Abstract
The Tax transactivator protein of human T-cell leukemia virus type 1 (HTLV-1) plays a central role in the activation of viral gene expression. In addition, Tax is capable of activating the expression of specific cellular genes and is involved in the transformation of T-lymphocytes resulting in the development of adult T-cell leukemia. Tax is a phosphoprotein that colocalizes in nuclear bodies with RNA polymerase II, splicing complexes, and specific transcription factors including members of the ATF/CREB and NF-κB families. In this study, we identified adjacent serine residues at positions 300 and 301 in the carboxy terminus of Tax as the major sites for phosphorylation. Phosphorylation of at least one of these serine residues is required for Tax localization in nuclear bodies and for Tax-mediated activation of gene expression via both the ATF/CREB and NF-κB pathways. Introduction of amino acid substitutions which are phosphoserine mimetics at positions 300 and 301 restored the ability of a phosphorylation-defective Tax mutant to form nuclear bodies and to activate gene expression. These studies define sites for regulatory phosphorylation events in Tax which are critical for its ability to activate gene transcription.
The human T-cell leukemia virus types 1 and 2 (HTLV-1 and HTLV-2) are closely related human retroviruses. HTLV-1 is the causative agent for adult T-cell leukemia/lymphoma (23, 39), while HTLV-2 is associated with a rare form of human hairy-cell leukemia (25). Both viruses encode potent activators of viral transcription known as Tax (9, 11, 41, 48). Not only does Tax activate viral gene expression, but it also activates the expression of specific cellular genes involved in normal T-cell activation and proliferation (4, 13, 31, 46), and this activity has been implicated in Tax transforming activity. Tax transforms lymphocytes and fibroblasts (10, 18, 34, 40, 51) and induces tumors in transgenic mice (19, 36).
Tax colocalizes in discrete nuclear bodies with cellular factors essential for its transcriptional activities (6, 7, 45), including RNA polymerase II, components of the splicesome, and specific members of the ATF/CREB and NF-κB families of transcription factors, including ATF-1, the two subunits of NF-κB p50 and RelA, and the two transcriptional coactivators CBP and p300. Tax activates HTLV-1 gene expression via interactions with ATF/CREB proteins (14, 15, 38, 54, 55) and the transcriptional coactivator CBP (16, 27), resulting in increased binding of these factors to three 21-bp repeats present in the viral long terminal repeat (LTR).
Tax is also capable of increasing the expression of other viral and cellular genes, such as the genes coding for interleukin-2 (IL-2), IL-2 receptor α, and the human immunodeficiency virus, by regulating NF-κB activation (4, 26, 31, 46). NF-κB is a heterodimeric complex containing two DNA-binding proteins termed p50 and RelA (3) which, in the absence of specific inducers, is sequestered in the cytoplasm through high-affinity binding with the labile cytoplasmic inhibitors IκBα and IκBβ (2, 22). NF-κB is constitutively activated in Tax-expressing cells and in HTLV-1-infected cells (17, 28). This constitutive activation is at least in part due to Tax-induced phosphorylation and subsequent proteolytic breakdown of IκBα and IκBβ with release of the RelA subunit from cytoplasmic sequestration and translocation of RelA into the nucleus (8, 26, 30, 35, 49). In addition to altering the stability of IκBα and IκBβ, Tax has also been shown to physically associate with the RelA subunit of NF-κB (29, 50) and to colocalize with both p50 and RelA in nuclear bodies (6). Tax mutants unable to activate gene expression via the NF-κB and/or the ATF/CREB pathways and defective for cellular transformation have been described previously (44, 47, 53).
Tax is phosphorylated on serine residues that map on a single tryptic peptide, and Tax phosphorylation in human lymphocytes is increased by a treatment of the cells with phorbol esters in a time- and dose-dependent manner (12). However, the localization of the phosphoserine residue(s) and the role of phosphorylation in Tax function are unknown. Since protein phosphorylation modulates the activity of a number of cellular factors (24), we characterized the role of Tax phosphorylation on its ability to activate gene expression. In the present study we identified two serine residues at positions 300 and 301 as the major sites for Tax phosphorylation and we demonstrated that phosphorylation is critical for transcriptional activation by Tax.
MATERIALS AND METHODS
Cell culture.
Cell lines were obtained from the American Type Culture Collection. BHK21 (hamster kidney) cells (ATCC CRL 8544) were grown in Glasgow minimum essential medium (Gibco BRL, Gaithersburg, Md.) supplemented with 10% tryptose phosphate, 20 mM HEPES buffer, and 5% fetal bovine serum. Jurkat cells were grown in RPMI 1640 medium (Gibco BRL) supplemented with 10% fetal bovine serum, 100 U of penicillin G sodium per ml, 100 μg of streptomycin sulfate per ml, and 2 mM glutamine.
Plasmids.
The wild-type tax cDNA fragment (Genbank accession no. P14079 [33]) was cloned in the pSFV3 vector (32) as described previously (6). Mutations F2 (Ser300 converted into Leu [S300L] and S301A), F3 (S301A), F4 (S300L, S301A, and N304S), F6 (S300L), and F9 (S300D and S301D) were introduced into the tax gene by site-directed mutagenesis and then transferred to the vector pSFV3. The wild-type and mutated tax cDNAs were also inserted into the pDP expression vector under the control of the Rous Sarcoma Virus promoter. The HIV type 1 (HIV-1) LTR chloramphenicol acetyltransferase (CAT) reporter plasmid contains an EcoRV (−343)/HindIII (+80) fragment from the HIV-1 LTR of ARV2 upstream of the CAT gene (21), and the HTLV-I LTR CAT reporter plasmid (43) was described previously.
Establishment of SFV-Tax recombinant stocks.
The Semliki Forest virus (SFV) expression system was described previously (5, 32). Briefly, SFV vector RNA and wild-type or mutant recombinant SFV-Tax RNAs were synthesized by using an SP6 in vitro transcription system. Each of these RNAs was cotransfected with SFV helper 2 RNA into BHK21 cells by electroporation (Bio-Rad, Hercules, Calif.). This generated replication-defective virus stocks for either control SFV or the SFV recombinants expressing wild-type Tax or the Tax mutants F2, F3, F4, F6, and F9. These viruses were concentrated 100-fold by ultracentrifugation, resuspended in TNE buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 0.5 mM EDTA), and stored at −80°C. The virus stocks were activated by chymotrypsin treatment (5) and titrated by immunofluorescence staining of BHK21 cells infected with various dilutions of these SFV stocks. Typically, the recombinant virus stocks, the concentrations of which ranged from 109 to 1010 PFU/ml, were used to infect cells at a multiplicity of infection of 5.
Antibodies.
Monoclonal antibody 168-A51, which recognizes a C-terminal epitope of Tax (NIH AIDS Research and Reagent Program), was used for immunoprecipitation and immunofluorescence staining to detect the Tax protein. The secondary antibody, a goat anti-mouse fluorescein isothiocyanate conjugate, was purchased from Jackson ImmunoResearch, West Grove, Pa.
Immunocytochemistry and confocal microscopy.
Cells cultured on coverslips were infected at a multiplicity of infection of 5 with the different recombinant SFVs, washed with phosphate-buffered saline (PBS), and fixed with methanol at −20°C for 6 min. The cells were washed twice with PBS, blocked for 30 min in PBS containing 0.5% gelatin and 0.25% bovine serum albumin, and incubated for 1 h at room temperature with the primary antibody. The preparations were washed three times with 0.2% gelatin in PBS and incubated for 1 h with the secondary antibody. Samples were washed three times and then mounted in a solution of 1 mg of p-phenylenediamine per ml in 90% glycerol. The preparations were examined on an MRC1024 laser scanning confocal microscope (Bio-Rad, Microscience Division; Cambridge, Mass.) equipped with a 15-mW air-cooled krypton-argon laser (Ion Laser Technology; Salt Lake City, Utah) as a light source. The images were constructed from greyscale confocal fluorescence images with Adobe Photoshop software.
In vivo incorporation of 32Pi and 35S-labeled methionine and cysteine.
BHK21 or Jurkat cells (106 or 107 cells, respectively) were infected with the control SFV or the different SFV-Tax recombinants for 5 h at a multiplicity of infection of 5. The cells were washed twice with PBS and incubated in starvation medium (minimal Eagle medium without methionine and cysteine or without phosphate) for 30 min which was then replaced by the same medium containing either 100 μCi of [35S]methionine and [35S]cysteine (Translabel; NCI Pharmaceuticals Inc., Costa Mesa, Calif.) or 0.5 mCi of [32P]orthophosphate (NEN, Boston, Mass.). The labeling was continued for 5 h, the cells were washed twice with PBS, and they were lysed with RIPA buffer (Tris-HCl 50 mM [pH 8.0], NaCl 150 mM, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS). The Tax protein was then immunoprecipitated with a monoclonal antibody directed against Tax.
Transfections and CAT assays.
Jurkat (5 × 106) cells were transfected by lipofection with expression vectors containing wild-type or mutant tax cDNAs (1 μg) under the control of the Rous sarcoma virus promoter and 1 μg of either the HIV-1 LTR CAT or HTLV-I LTR CAT reporter plasmids (Lipofectamine; Gibco BRL). The cells were collected at 32 h post-transfection, and the cell extracts were assayed for determination of the CAT activity by separation of acetylated chloramphenicol by thin-layer chromatography. The percentage of acetylated chloramphenicol was quantitated by a PhosphorImager.
Protein tryptic digestion.
The Tax protein, which was labeled by incorporation of [32P]orthophosphate, was purified by immunoprecipitation, separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE), and electroblotted on a nitrocellulose membrane. After autoradiography, the piece of membrane carrying the labeled Tax protein was excised; washed twice for 30 min in 200 μl of 100 mM Tris HCl, pH 8.5, containing 50% acetonitrile; and incubated for 20 h at 37°C in digestion solution (100 mM Tris HCl [pH 8.5], 2 mM CaCl2, 5% acetonitrile, 0.2% Tween 20, 0.1 μg of trypsin [sequencing grade; Sigma]). The digestion solution was collected, and extraction of the digested peptides was continued for 1 h in 200 μl of 0.1% trifluoroacetic acid (TFA). The combined extracts were concentrated by lyophilization. The tryptic peptides were resolved by electrophoresis on a Tris-Tricine SDS–16.5% PAGE gel (42) and by reverse-phase high-performance liquid chromatography (HPLC).
Separation of tryptic peptides by reverse-phase HPLC.
The tryptic peptides were separated by reverse-phase HPLC on a PE Applied Biosystems model 17A Capillary LC/Microblotter (C18 column [0.5 by 150 mm]). The peptides were eluted with a linear gradient of 5 to 45% solvent B (acetonitrile containing 0.075% TFA) in solvent A (0.1% TFA in HPLC-grade water) and collected on a polyvinylidene difluoride (PVDF) membrane. Peptide elution was monitored by UV absorbance at 214 nm. The PVDF membrane containing the peptides was stored at −80°C.
Amino acid microsequence analysis.
Amino acid microsequence analysis of the peptides was performed by automated Edman degradation on an LF3400 protein-peptide microsequencer (Beckman Instruments, Inc., Fullerton, Calif.) equipped with an on-line gold 126 microgradient HPLC system and a Diode Array detector (model 168; Beckman) (52). To identify phosphoserine residues, phosphoserines were converted into S-ethylcysteine as described elsewhere (37). Microsequencing of peptides containing converted phosphoserines yields well-separated peaks corresponding to phenylthiohydantoin derivatives of S-ethylcysteine which elute just before the elution position of N,N′-diphenylthiourea.
RESULTS
Tryptic peptide analysis of in vivo-phosphorylated Tax protein.
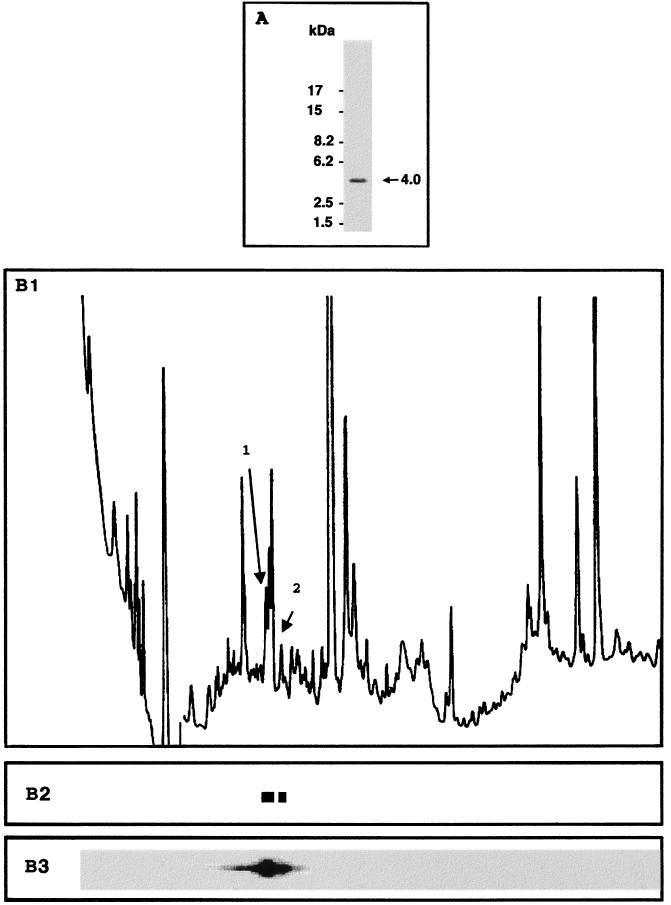
Previous studies have suggested that Tax was phosphorylated on a serine residue(s) that maps on a single tryptic peptide (12). To identify the phosphorylated peptide, tryptic digests from in vivo-phosphorylated Tax were analyzed by Tris-Tricine SDS-PAGE and resolved by reverse-phase HPLC. Infection of BHK21 cells with a recombinant SFV vector expressing the tax gene has previously been shown to result in high-level expression of transcriptionally active Tax protein (6). This expression system was used to produce in vivo 32P-labeled Tax protein that was purified by immunoprecipitation with a monoclonal antibody directed against Tax. The 40-kDa 32P-labeled Tax species was transferred to nitrocellulose membrane and subjected to digestion with trypsin.
Tris-Tricine SDS-PAGE of the tryptic digest and autoradiography identified a unique radioactive species with an apparent molecular mass of 4,000 Da (Fig. 1A). However, resolution of the same tryptic digest by reverse-phase HPLC revealed two 32P-labeled peaks after collection of the fractions on a PVDF membrane and autoradiography (Fig. 1B). The radioactive fractions corresponding to the two peaks were excised and analyzed by amino acid microsequencing to identify the phosphorylated peptides present in the two peaks. Each 32P-labeled fraction contained a Tax tryptic peptide. Both peptides were characterized by the same amino-terminal sequence, Ala-Tyr-His-Pro-Ser-Phe-Leu, which characterizes the 40-amino-acid Tax tryptic peptide spanning amino acids 285 to 324. Two phosphoserine residues at positions 300 and 301 were detected by microsequence analysis of this peptide after conversion of phosphoserine residues into S-ethylcysteines. These results demonstrated that the HTLV-1 Tax protein was phosphorylated on two adjacent serine residues, at positions 300 and 301. The resolution of the phosphorylated tryptic peptide into only one 4,000-Da band by electrophoresis on a Tris-Tricine gel and its resolution by HPLC into two fractions of different hydrophobicity suggested that Tax may be produced in vivo as a mixture of molecules phosphorylated on both serine residues 300 and 301 and molecules phosphorylated on either of these two serine residues.
FIG. 1.
Tryptic peptide analysis of in vivo-phosphorylated Tax. The tryptic digest of 32P-labeled Tax was analyzed by Tris-Tricine SDS–16.5% PAGE and autoradiography (A) and by reverse-phase HPLC (B1 to B3). The HPLC elution profile was monitored by absorbance at 220 nm (B1) and by autoradiography of the PVDF membrane containing the fractions (B3). (B2) The region of the PVDF membrane that was excised for amino acid sequencing is shown.
Tax is phosphorylated on serine residues 300 and 301.
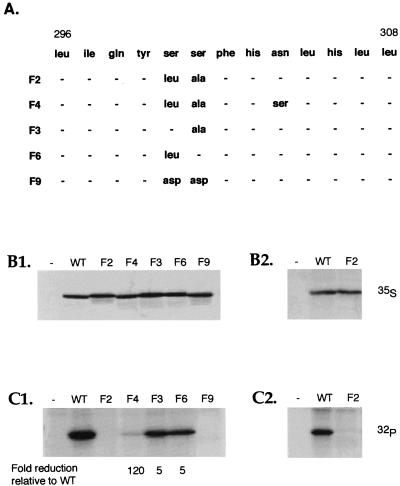
To test the phosphorylation status of serine residues 300 and 301 and analyze its role in Tax activation of gene expression, amino acid substitutions were introduced at positions 300 and 301. Site-directed mutagenesis was performed on a wild-type tax cDNA to create three Tax mutants which had either serine residue 300 replaced by leucine (F6), serine residue 301 replaced by alanine (F3), or both serine residues 300 and 301 replaced by leucine and alanine, respectively (F2) (Fig. 2A). To study the effects of these mutations on Tax phosphorylation, we expressed these three Tax mutants in an SFV vector. BHK21 cells infected with SFV recombinants expressing wild-type Tax or the Tax mutants F2, F3, or F6 were cultured in medium containing either [32P]orthophosphate or [35S]methionine and [35S]cysteine. Tax proteins were immunoprecipitated with a monoclonal antibody directed against Tax, separated by SDS–10% PAGE, and analyzed by autoradiography (Fig. 2B1 and C1).
FIG. 2.
Tax is phosphorylated on serine residues 300 and 301. (A) A schematic of the 353-amino-acid Tax protein with the positions of the amino acid changes in the Tax mutants used in this study is shown. BHK21 cells (B1 and C1) or Jurkat cells (B2 and C2) were infected for 5 h with the control SFV or the SFV recombinants expressing wild-type (WT) Tax or the Tax mutants F2, F4, F3, F6, or F9. The infected cells were then incubated for 5 h in medium containing [35S]methionine and [35S]cysteine (B1 and B2) or [32P]orthophosphate (C1 and C2). The cell extracts were immunoprecipitated with a monoclonal antibody directed against Tax followed by SDS–10% PAGE and autoradiography.
[35S]methionine and [35S]cysteine incorporation demonstrated that the mutant Tax proteins were present at steady-state levels similar to those of wild-type Tax (Fig. 2B1). Incorporation of [32P]orthophosphate also led to the presence of a prominent species migrating at 40 kDa at the same position as the 35S-labeled species. This species was seen with wild-type Tax and the two Tax mutants F3 and F6 but was not detected in extracts prepared from cells expressing the Tax mutant F2 (Fig. 2C1). Quantitation of the intensities of these species by PhosphorImager and normalization for equal amounts of Tax protein indicated that the Tax mutant F2 was not phosphorylated at a detectable level while phosphorylation of mutants F3 and F6 was reduced by a factor of 5 relative to phosphorylation of wild-type Tax. The effect of the other Tax mutants, F4 and F9, on Tax phosphorylation is discussed below.
To determine whether phosphorylation of Tax was a property independent of the cell line used, Jurkat cells were infected with SFV-Tax in the presence of [35S]methionine and [35S]cysteine or [32P]orthophosphate (Fig. 2B2 and C2). The results demonstrated that Tax was phosphorylated in T lymphocytes and that phosphorylation of the Tax F2 mutant was reduced to undetectable levels in this cell line, confirming the results obtained in BHK21 cells. These results support the results of the amino acid microsequencing analysis, demonstrating that the two serine residues at positions 300 and 301 are major sites for Tax phosphorylation. They also indicated that each of these two serine residues could be independently phosphorylated in vivo.
Serine residues 300 and 301 are essential for Tax transcriptional activity.
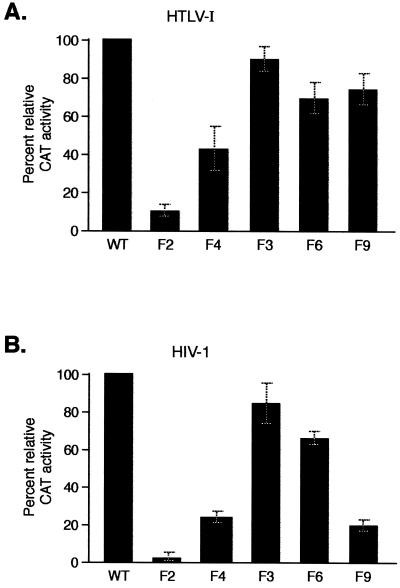
Tax activates viral and cellular gene expression via modulation of the activity of either the ATF/CREB or NF-κB activation pathways (44, 47, 53). To characterize the ability of the Tax mutants F2, F3, and F6 to activate gene expression via these pathways, expression vectors carrying either the wild-type tax gene or the mutated tax genes F2, F3, or F6 were utilized. These expression vectors were cotransfected into Jurkat cells with an HIV-1 LTR CAT reporter which contains two NF-κB sites to assay for the effects of Tax on the NF-κB pathway or an HTLV-1 LTR CAT reporter which contains three unique CRE sites known as 21-bp repeats to assay for the effects of Tax on the ATF/CREB pathway.
Wild-type Tax activated gene expression from both the HTLV-1 (40-fold) and the HIV-1 LTR (10-fold). Tax did not activate HIV-1 gene expression when both NF-κB sites in the HIV-1 promoter were mutated (data not shown). The Tax mutant F2, whose phosphorylation was reduced to undetectable levels as a result of mutations at both serine residues 300 and 301, exhibited only 10% (HTLV-1) and 3% (HIV-1) Tax activation relative to that seen with wild-type Tax (Fig. 3). The Tax mutants F6 and F3, which had either of the above serine residues substituted, activated gene expression from both the HIV-1 and the HTLV-1 promoters to a much greater extent than the Tax mutant F2. The activity of the Tax mutant F3 was 92% (HTLV-1) and 85% (HIV-1) of the activity of wild-type Tax while the Tax mutant F6 gave 73% (HTLV-1) and 66% (HIV-1) of the activity of wild-type Tax (Fig. 3). The effect of the Tax mutants F4 and F9 on activation of HIV-1 and HTLV-1 gene expression is discussed below. Thus, the Tax mutant F2 whose phosphorylation was decreased to undetectable levels was defective for its ability to activate gene expression from both the HIV-1 and the HTLV-1 promoters. Although mutations of either of the two serine residues at positions 300 and 301 reduced phosphorylation by a factor of 5, they resulted in Tax proteins with nearly wild-type levels of activation of gene expression.
FIG. 3.
Phosphorylation of Tax on serine residues 300 or 301 is essential for Tax transcriptional activity. Jurkat cells were cotransfected with either the HTLV-I LTR CAT (A) or the HIV-1 LTR CAT (B) reporter plasmid, and vectors expressing either wild-type (WT) Tax or the Tax mutant F2, F4, F3, F6, or F9. CAT activity was measured 32 h after transfection and is shown as a percentage of the activity relative to that seen with WT Tax. The values shown represent the averages of three independent experiments, with the standard deviations indicated by error bars.
Phosphorylation of Tax is involved in the formation of Tax-containing nuclear bodies.
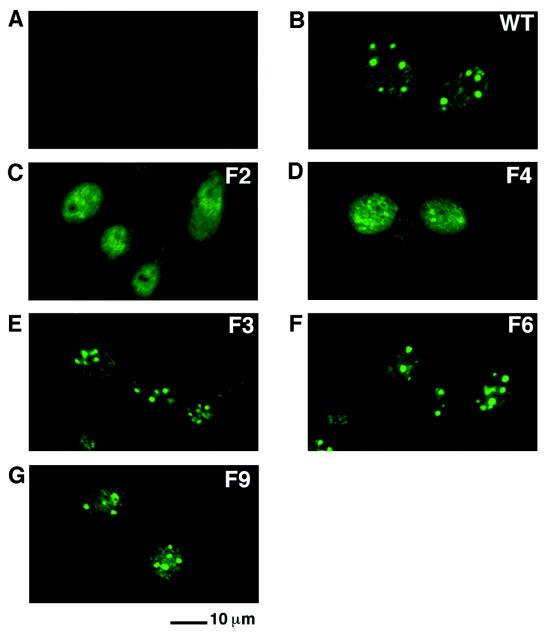
Previous studies indicated that Tax is distributed in discrete nuclear bodies both in HTLV-1-transformed lymphocytes and in cells infected with an SFV-Tax recombinant. Tax also colocalizes in these nuclear structures with cellular factors essential for its transcriptional activities, suggesting that the nuclear bodies containing Tax are involved in Tax-mediated activation of gene expression (6, 7, 45). We next asked whether phosphorylation of Tax on serine residues 300 and 301 altered the intracellular localization of Tax. BHK21 cells infected with SFV recombinants expressing wild-type Tax or the Tax mutants F2, F3, or F6 were fixed and stained with a monoclonal antibody directed against Tax and an anti-mouse immunoglobulin antibody conjugated to fluorescein isothiocyanate followed by analysis using confocal microscopy.
Wild-type Tax localized in nuclear bodies as previously demonstrated (Fig. 4). The Tax mutants F3 and F6 gave a similar nuclear localization (Fig. 4). In contrast, the Tax mutant F2, which was not efficiently phosphorylated, displayed a diffuse nuclear distribution (Fig. 4). This result indicated that phosphorylation of Tax was likely involved in the assembly of the Tax-containing nuclear bodies but was not required for the transport of Tax into the nucleus.
FIG. 4.
Phosphorylation of Tax on serine residues 300 or 301 is involved in Tax localization in nuclear bodies. BHK21 cells were infected for 18 h with either the control SFV (A) or SFV expressing wild-type Tax (B) or the Tax mutant F2 (C), F4 (D), F3 (E), F6 (F), or F9 (G). The cells were fixed, stained by immunofluorescence with a monoclonal antibody directed against Tax, and analyzed by confocal microscopy.
The variant serine residue at position 304 is a minor site for Tax phosphorylation.
Alignments of closely related Tax variants from HTLV-1 and HTLV-2 indicate that, although serine residues at positions 300 and 301 are conserved in each of these variants, a third serine residue is present at position 304 in 9 of 14 HTLV-1 Tax variants but is replaced by an asparagine residue in 5 of 14 HTLV-1 Tax variants. Asparagine is the only amino acid present at this position in 17 variants of HTLV-II Tax. The tax gene used in this study codes for an asparagine residue at position 304. To determine whether the serine residue at position 304 influenced Tax phosphorylation, we substituted a serine residue for the asparagine residue in the mutant F2 to generate the Tax mutant F4. Thus, the Tax mutant F4 had serine 300, serine 301, and asparagine 304 replaced by leucine, alanine, and serine, respectively (Fig. 2A).
Experiments to characterize the phosphorylation of this Tax mutant, its ability to activate gene expression, and its intracellular localization are included in Fig. 2 to 4. Phosphorylation of the Tax mutant F4 was reduced by a factor of 120 relative to phosphorylation of wild-type Tax in contrast to the phosphorylation of the Tax mutant F2, which was undetectable (Fig. 2C). The Tax mutant F4 had an increased ability to activate gene expression from both the HTLV-1 and the HIV-1 promoters compared to mutant F2. The activation of gene expression from the HTLV-1 promoter by F4 was 44% and that of the HIV-1 promoter was 24% of the activity of wild-type Tax (Fig. 3). Although both the F2 and F4 mutants were present in the nucleus, their nuclear distributions were different. While the Tax mutant F2 displayed a diffuse nuclear distribution, the Tax mutant F4 was associated with nuclear speckles superimposed on a diffuse nucleoplasmic staining. However the Tax mutant F4 did not form the prominent nuclear bodies observed with wild-type Tax (Fig. 4). These results indicated that the serine residue at position 304 was a minor site for Tax phosphorylation and that phosphorylation of this serine resulted in a Tax protein with a speckled nuclear distribution and an increased ability to activate both HIV-1 and HTLV-1 gene expression.
Introduction of phosphoserine mimetics at positions 300 and 301 corrects the functional defect of the Tax mutant F2.
To analyze whether serine residues 300 and 301 constitute a site for regulatory phosphorylation events that allow Tax-induced activation of gene expression, we replaced leucine 300 and alanine 301 in mutant F2 by charged amino acids that can function as phosphoserine mimetics (1, 20). Aspartic acid residues were introduced at positions 300 and 301 in mutant F2 to generate mutant F9. Like mutant F2, mutant F9 displayed an undetectable level of phosphorylation (Fig. 2C). The Tax mutant F9 was nevertheless able to associate with nuclear bodies (Fig. 4) and was also able to activate gene expression from the HTLV-1 promoter to nearly a wild-type level (75% relative to wild-type Tax) (Fig. 3A). However, introduction of the aspartic acid residues at positions 300 and 301 only partly restored the ability to activate gene expression from the HIV-1 promoter (20% relative to wild-type Tax) (Fig. 3B). These results indicated that serine residues 300 and 301 function as regulatory sites for phosphorylation which are critical for the ability of Tax to associate with nuclear bodies and for the ability of Tax to activate gene expression via both the ATF/CREB and NF-κB activation pathways.
DISCUSSION
Cytoplasmic and nuclear events are involved in Tax activation of gene expression. In the cytoplasm, Tax induces the release of NF-κB from sequestration and its translocation to the nucleus by acting on the stability of NF-κB inhibitors (8, 26, 30, 35, 49). In the nucleus, Tax associates with members of the ATF/CREB and NF-κB families of transcription factors and with the transcriptional coactivators CBP and p300 to assemble enhancer complexes on the regulatory sequences in the HTLV-1 promoter and in the promoters of select cellular genes. These cellular transcription factors colocalize with wild-type Tax in nuclear bodies that also include components of the general transcription and splicing complexes (6, 7, 45). These nuclear structures are labeled by a short pulse of 5-bromouridine 5′-triphosphate (45) and contain the mRNA from a gene specifically activated by Tax (6), suggesting that these structures are involved in Tax-mediated activation of gene expression.
Here we demonstrate that nuclear events resulting in the formation of nuclear structures containing Tax and subsequent events leading to activation of gene expression via the ATF/CREB and NF-κB pathways depend on phosphorylation of Tax on either of two critical serine residues at positions 300 and 301. Phosphorylation of at least one of these serine residues is required for Tax-mediated activation of gene expression and for Tax localization in nuclear bodies but not for Tax transport into the nucleus. Tryptic peptide mapping, amino acid microsequencing, and mutational analysis indicate that serine residues at positions 300 and 301 can be independently phosphorylated in vivo, resulting in production of Tax as a mixture of molecules phosphorylated on both serine residues as well as molecules phosphorylated on either of these two serine residues. Our results support the observations by Fontes et al. (12) that Tax is phosphorylated on serine residues and that these phosphorylated serines are present on a unique tryptic fragment. Moreover, they demonstrate that Tax phosphorylation is involved in its punctate distribution in the nucleus and in its transcriptional activity.
The argument that phosphorylation events occurring at serine residues 300 and 301 play a critical role is further supported by the fact that introduction of phosphoserine mimetics at position 300 and 301 (F9 [S300D and S301D]) restored the ability of the F2 mutant (S300L and S301A) to form Tax-containing nuclear bodies and to activate gene expression. Although activation of HTLV-1 gene expression was restored to nearly wild-type levels, activation of gene expression from the HIV-1 promoter was only partial. This observation suggests that phosphoserine residues at positions 300 and 301 are required for more than one step during the process of Tax activation of gene expression via the NF-κB pathway.
Tax genes isolated from different HTLV-1 and HTLV-2 genomes have either an asparagine or a serine residue at position 304. It is interesting that when a serine residue was substituted for the asparagine residue at position 304 in mutant F2 (S300L, S301A, N304) to generate mutant F4 (S300L, S301A, N304S), phosphorylation as well as the ability of Tax to activate gene expression from both the HTLV-1 and the HIV promoters was partly restored. This result indicates that the serine residue at position 304 present in some Tax variants is a minor site for phosphorylation and that phosphorylation of this serine residue can partly rescue the functional defect of the serine 300 and 301 F2 mutant. A similar Tax mutant with alanine substitutions at serine residues 300 and 301 and a serine residue at position 304 was described previously (44). As expected due to the presence of a serine residue at position 304, this Tax mutant was able to activate gene expression from both the HIV and HTLV-1 promoters (44) and displayed a phenotype comparable to that of our F4 mutant (S300L, S301A, N304S). Thus, Tax contains three neighboring serine residues that can be independently phosphorylated. We speculate that, due to the critical role of phosphorylation for Tax function, several redundant phosphorylation sites have been selected during evolution.
Modification of proteins by phosphorylation is a major process involved in the modulation of protein activity and/or nuclear translocation (24). Our results indicate that phosphorylation is not critical for Tax nuclear translocation but is required for its ability to activate gene expression. Besides the mutants described in this work, other Tax mutants were analyzed for the ability to be phosphorylated in vivo, including Tax M148 (53) and Tax M47 (47), which are selectively able to activate gene expression via either the ATF/CREB or the NF-κB pathway. Both these mutants were phosphorylated to nearly wild-type levels (5a) and associated with nuclear structures (7). These observations indicate that phosphorylation of Tax and its association with nuclear structures are factors important for the ability of Tax to activate gene expression via either the NF-κB or the ATF/CREB pathway. Other critical steps for Tax transcriptional activity are its ability to induce the release of NF-κB from cytoplasmic sequestration by the action of Tax on the phosphorylation and degradation of IκB inhibitors and nuclear events leading to the assembly of transcriptionally active complexes containing members of both the ATF/CREB and the NF-κB families of transcription factors. Further analysis will be needed to address the cellular kinase that is involved in modulating Tax phosphorylation and its subsequent ability to form nuclear bodies and to activate gene expression via the ATF/CREB and the NF-κB pathways.
ACKNOWLEDGMENTS
We thank the NIH AIDS Research and Reagent Program for providing the Tax monoclonal antibody. Sylvie Lebrun is acknowledged for her skilled technical assistance.
This work was supported by grants from the Belgian Fonds National de la Recherche Scientifique, Télévie, the Fonds de Financement de la Recherche Cancérologique de la CGER Assurances, the NIH, and the Council of Tobacco Research.
REFERENCES
- 1.Adams A. Replication of latent Epstein-Barr virus genomes in Raji cells. J Virol. 1987;61:1743–1746. doi: 10.1128/jvi.61.5.1743-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin J A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I Tax induces cellular proteins that activate the κB element in the IL-2 receptor α gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 5.Berglund P, Sjöberg M, Garoff H, Atkins G J, Sheahan B J, Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Bio/Technology. 1993;11:916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- 5a.Bex, F. Unpublished data.
- 6.Bex F, McDowall A, Burny A, Gaynor R B. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-κB proteins. J Virol. 1997;71:3484–3497. doi: 10.1128/jvi.71.5.3484-3497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bex F, Yin M-J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockman J A, Scherer D C, MsKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cann A J, Rosenblatt J D, Wachsman W, Shah N P, Chen I S Y. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen I S, Quan S G, Golde D N. Human T-cell leukemia virus type II transforms normal human lymphocytes. Proc Natl Acad Sci USA. 1983;80:7006–7009. doi: 10.1073/pnas.80.22.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felber B K, Paskalis H, Kleinman-Ewing D, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 12.Fontes J D, Strawhecker J M, Bills N D, Lewis R E, Hinrichs S H. Phorbol esters modulate the phosphorylation of human T-cell leukemia virus type I Tax. J Virol. 1993;67:4436–4441. doi: 10.1128/jvi.67.7.4436-4441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii M, Sassone-Corsi P, Verma I M. c-fos promoter trans-activation by the tax 1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisawa J, Seiki M, Sato M, Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for transactivation mediated by p40x of HTLV-I. EMBO J. 1986;5:713–718. doi: 10.1002/j.1460-2075.1986.tb04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisawa J-I, Toita M, Yoshida M. Tax of HTLV-1 interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21 base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1994;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Good L, Sun S C. Persistent activation of NF-κB B/Rel by human T-cell leukemia virus type 1 Tax involves degradation of IκB. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M, Sodroski J, Haseltime W. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman W J, Kimata J T, Wong F-H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammerschmidt W, Sugden B. Identification and characterization of orilyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988;55:427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 21.Harrich D, Garcia J, Mitsuyasu R, Gaynor R. TAR independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 1990;9:4417–4423. doi: 10.1002/j.1460-2075.1990.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben N Y, Baeuerle P A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 23.Hinuma Y, Komoda H, Chosa T, Kondo T, Kohakura M, Takenaka T, Kikuchi M, Ichimaru M, Yunoki K, Sato I, Matsuo R, Takiuchi Y, Uchino H, Hanaoka M. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide seroepidemiologic study. Int J Cancer. 1982;29:631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- 24.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 25.Kalyanaraman V S, Sarngadharan M G, Robert-Guroff M, Mujoshi M, Golde D, Gallo R C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982;218:571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- 26.Kanno T, Brown K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type I Tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 28.Lacoste J, Cohen L, Hiscott J. NF-κB activity in T-cells stably expressing the Tax protein of human T-cell lymphotropic virus type I. Virology. 1991;184:553–562. doi: 10.1016/0042-6822(91)90425-b. [DOI] [PubMed] [Google Scholar]
- 29.Lacoste J, Lanoix J, Pepin N, Hiscott J. Interactions between HTLV-I Tax and NF-κB/Rel proteins in T-cells. Leukemia. 1994;8:71–76. [PubMed] [Google Scholar]
- 30.Lacoste J, Petropoulos L, Pépin N, Hiscott J. Constitutive phosphorylation and turnover of IκBα in human T-cell leukemia virus type I-infected and Tax-expressing T cells. J Virol. 1995;69:564–569. doi: 10.1128/jvi.69.1.564-569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung K, Nabel G J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 32.Liljeström P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 33.Malik K T, Even J, Karpas A. Molecular cloning and complete nucleotide sequence of an adult T-cell leukaemia virus/human T-cell leukaemia virus type I (ATLV/HTLV-I) isolate of Caribbean origin: relationship to other members of the ATLV/HTLV-I subgroup. J Gen Virol. 1988;69:1695–1710. doi: 10.1099/0022-1317-69-7-1695. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K, Shibata H, Fujisawa J-I, Inoue H, Hakura A, Tsukahara T, Fujii M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the Tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nerenberg M, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 37.Meyer H E, Hoffmann-Posorske E, Korte H, Heilmeyer L M G., Jr Sequence analysis of phosphoserine-containing peptides. FEBS Lett. 1986;204:61–66. doi: 10.1016/0014-5793(86)81388-6. [DOI] [PubMed] [Google Scholar]
- 38.Paskalis H, Felber B K, Pavlakis G N. Cis-acting sequences responsible for the transcriptional activation of a human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci USA. 1986;83:6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poiesz B J, Ruscetti R W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen C A, Sodroski J G, Kettman R, Haseltine W A. Activation of enhancer sequences in type II human T-cell leukemia virus and bovine leukemia virus long terminal repeats by virus-associated trans-acting regulatory factors. J Virol. 1986;57:738–744. doi: 10.1128/jvi.57.3.738-744.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–397. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 43.Seeler J S, Muchardt C, Podar M, Gaynor R B. Regulatory elements involved in tax-mediated transactivation of the HTLV-I LTR. Virology. 1993;196:442–450. doi: 10.1006/viro.1993.1500. [DOI] [PubMed] [Google Scholar]
- 44.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semmes O J, Jeang K-T. Localization of human T-cell leukemia virus type 1 Tax to subnuclear compartments that overlap with interchromatin speckles. J Virol. 1996;70:6347–6357. doi: 10.1128/jvi.70.9.6347-6357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siekevitz M, Feinberg M B, Holbrook N, Wong-Staal F, Greene W C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the transactivator (tat) gene product of human T-cell leukemia virus type 1. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 48.Sodroski J, Rosen C, Goh W C, Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985;228:1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- 49.Sun S-C, Elwood J, Béraud C, Greene W C. Human T-cell leukemia virus type I Tax activation of NF-κB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol. 1994;14:7377–7384. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki T, Hirai H, Yoshida M. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-κB p65 and c-Rel proteins bound to the NF-κB binding site and activates transcription. Oncogene. 1994;9:3099–3105. [PubMed] [Google Scholar]
- 51.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X-M, Wattiez R, Mock M, Falmagne P, Ruysschaert J M, Cabiaux V. Structure and interaction of PA63 and EF of Bacillus anthracis with lipid membrane. Biochemistry. 1997;36:14906–14913. doi: 10.1021/bi971661k. [DOI] [PubMed] [Google Scholar]
- 53.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- 54.Yin M-J, Gaynor R B. HTLV-I 21bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L J, Giam C Z. Interaction of the human T-cell lymphotropic virus type I (HTLV-I) transcriptonal activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]