diagnosis of more than 180 days would likely be considered unacceptable by most clinicians.
Cancer diagnosis combined with treatment at an earlier stage may be associated with improved clinical and economic outcomes, including decreased morbidity and mortality as well as lower resource utilization and costs.1,7,37-41 For example, estimates using Surveillance, Epidemiology, and End Results Program 2006-2015 data suggest that the identification of multiple cancer types earlier than stage IV could reduce cancer-related deaths in the US by 15% within 5 years.37 Another study evaluating the potential treatment cost savings from early cancer diagnosis using published 2017 cost estimates and incidence rates by stage at diagnosis, estimated that the US national cost savings from early cancer diagnosis was approximately $26 billion.38
More comprehensive diagnostic pathway guidelines as implemented in other countries12-18 or medical innovations that help triage the cancer diagnostic pathways may contribute to an efficient, streamlined diagnosis process. Guideline features that may improve efficiency include setting a maximum threshold to reach points along the diagnostic pathway,42,43 specifying a shorter referral timeline for patients suspected of having cancer, 42,43 and providing options for patients with nonspecific signs and symptoms.44 Research on the impact of diagnostic guidelines implemented in other countries suggest these guidelines may aid in shortening the diagnostic process42,45-48 and reducing waiting times45,49-51 and ultimately improve tumor detection,52 patient diagnosis at earlier cancer stages,50 health-related quality of life,53 and survival.48,54 However, concerns over potential increases in resource utilization, radiation exposure risk, and overdiagnosis55 and inadequacies in guidelines to optimally benefit patients presenting with nonspecific signs and symptoms still need to be addressed.53
Diagnostic-related HRU increased with a longer time to diagnosis for most diagnostic categories and most selected cancers of interest. Colorectal cancer was an exception with relatively small percentage decreases in use for several categories indicating more constant resource use across time to diagnosis cohorts and perhaps suggesting that colorectal cancer may be more difficult to pinpoint given the potential noise of a range of potential nonspecific signs and symptoms to diagnostically assess. Some of the larger percentage decreases (≥ 26.1%) in PET/PET-CT, nuclear medicine, and genetic tests possibly reflect nuances in the population with colorectal cancer presenting for care and the patterns of care for diagnosis. One study based on Veterans Affairs data (2003-2005, n = 449) suggests that colorectal cancer diagnostic-related HRU may be highly variable dependent of facility, patient age, and clinical presentation.56 Unlike this study, in which some of these key facility and patient characteristics are very different, increased resource use among patients with colorectal cancer was found to be highly correlated with increased time to diagnosis (similar to trends found in the other cancer types of interest). Resource use by time to diagnosis were also relatively small for prostate cancer, perhaps reflecting that prostate cancer diagnosis is more straightforward and systematic, and tends to implement a “watch and wait” approach that extends more constant resource use across the time to diagnosis categories.
Some challenges exist in comparing the study results with the published literature. In addition to the US literature being scarce, studies tend to focus on a few cancer types, such as lung and breast, have different unit of measurement (eg, per person per month), be limited in generalizability, vary in insurance coverage population mixes, and use a variety of definitions for the diagnostic period.24-32 For instance, some researchers have assessed from the presentation of symptoms to diagnosis, whereas others have focused in on the period from the use of the first diagnostic test or a positive test to diagnosis. Defining from the first “positive” diagnostic test may underestimate the time to diagnosis and HRU, as there may have been multiple tests run prior to the “positive” test, especially among those with nonspecific signs and symptoms or more complex presentations.
Results from the limited published US literature on the time to diagnosis in the cancers of interest are highly variable. However, published results seem generally in line with the study findings within the context of population and methodology differences. An older study assessing Surveillance, Epidemiology, and End Results data from 1992 to 2002 reported that 18% of the population with bladder cancer experienced a diagnostic delay greater than or equal to 180 days28 compared with 36.9% in our study. However, the aforementioned study truncated the results at the end of 12 months and limited the diagnostic period from hematuria claim to diagnosis. Similarly, breast cancer literature is sparse and older; one study (2010-2012, n = 120) found a much smaller median of 23 days29 vs 100 days in this study, but the study methodology only considered the period between presentation and diagnostic biopsy. Not all patients diagnosed with breast cancer will receive a diagnostic biopsy (eg, because of safety or logistical concerns); thus, other potential diagnostic test methods, such as a breast ultrasound or MRI or a diagnostic mammogram, should be included as an option to define the breast cancer diagnosis date. Published colorectal cancer time to diagnosis data generally indicated relatively similar times; a study assessing 2008 to 2020 data (n = 252) reported an average time to diagnosis of 141 days31 vs 168 days in this study. Research on Veterans Administration data (2003-2005, n = 447) reported a median time to diagnosis of 73 to 91 days26 compared with 136 days in our study, but the timing of the initial event starting the diagnostic period varied and sometimes was only assessed from an abnormal screening test result. And an intervention study using primary care provider data (2011-2012, n = 557) recorded a median time to diagnosis of 104 to 200 days30 from a trigger of a positive or suspicious test in line with this study’s results. Published data on lung cancer time to diagnosis generally indicate a shorter time to diagnosis than our study results (mean: 168 days; median: 100 days). In contrast, a study on data from Wisconsin and North Carolina sites (2012-2014, n = 347), reported a mean time to diagnosis of 85 days32; research on the Veterans Affairs Connecticut Healthcare System hospital (2005-2010, n = 352) had a mean time to diagnosis of 53-76 days dependent on participation in a care coordination intervention24 but started the time to diagnosis assessment from an abnormal finding; and another study (2011-2012, n = 19) recorded a median time to diagnosis of 65-93 days dependent on the use of a trigger-based intervention,30 but the assessment of time to diagnosis only occurred from a trigger of a positive or suspicious test. One study on patients with ovarian cancer that evaluated the time between initial gastrointestinal symptoms and diagnosis using Truven MarketScan data (2014-2018, n = 5,865) found a similar mean time to diagnosis (147 days27) to our study (152 days). The trigger-based intervention study conducted in patients with colorectal and lung cancer was also done in patients with prostate cancer (n = 157) with a recorded median time to diagnosis of 144-192 days,30 relatively similar to our study (139 days). Intervention study data24-25,30 further support the idea that implemented care guidelines outlining a recommended diagnosis process can decrease the time to diagnosis.
LIMITATIONS
Our study captures individuals with commercial or Medicare Advantage health coverage in the United States with data available in the Optum Clinformatics Data Mart database. Thus, results of our analysis may not be generalizable to patients not captured in the database (eg, with other insurance or without health insurance coverage). Cancer diagnoses in our analyses are dependent on the ICD classification system, specifically ICD-10 diagnosis codes for cancer, and clinician consistent and appropriate code use. Limited research on the validation of ICD codes use for cancer diagnosis suggests that ICD diagnosis code use may misclassify or miss diagnoses for some cancers in comparison with the medical record.33,57,58 Thus, the study population may include some individuals not diagnosed with cancer or may not represent all eligible patients diagnosed with cancer. As patients with multiple cancer diagnosis codes for cancer on the diagnosis date were excluded, the results are not generalizable to this population. Additionally, the use of a 60-day gap algorithm between diagnostic tests is not validated and some time to diagnosis and HRU calculations may not capture the diagnosis process’ full duration. Resource use analyses include diagnostic-related codes that could be applied to diagnosis procedures for non–cancer-related conditions. For instance, a biopsy recorded during the colorectal cancer diagnosis period may be related to infection or an inflammatory/autoimmune disorder assessment and not to the cancer diagnosis. The current study primarily (71%) includes a population measured from the first office visit. However, 29% of patients did not have an office visit within the 4-week period prior to a diagnostic test and the first diagnostic test date was used as the index, which may underestimate the calculated time to diagnosis. Furthermore, the scope of these analyses only covers from the index date to cancer diagnosis and does not capture the time between the onset of suspicious symptoms and presenting into the health care system or outline the impact of a direct or delayed referral for diagnostic testing. Unfortunately, it is unknown if the commercial plans captured in the study’s analysis population had diagnostic guidelines established or if they impacted time to diagnosis or resource utilization. Other factors potentially contributing to diagnostic error, delay, or lengthening of the diagnostic pathway need further exploration.
Conclusions
A clinically and economically meaningful proportion of patients diagnosed with cancer experienced a diagnostic process greater than 180 days. Taken in concert with findings of greater diagnostic-related HRU trending with a longer time to diagnosis, the study results highlight inefficiencies in the US cancer diagnostic process. The development or modification of policies, guidelines, or medical interventions that streamline cancer diagnostic pathways are needed to optimize patient outcomes as well as reduce resource burden and cost to the health care system.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2022. American Cancer Society; 2022. [Google Scholar]
- 3.National Cancer Institute. Cancer trends progress report. National Institutes of Health. Accessed January 20, 2022. https://progressreport.cancer.gov
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. doi:10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 5.Sarma EA, Kobrin SC, Thompson MJ. A. proposal to improve the early diagnosis of symptomatic cancers in the United States. Cancer Prev Res (Phila). 2020;13(9):715-20. doi:10.1158/1940-6207.CAPR-20-0115 [DOI] [PubMed] [Google Scholar]
- 6.Sarma EA, Walter FM, Kobrin SC. Achieving diagnostic excellence for cancer: symptom detection as a partner to screening. JAMA. 2022;328(6):525-6. doi:10.1001/jama.2022.11744 [DOI] [PubMed] [Google Scholar]
- 7.Neal RD, Tharmanathan P, France B, et al. . Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(Suppl 1):S92-107. doi:10.1038/bjc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna TP, King WD, Thibodeau S, et al. . Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi:10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen MM, Silverstein SC, Quinn M, et al. . Timeliness of access to lung cancer diagnosis and treatment: a scoping literature review. Lung Cancer. 2017;112:156-64. doi:10.1016/j.lungcan.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 10.Weller D, Vedsted P, Rubin G, et al. . The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106:1262-7. doi:10.1038/bjc.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman-Toker DE, Wang Z, Zhu Y, et al. . Rate of diagnostic errors and serious misdiagnosis-related harms for major vascular events, infections, and cancers: towards a national incidence estimate using the “Big Three”. Diagnosis. 2021;8(1):67-84. doi:10.1515/dx-2019-0104 [DOI] [PubMed] [Google Scholar]
- 12.Canadian Partnership Against Cancer. Canadian strategy for cancer control. Accessed February 22, 2022. https://www.partnershipagainstcancer.ca/cancer-strategy/ [Google Scholar]
- 13.Cancer Council. Optimal care pathways. Accessed February 22, 2022. https://www.cancer.org.au/health-professionals/optimal-cancer-care-pathways [Google Scholar]
- 14.National Institute for Health and Care Excellence. Suspected cancer: recognition and referral. June 23, 2015. Accessed March 20, 2023. https://www.nice.org.uk/guidance/ng12 [Google Scholar]
- 15.Emery J, Vedsted P. New NICE guidance on diagnosing cancer in general practice. Br J Gen Pract. 2015;65(638):446-7. doi:10.3399/bjgp15X686401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson BD, Oke J, Friedemann Smith C, et al. . The Suspected CANcer (SCAN) pathway: protocol for evaluating a new standard of care for patients with nonspecific symptoms of cancer. BMJ Open. 2018;8(1):e018168. doi:10.1136/bmjopen-2017-018168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vedsted P, Olesen F.. A differentiated approach to referrals from general practice to support early cancer diagnosis – the Danish three-legged strategy. Br J Cancer. 2015;112(Suppl 1):S65-9. doi:10.1038/bjc.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkens J, Thulesius H, Schmidt I, Carlsson C. The 2015 National Cancer Program in Sweden: introducing standardized care pathways in a decentralized system. Health Policy. 2016;120(12):1378-82. doi:10.1016/j.healthpol.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Breast cancer screening and diagnosis (version 1.2022). Accessed February 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Colorectal cancer screening (version 3.2022). Accessed February 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf [Google Scholar]
- 21.National Comprehensive Cancer Network. Lung cancer screening (version 1.2023). Accessed February 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf [Google Scholar]
- 22.National Comprehensive Cancer Network. Prostate cancer early detection (version 1.2023). Accessed February 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf [DOI] [PubMed] [Google Scholar]
- 23.Al Achkar M, Zigman Suchsland M, Walter FM, Neal RD, Goulart B, Thompson MJ. Experiences along the diagnostic pathway for patients with advanced lung cancer in the USA: a qualitative study. BMJ Open. 2021;11(4):e045056. doi:10.1136/bmjopen-2020-045056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsamarai S, Yao X, Cain HC, et al. . The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer. 2013;14:527-34. doi:10.1016/j.cllc.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo SM, Geronimo MCM, Putscher TMJ, et al. . Improved coordination of care for patients with abnormal chest imaging: the rapid access chest and lung assessment program. J Clin Outcomes Manag. 2014;21:453-8. [Google Scholar]
- 26.Fisher DA, Zullig LL, Grambow SC, Abbott DH, et al. . Determinants of medical system delay in the diagnosis of colorectal cancer within the Veteran Affairs Health System. Dig Dis Sci. 2010;55(5):1434-41. doi:10.1007/s10620-010-1174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrow B, Perhanidis J, Chase D. Do gastrointestinal symptoms delay the diagnosis of ovarian cancer? Value Health. 2019;22(Suppl2):S84. doi:10.1016/j.val.2019.04.275 [Google Scholar]
- 28.Hollenbeck BK, Dunn RL, Ye Z, et al. . Delays in diagnosis and bladder cancer mortality. Cancer. 2010;116(22):5235-42. doi:10.1002/cncr.25310 [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal K, Hull M, Furniss AL, Doyle R, Gayou N, Bayliss E. Delays in diagnosis and treatment of breast cancer: a safety-net population profile. J Natl Compr Canc Netw. 2018;16(12):1451-7. doi:10.6004/jnccn.2018.7067 [DOI] [PubMed] [Google Scholar]
- 30.Murphy DR, Wu L, Thomas EJ, et al. . Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized control trial. J Clin Oncol. 2015;33(31):3560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siminoff LA, Rogers HL, Harris-Haywood S. Missed opportunities for the diagnosis of colorectal cancer. Biomed Res Int. 2015;2015:285096. doi:10.1155/2015/285096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidaver RM, Shershneva MB, Hetzel SJ, Holden TR, Campbell TC. Typical time to treatment of patients with lung cancer in a multisite, US-based study. J Oncol Pract. 2016;12(6):e643-53. doi:10.1200/JOP.2015.009605 [DOI] [PubMed] [Google Scholar]
- 33.Whyte JL, Engel-Nitz NM, Teitelbaum A, Gomez Rey G, Kallich JD. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care. 2015;53(7):e49-57. doi:10.1097/MLR.0b013e318289c3fb [DOI] [PubMed] [Google Scholar]
- 34.Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A. snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-5. [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence. Suspected cancer: recognition and referral. Non–site-specific symptoms. NICE guideline [NG12.1.13]. Accessed February 20, 2023. https://www.nice.org.uk/guidance/ng12/chapter/Recommendations-organised-by-site-of-cancer#non-site-specific-symptoms [Google Scholar]
- 36.Emery J, Lee-Bates B, Brown G, et al. . Optimal maximum time from referral to diagnosis and treatment. Cancer Council Australia. Accessed February 20, 2023. https://wiki.cancer.org.au/australia/Clinical_question:Diagnosis_interval_in_colorectal_cancer [Google Scholar]
- 37.Clarke CA, Hubbell E, Kurian AW, Colditz GA, Hartman AR, Gomez SL. Projected reductions in absolute cancer-related deaths from diagnosing cancers before metastasis, 2006-2015. Cancer Epidemiol Biomarkers Prev. 2020;29(5):895-902. doi:10.1158/1055-9965.EPI-19-1366 [DOI] [PubMed] [Google Scholar]
- 38.Kakushadze Z, Raghubanshi R, Willie Yu. Estimating cost savings from early cancer diagnosis. Data. 2017;2(3):30. doi:10.3390/data2030030 [Google Scholar]
- 39.World Health Organization. Guide to cancer early diagnosis. Published February 16, 2017. Accessed February 16, 2023. https://www.who.int/publications/i/item/9789241511940 [Google Scholar]
- 40.McGarvey N, Gitlin M, Fadli E, Chung KC. Increased healthcare costs by later stage cancer diagnosis. BMC Health Serv Res. 2022;22(1):1155. doi:10.1186/s12913-022-08457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49(9):2187-98. doi:10.1016/j.ejca.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 42.Neal RD, Din NU, Hamilton W, et al. . Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110(3):584-92. doi:10.1038/bjc.2013.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prades J, Espinàs JA, Font R, Argimon JM, Borràs JM. Implementing a Cancer Fast-track Programme between primary and specialised care in Catalonia (Spain): a mixed methods study. Br J Cancer. 2011;105(6):753-9. doi:10.1038/bjc.2011.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolly SO, Jones G, Allchorne P, et al. . The effectiveness of the Guy’s Rapid Diagnostic Clinic (RDC) in detecting cancer and serious conditions in vague symptom patients. Br J Cancer. 2021;124(6):1079-87. doi:10.1038/s41416-020-01207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyrop HB, Safwat A, Vedsted P, et al. . Cancer patient pathways shortens waiting times and accelerates the diagnostic process of suspected sarcoma patients in Denmark. Health Policy. 2013;113(1-2):110-7. doi:10.1016/j.healthpol.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 46.Lyhne NM, Christensen A, Alanin MC, et al. . Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer. 2013;49(7):1627-33. doi:10.1016/j.ejca.2012.11.034 [DOI] [PubMed] [Google Scholar]
- 47.Jensen H, Tørring ML, Olesen F, Overgaard J, Fenger-Grøn M, Vedsted P. Diagnostic intervals before and after implementation of cancer patient pathways - a GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer. 2015;15:308. doi:10.1186/s12885-015-1317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen KH, Maina PJ. Cancer pathways are associated with improved long-term survival. Dan Med J. 2015;62(2):A5000. [PubMed] [Google Scholar]
- 49.Martínez MT, González I, Tarazona N, et al. . Implementation and assessment of a fast-track programme to improve communication between primary and specialized care in patients with suspected cancer: how to shorten time between initial symptoms of cancer, diagnosis and initiation of treatment. Clin Transl Oncol. 2015;17(2):167-72. doi:10.1007/s12094-014-1209-3 [DOI] [PubMed] [Google Scholar]
- 50.Nilssen Y, Brustugun OT, Tandberg Eriksen M, et al. . Decreasing waiting time for treatment before and during implementation of cancer patient pathways in Norway. Cancer Epidemiol. 2019;61:59-69. doi:10.1016/j.canep.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 51.Dahl TL, Vedsted P, Jensen H. The effect of standardised cancer pathways on Danish cancer patients’ dissatisfaction with waiting time. Dan Med J. 2017;64(1):A5322. [PubMed] [Google Scholar]
- 52.Chng M, Coulter Ic, Surash S.. Impact of the updated NICE referral pathway for patients with suspected brain cancer on a neuroscience service. Br J Neurosurg. 2022;36(1):11-5. doi:10.1080/02688697.2020.1823317 [DOI] [PubMed] [Google Scholar]
- 53.Moseholm E, Lindhardt BØ. Patient characteristics and cancer prevalence in the Danish cancer patient pathway for patients with serious non-specific symptoms and signs of cancer-a nationwide, population-based cohort study. Cancer Epidemiol. 2017;50(Pt A):166-72. doi:10.1016/j.canep.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 54.Jensen H, Vedsted P. Exploration of the possible effect on survival of lead-time associated with implementation of cancer patient pathways among symptomatic first-time cancer patients in Denmark. Cancer Epidemiol. 2017;49:195-201. doi:10.1016/j.canep.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 55.Næser E, Fredberg U, Møller H, Vedsted P. Clinical characteristics and risk of serious disease in patients referred to a diagnostic centre: a cohort study. Cancer Epidemiol. 2017;50(Pt A):158-65. doi:10.1016/j.canep.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 56.Srygley FD, Abott DH, Grambow SC, Provenzale D, Sandler RS, Fisher DA. Variability in resource use: diagnosing colorectal cancer. Am J Manag Care. 2013;19(5):370-6. [PubMed] [Google Scholar]
- 57.Baldi I, Vicari P, Di Cuonzo D, et al. . A high positive predictive value algorithm using hospital administrative data identified incident cancer cases. J Clin Epidemiol. 2008;61(4):373-9. doi:10.1016/j.jclinepi.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 58.Park SL, Tate JP, Rodrigues-Barradas MC, et al. . Cancer incidence in HIV-infected versus uninfected veterans: comparison of cancer registry and ICD-9 code diagnoses. J AIDS Clin Res. 2014;5(7):1000318. doi:10.4172/2155-6113.1000318 [DOI] [PMC free article] [PubMed] [Google Scholar]


