Abstract
Nuclear receptors are a superfamily of transcription factors regulated by a wide range of lipids that include phospholipids, fatty acids, heme-based metabolites, and cholesterol-based steroids. Encoded as classic two-domain modular transcription factors, nuclear receptors possess a DNA-binding domain (DBD) and a lipid ligand-binding domain (LBD) containing a transcriptional activation function. Decades of structural studies on the isolated LBDs of nuclear receptors established that lipid–ligand binding allosterically regulates the conformation of the LBD, regulating transcriptional coregulator recruitment and thus nuclear receptor function. These structural studies have aided the development of several FDA-approved drugs, highlighting the importance of understanding the structure-function relationships between lipids and nuclear receptors. However, there are few published descriptions of full-length nuclear receptor structure and even fewer descriptions of how lipids might allosterically regulate full-length structure. Here, we examine multidomain interactions based on the published full-length nuclear receptor structures, evaluating the potential of interdomain interfaces within these nuclear receptors to act as inducible sites of allosteric regulation by lipids.
Supplementary key words: Full-length nuclear receptor, lipid structural biology, lipid regulation of full-length nuclear receptor, structural interfaces, lipid regulation of transcription
Overview of nuclear receptor regulation
Nuclear receptors can be divided into three general classes: endocrine receptors, orphan receptors, and adopted orphan receptors (1, 2). Historically, the discovery and cloning of the first nuclear receptors and the identification of their lipophilic ligands are what led to the three-class classification system used in the field. Genes encoding receptors for steroids were isolated based on their ability to bind steroid hormones and called “Endocrine” receptors, while homologous genes with unidentified ligands were then identified and referred to collectively as “Orphan” receptors (2). Endocrine receptors generally have high-affinity, cholesterol-based ligands such as cortisol, aldosterone, and estradiol which bind nuclear receptors such as estrogen receptor (ER), progesterone receptor (PR), and glucocorticoid receptor (GR) (3). In the absence of ligand, endocrine receptors are usually monomers in the cytoplasm bound by heat-shock protein chaperones (4). Upon lipid binding, endocrine receptors dissociate from chaperones, homodimerize, and translocate into the nucleus where they bind and activate transcription of their respective target genes (5). In contrast, orphan nuclear receptors, such as steroidogenic factor-1 (SF-1), and adopted orphan nuclear receptors, such as RAR-related orphan receptor α (RORα) and vitamin D receptor (VDR), generally have lower affinity fatty acid (6, 7, 8, 9) or phospholipid-based ligands (10, 11). Orphan receptors are often constitutively nuclear and often heterodimerize with a common nuclear receptor called the retinoid X receptor (RXR) (2). Lipid binding to Orphan receptors often alters the subset of transcriptional co-regulators recruited to the receptor (3, 12, 13, 14). This lipid-induced shift serves to alter the transcriptional profile depending on the receptor and the genetic locus, tissue, developmental stage, milieu of coregulators, and the signaling state of the cell (15). The common thread throughout nuclear receptor regulation is 1) an ability to bind a hydrophobic, lipid-based ligand, resulting in 2) an altered conformation of the nuclear receptor LBD which in turn 3) alters the coactivator recruitment and localization of the receptor, which 4) changes the resultant transcriptome.
Nuclear receptor primary structure
The nuclear receptor superfamily is composed of 48 distinct genes in humans, each with a slightly different primary structure. Overall, most members of the superfamily have one DNA-binding and one ligand-binding domain, decorated with various disordered/low-complexity tethers of various length, structure, and function (Fig. 1A). Most nuclear receptors have an N-terminal “A/B” domain that is disordered in all crystal structures to date, has low sequence complexity, and often function as a transcriptional activation domain. The region of the disordered N-terminal A/B domain which carries transcriptional regulatory activity is called the Activation Function-1 (AF1). The N-terminal A/B domain is usually followed by the “C” domain, a DNA-binding domain (DBD) containing two standard Cys-based Zinc finger motifs and often a C-terminal extension (Fig. 1B). An exception is the NR5A monomeric nuclear receptors, which also contain a helical FTZ-F1 domain (Fig. 1B) recently shown to stabilize LBD–DBD interaction (16). The DBD is followed by the “D” domain, which is a central hinge tethering the DBD to the ligand-binding domain (LBD). The “E/F” domain is the LBD and lipid-binding site, which interacts in 1:1 stoichiometry with lipid ligands (Fig. 1C–E). The LBD contains the Activation Function-2 (AF2), a surface on the LBD that interacts with coregulators and is largely responsible for transcriptional regulatory activity. From a structural perspective, nuclear receptors are very much like other allosteric switches such as G-protein coupled receptors, in that lipid hormone binding alters LBD conformation, directly regulating the ability of the LBD to interact with transcriptional co-regulator protein effectors via the AF2 site, and thus altering the biological activity of the nuclear receptor. The AF2 surface is allosterically regulated by lipid binding, and the last helix of the 12-helix bundle that makes up the LBD called helix 12 (H12) is critical for both interaction with coregulators and allosteric response to lipid ligands (Fig. 2A, B). These aspects of nuclear receptor regulation are well-validated and have informed the development of several FDA-approved drugs. Thus, our understanding of isolated LBD structure and function has had great utility in informing drug design as well as more fundamental aspects of our understanding of nuclear receptor biology. However, the behavior of isolated LBDs alone does not completely recapitulate the regulatory capacity of many intact full-length nuclear receptors that have been studied to date; as such, there have been calls for new approaches in drug discovery and development (17). Here we review these structures and ask how lipid ligands might regulate their structure.
Fig. 1.
Structural organization of nuclear receptors. A: General domain structure of nuclear receptors is composed of a disordered “A/B” domain which carries the Activation Function-1 (AF1) region, a DNA-binding domain (“C”), the disordered hinge region (“D”), and a ligand-binding domain that carries Activation Function-2 (AF2) region (“E/F”). B: NMR structure of the DNA-binding domain of SF-1 (PDB: 2FF0) consists of a core unit of two α-helices with two standard Cys-based Zinc finger motifs, often extended with the C-terminal extension (CTE). The FTZ-F1 helix shown is C-terminal the CTE and is unique to NR5A1 and NR5A2 nuclear receptors. C: Overall architecture of the ligand-binding domain (LBD) of SF-1 (NR5A1, PDB: 4QJR), showing the twelve α-helical bundle and PGC1α coregulator peptide (cyan) and dipalmitoyl PI(3,4,5)P3 ligand. D: Helices 1–3 of the LBD of SF-1 (PDB: 4QJR) with PGC1α coactivator peptide (cyan), and (E) the opposite face of the LBD showing helices 4–12 of SF-1 (PDB: 4QJR).
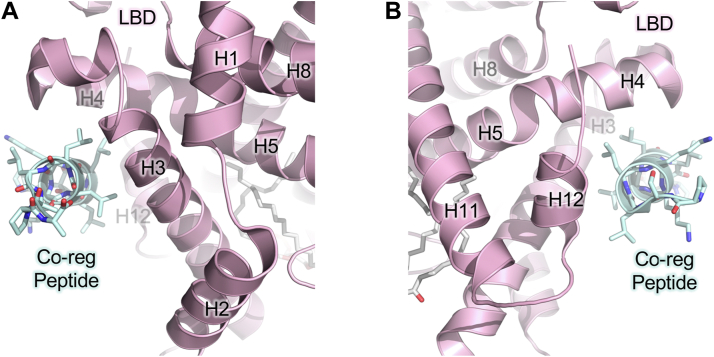
Fig. 2.
Structure of the coregulator binding site and Activation Function-2 (AF2) in the ligand-binding domain (LBD) (PDB: 4QJR). A: Coregulator peptide (cyan ribbon) bound to the AF2 site in the LBD. In most nuclear receptors binding of coregulator peptide to the nuclear receptor is induced by a lipid ligand. SF-1 LBD (pink cartoon) shown bound by PGC1α coregulator peptide (cyan ribbon) and the dipalmitoyl PIP3(16:0/16:0) phospholipid ligand (atom-colored sticks) B: Opposing view of A., showing Helix 12 (H12) which helps form the lipid-ligand regulated AF2 site. The AF2 site is critical for lipid-induced allosteric structural change to the AF2 facilitating coregulator binding to nuclear receptors.
Materials and methods
None aside from review of the published literature.
Results
Full-length nuclear receptor structures
Eight nuclear receptors have high-confidence models describing the molecular interfaces between the domains. These domain-domain interfaces represent one of the most important aspects of understanding how full-length nuclear receptor regulation can differ from what we already know about the regulation of isolated domains. Most of the full-length nuclear receptor structural models are based on X-ray diffraction data, but several groups have used other approaches that integrate structural data collected from orthogonal biophysical analyses. To our knowledge, no sidechain-resolved cryo-EM structures of a full-length nuclear receptor have been reported as yet. Here, we review the details for each of these eight important structures and illustrate how events in the LBD and/or the DBD can influence the structure or function at the interfaces of these transcription factors, highlighting the evidence suggesting lipids might have activity to influence those interdomain interactions.
PPARγ, Peroxisome proliferator-activated receptor γ
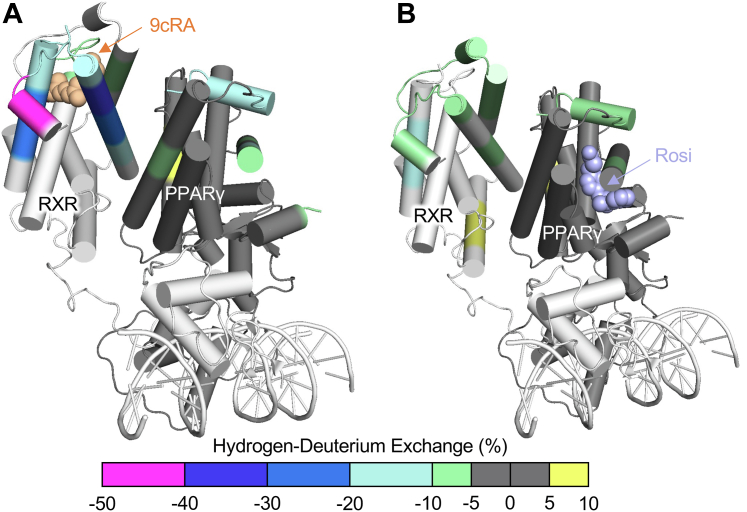
Peroxisome proliferator-activated receptor γ (PPARγ; NR1C3) heterodimerized with Retinoid X Receptor-α (RXRα; NR2B1) was the first model of a full-length, side chain-resolved nuclear receptor structure to be reported (3, 18) with important roles in lipid and carbohydrate metabolism (19, 20, 21). Drug targeting of PPARγ has focused on hyperglycemia, hyperinsulinemia, atherosclerosis, inflammation, and hypertension and has been based on the model that the isolated LBD recapitulates the major functions of the full-length protein (18, 22, 23, 24, 25, 26). The full-length crystal structure included the synthetic ligand rosiglitazone (Rosi) and the lipid 9-cis-retinoic acid bound to RXRα, with relevant coactivator peptides (Fig. 3A) (18). These ligands have all been used in previous structural studies of the isolated domains (27, 28), but the interfaces between domains in the full-length complex suggest how lipids might participate in allosteric communication between domains (Fig. 3B). A lipid ligand has potential to regulate three distinct heterodimerization interfaces: 1) an interface between the DBDs, 2) an interface between the LBDs, and 3) an interface between the PPARγ LBD and the RXRα DBD C-terminal extension (CTE) (Fig. 3B). 9-cis retinoic acid binding to RXRα affects coregulator recruitment to PPARγ, while Rosiglitazone biding to PPARγ affects coregulator recruitment to RXRα, albeit less dramatically (29). Consistent with this observation, 9-cis retinoic acid binding to RXRα altered HDX at several functionally important surfaces of PPARγ LBD (Fig. 4A), whereas binding of the PPARγ ligand Rosiglitazone led to comparably fewer HDX changes in the RXRα LBD (Fig. 4B). HDX studies on the isolated PPARγ LBD suggest Rosi-induced HDX protection in residues which map to the LBD–LBD dimerization interfaces in the full-length structure, while a partial agonist (MRL24) induced protection in distinct regions (the β-sheet and helix 6 regions) which map to the PPARγ LBD-RXRα DBD interface in the full-length model (30). Together, these studies are consistent with lipid and other small molecule ligands regulating structural and functional communication between the domains of the PPARγ-RXRα complex.
Fig. 3.
Crystal structure of PPARγ/RXRα with rosiglitazone and 9-cis-retinoic acid (PDB: 3DZY). A: Overall architecture of the co-complex containing hydrophobic ligands rosiglitazone (Rosi, black atom-colored sticks) for PPARγ (blue LBD and green DBD) and 9-cis retinoic acid (9cRA, black atom-colored sticks) for RXRα (pink LBD and brown DBD), with N-CoA2 coactivator peptides (black ribbon) and DNA oligo (grey). The RXRα DBD and LBD are intersected by the PPARγ LBD. B: Three heterodimerization interfaces between the two receptors include an interface between the two DBDs, an interface between the two LBDs, and an interface between the PPARγ LBD and the C-terminal extension (CTE) of the DBD of RXRα. The interface between the two DBDs contains 30 Å2 of interaction surface. C: The interface between two LBDs contains 2100 Å2 of interaction surface (18).
Fig. 4.
Crystal structure of PPARγ/RXRα with rosiglitazone and 9-cis-retinoic acid (PDB: 3DZY) with Mapped HDX-mass spectrometry. A: 9cRA- or (B) Rosiglitazone-induced HDX-MS data collected from the isolated ligand binding domain mapped onto the full-length crystal structure of PPARγ and RXRα. Sites of altered HDX map close to the interfaces between RXRα and PPARγ, suggesting ligand regulation of the interfaces (29).
LXRβ, liver X receptor-β
Liver X receptor-β (LXRβ; NR1H2) also functions as a heterodimer with RXRα (31, 32). LXRβ is found in most tissues (33) and is a “master regulator” of cholesterol and glucose homeostasis, with roles in adipocyte growth (34), pancreatic β cells (34, 35, 36), and atherosclerosis (3, 35, 37, 38). A crystal structure of the RXRα/LXRβ heterodimer co-complex was solved with 9-cis retinoic acid bound to RXRα and synthetic GW3965 bound to LXRβ, along with coactivator peptides derived from Nuclear Receptor Coactivator 2 (N-CoA2) and a DNA oligonucleotide (36). The two receptors are linked by a nearly symmetric interaction around helix 1 of both receptors (Fig. 5A). The LBDs share an interface that buries a relatively large ∼2,200 Å2 of solvent-accessible surface; however, recent studies of the isolated LXRβ LBD did not find large changes in Helix 1 induced by LXRβ ligands in HDX studies, while HDX changes were found in Helix 1 of LXRα (39). In contrast to the LBDs, the DBDs in the heterodimer have no confirmed interaction with each other, only putative surfaces that may exist upon rearrangements of the RXRα/LXRβ complex, although DNA recognition and dimer assembly are largely DNA dependent (Fig. 5B), consistent with previous analyses of nuclear receptors (40). Except for six residues of the LXRβ N-terminal domain (NTD) are observed to interact with the minor groove of DNA with the 4-nucleotide long spacer (Fig. 5C), however, there are little interdomain contacts evident from the structures. However, there is one salt bridge that forms between the LXRβ LBD and RXRα DBD between Q413 and R172 which is likely to stabilize the complex. It remains to be determined if this interdomain salt bridge can be regulated by lipid binding to LXRβ.
Fig. 5.
Crystal structure of RXRα/LXRβ with 9-cis-retinoic acid and synthetic GW3965 LXRβ ligand (PDB: 4NQA). A: Overall architecture of the RXRα/LXRβ complex with 9-cis retinoic acid (9cRA) and the GW3965 synthetic ligand (black atom-colored sticks) N-CoA2 coactivator peptide (black ribbon) and DNA oligo (grey). The dimerized receptors are linked by a nearly symmetric interaction centered on helix 1 of both LBDs in a slanted “X” shape. B: Interfaces between the opposing LBD and DBD in the heterodimer. C: Six residues (K75–K80) of the LXRβ N-terminal domain showing backbone and side chain atoms (green atom-colored sticks) and interaction with the minor groove of the co-crystalized DNA oligo (36).
RARβ, retinoic acid receptor β
Retinoic acid receptor β (RARβ; NR1B2) heterodimerizes with RXRα (41, 42, 43) and has roles in cell growth, differentiation, embryonic development, and apoptosis (42, 44, 45) with synthetic ligands studied as cancer therapeutics (45). The crystal structure of the RARβ/RXRα heterodimer (43) included 9-cis retinoic acid bound to RXRα and all-trans retinoic acid bound to RARβ, along with a DNA response element and coactivator peptides from SRC-2 (Fig. 6A). The authors compared the crystal structure of the heterodimer to published structures of the individual domains and found little change between the structures, suggesting the isolated LBD structures accurately model the properties of this full-length complex (46, 47). However, the full-length structure shows that RARβ LBD and DBDs are spatially adjacent, revealing an interface between the DBD and LBD of RARβ (Fig. 6B, C). This RARβ interface is between DBD helix 1 and LBD loop between helices 9 and 10 (Fig. 7A), which buries ∼312 Å2 from the DBD and 345 Å2 from the LBD. Interdomain hydrogen bonds are also found in this region, and several of these residues are highly conserved between species. Several lines of evidence suggest that the RARβ inter-domain interface is regulated by lipid binding. Two distinct RARβ ligands (all-trans retinoic acid and the synthetic antagonist BMS-189453) induce significant changes to HDX solvent accessibility in the hinge and in the DBD itself (43), suggesting structural information is translated across the entire RARβ/RXRα complex (Fig. 7B). Functional DNA-binding analyses showed altered DNA affinities when RARβ is bound to different ligands, attributed to a more dynamic state the DBD, further supported by HDX studies (43). Similar changes to interdomain communication were also observed in the full-length LRH-1 monomer between the LBD and DBD with and without coregulator peptides bound to the LRH-1 LBD (16). Together, the data suggest interdomain communication between the RARβ/RXRα heterodimer domains occurs and can be regulated by lipid occupancy of the LBD.
Fig. 6.
Crystal structure of the RARβ/RXRα with all-trans retinoic acid and 9-cis retinoic acid (PDB: 5UAN). A: Overall architecture of the complex with 9-cis retinoic acid (9cRA) for RXRα and all-trans retinoic acid (ATRA) for RARβ (both indicated as black atom-colored sticks), DR1 DNA oligo containing direct repeats of two half-sites (AGGTCA) separated by one base pair, and N-CoA2 coactivator peptides (black ribbons). Zinc is shown as cyan spheres. B: The DBD and LBD of RARβ are spatially adjacent and share an interface between the α1 helix of the DBD and the LBD loop between helices α9 and α10 (9–10 loop), where hydrogen bonds between R359 with N112 and I114 are found. C: The DBD of RXRα is almost entirely separated from the rest of the heterodimer, located on the other side of the DNA oligo (46).
Fig. 7.
Crystal structure of the RARβ/RXRα with all-trans retinoic acid and 9-cis retinoic acid (PDB: 5UAN) with mapped HDX-MS data. A: The interface between RARβ DBD and LBD buries a large surface area of contact, with hydrogen bonds between side chains of S362 (LBD) and T116 (DBD), salt bridge between main chains of R359 (LBD) and I114 (DBD) and another salt bridge between side chain of R359 and main chain of N112 (DBD) stabilizing this interdomain interaction. B: Ligand binding to RARβ LBD leads to a significant change to solvent accessibility in other domains of the full-length proteins. Replacing all-trans retinoic acid with a synthetic antagonist (BMS-189453) leads to significant changes in HDX in the hinge region and in the DBD, these regions are colored red.
VDR, vitamin D receptor
Vitamin D Receptor (VDR, NR1I1) heterodimerizes with RXRα (48), and its cognate ligands are Vitamin D3, or the active form of Vitamin D, calcitriol (1α,25(OH)2D3). VDR is expressed in the pancreas, kidney, intestines, prostate, ovary, and skin (49, 50) and dysregulation of VDR has been linked to breast and prostate cancers as well as type 1 diabetes, multiple sclerosis, and rheumatoid arthritis (48, 51, 52). VDR cryo-electron microscopy (cryo-EM) (48) produced a 10–12 Å resolution map used with crystal structures of the individual VDR and RXRα domains to reconstruct the domain organization. The complex included VDR/RXRα bound to cognate Vitamin D3 for VDR, 9cRA for RXRα, and a DNA oligo, presenting an open architecture in which the LBD heterodimer is positioned perpendicular to the DBDs (Fig. 8A–C). Vitamin D3 binding VDR LBD led to altered HDX in VDR DBD, which is remotely located from the ligand binding pocket of LBD, suggesting interdomain communication between the LBD and DBD occurs upon ligand binding (54). On the other hand, the binding of 9-cis retinoic acid to RXRα LBD, while leaving its own DBD solvent accessibility unperturbed, led to a subtle yet significant change in the HDX pattern of the DBD of its dimer partner, VDR, suggesting the binding of 9-cis retinoic acid to RXRα LBD is communicated to the VDR DBD (54). Together, these data suggest lipid binding can orchestrate interdomain communication within VDR.
Fig. 8.
Cryo-EM structure of VDR/RXRα with Vitamin D3 and 9-cis retinoic acid (EMDB: EMD-1985). A: Overall architecture of the complex (blue surface representation) bound to vitamin D3 for VDR and 9cRA for RXRα, and a DNA response element, position of 3′ and 5′ ends of the DNA indicated. B: 3′ view down the DNA oligo showing the open architecture in which the LBD heterodimer is positioned perpendicular to DNA. C: Opposing view to (A), showing the opposite face of each LBD of the heterodimer located at the 5′ end of the DNA (53).
HNF4α, Hepatocyte Nuclear Factor α
Hepatocyte Nuclear Factor α (HNF4α, NR2A1) is an orphan nuclear receptor that binds DNA as a homodimer and is the most abundant DNA-binding protein found in the liver (55, 56). It has an important role in liver homeostasis associated with maturity-onset diabetes of the young, type 1 (MODY1), renal defects (57) and hyper-insulinemic hypoglycemia (58). The 2.9 Å crystal structure of HNF4α homodimer was solved bound to the lipid myristic acid (Fig. 9A), DNA, and coregulator peptide from N-CoA2 (SRC2, TIF2). The structure shows an interface between both LBDs and one DBD (Fig. 9B) referred to as the convergence center (Fig. 9B, C), suggested as a potential site for interdomain communication. Although an endogenous lipid ligand of HNF4α remains to be identified, several lines of evidence link changes in the LBD to altered function of the DBD in the full-length HNF4α. Human polymorphisms in the LBD (I314F, R324H) map to the convergence center in the full-length structure, and these polymorphisms alter HNF4α affinity for DNA. Two post-translational modifications of the LBD, methylation of Arg 91 and phosphorylation of Ser 78, regulate HNF4α DNA affinity (56), suggesting the LBD and DBD communicate with each other. The addition of the HNF4α LBD to a DBD-hinge construct (Fig. 9A) improved DNA affinity a remarkable 75-fold, highlighting the importance of the LBD for DBD function in HNF4α. Although purified HNF4α contains saturated and cis-monounsaturated fatty acids (59), several other lipid candidates have been suggested as endogenous ligands of HNF4α including fatty acyl-CoA thioesters (60) and linoleic acid (61). Regardless of the identity of the specific lipid ligand for HNF4α, the evidence suggests a potential regulatory role for lipid ligands in the regulation of full-length HNF4α structure and function.
Fig. 9.
Crystal structure of HNF4α homodimer with myristic acid (PDB: 4IQR). A: Overall architecture of the HNF4α homodimer (blue LBD, green DBD) each bound to a myristic acid (black atom-colored sticks) and N-CoA2 coactivator peptides (black ribbon) and DNA oligo (grey). B: The interface between both LBDs and between the DBD of the upstream subunit of the homodimer and the hinge of the downstream subunit at a central zone, referred to as the convergence center. Zinc atoms are shown as cyan spheres. C: Expanded view of the convergence center as in (B), highlighting complementary conformations of the LBDs and DBDs (56).
ERα, estrogen receptor α
Estrogen Receptor α (ERα; NR3A1) is a homodimer that binds cholesterol-based steroid hormones, the endogenous human ligand being 17β-estradiol (62). ERα has important roles in cell growth, development, metabolism, and survival (63) and is a key target in breast cancer and other estrogen-regulated diseases (64). While crystal structures of isolated LBD and DBD of Erα are available, no full-length Erα crystal structure has been reported. The Yang lab used an integrated approach to model the full-length ERα homodimer by combining hydroxyl radical-based protein footprinting coupled to mass spectrometry (HRPF-MS) with small-angle X-ray scattering (SAXS) (62, 65). Three bulky, hydrophobic residues (W200, I326, and W393) have been shown to be protected from HRPF in full-length ERα but are solvent exposed in the DBD crystal structure (62), suggesting these DBD residues are buried in a DBD–LBD interface in full-length ERα (Fig. 10A–C). This DBD–LBD interface was explored using contact of structural units analysis (66), also suggesting the interface consists mainly of hydrophobic interactions (Fig. 10D), consistent with a stable interface. Although mutational analyses showed this DBD–LBD interface is important for ERα function (62), it remains unclear how ligands might regulate the interface. The effects of ligands on HDX dynamics of the isolated ERα LBD have been studied by the Griffin lab, showing ERα ligands alter HDX of the isolated LBD at sites predicted to be in the LBD–DBD interface when mapped onto the full-length model (67). Specifically, ERα ligands tamoxifen and raloxifene decrease the level of deuteration in the β1-2 sheet region, shown to be required for full ERα transcriptional activity (62). On the other hand, the LBD–LBD dimerization interface (helix 10/loop region) is the only region that increased solvent accessibility upon ERα ligand binding when analyzing full-length protein. An earlier full-length ERα–coactivator transcriptional complex using cryo-EM provided lower-resolution details of the overall architecture (68); but atomic-resolution details of how ERα ligands affect the full-length receptor structure remain to be clarified. Still, these solution-based dynamic structural studies again suggest interdomain communication can be regulated by ERα ligands.
Fig. 10.
Integrated SAX model of ERα homodimer with estradiol (SASBDB: SASDDU8). A: Overall architecture of the full-length ERα homodimer complex (blue LBD and green DBD) complexed with N-CoA2 coregulator peptide (black ribbon), DNA and estradiol, showing highly overlapped LBD interfaces in the complex, and (B) the relatively distantly located DBDs. C: The ERα homodimer has an “L” shape or boot-like structure, where the two DBDs are offset from LBDs, with the DBDs forming the base of the “L” while the LBDs form a “neck”, positioned almost perpendicular to the DBDs. D: Closeup view of the mainly hydrophobic DBD-LBD interface which can mediate interdomain communication in full-length ERα, residues W200, I326 and W393 have been shown to be important in this DBD-LBD interface (62).
EcR and USP, The Ecdysone Receptor, and ultraspiracle
The Ecdysone Receptor (EcR; NR1H1) and Ultraspiracle Protein (USP; NR2B4) are arthropod nuclear receptors that heterodimerize (69). USP is often grouped with mammalian RXRs (as a part of NR2B subfamily) as it is considered an ortholog of mammalian RXRs (70, 71) while EcR is categorized together with LXRβ (as a part of NR1H subfamily). These structures reveal the topology of nuclear receptor binding to response elements, similar to those of the mammalian steroid hormone receptors. While there are crystallographic data for ligand binding domains of ECR and USP, no crystallographic data exist for full-length protein complex. The Klaholz lab reported an 11 Å model of the USP/EcR using small-angle X-ray scattering (SAXS), cryo-EM and 3D reconstruction, (72) using previously solved crystal structures of the individual domains. The DBDs resemble saddles that rest on either side of the DNA (Fig. 11A) (73), and the LBDs and DBDs share minimal contact with each other, displaying an open conformation bound to DNA (Fig. 11B). An interesting aspect of this model is that the USP LBD is located close to the DNA, and the LBD heterodimer is angled in the 5′ direction of the DNA. To examine the model further, the authors extended the DNA response element, which suggested a number of positively charged residues in the USP LBD (R360, K394, R404, and R405) could make contacts with the extended portion of DNA (Fig. 11C, D). Consistent with the idea of LBD residues interacting with DNA, mutating these basic residues to alanine increased transcriptional activation of USP, consistent with these residues participating in USP repression. The potential interaction between LBD and DNA must be studied further to establish the relationship. EcR has a short C-terminal extension on the DBD that is ordered when bound to certain DNA sequences, but disordered when bound to other sequences, suggesting DNA sequence can influence C-terminal extension structure. The data available suggest full-length EcR/USP complex is another full-length allosteric model that could reveal a role for lipids in the regulation of full-length EcR/USP structure.
Fig. 11.
Cryo-EM structure of USP/EcR with PE(18:1/16:0) and ponasterone A (PDB: 4UMM). A: Overall architecture of the USP (pink LBD and brown DBD)/EcR (blue LBD and green DBD) in complex with USP antagonist phospholipid oleoyl/palmitoyl phosphatidylethanolamine PE(18:1/16:0) (black atom-colored sticks) and the cholesterol-based EcR ligand ponasterone A (black atom-colored sticks). The DBDs are positioned to the side of the DNA while LBDs lie on the top, similar to the architecture observed for the ERα homodimer complex shown in Fig 8. B: The LBDs display a “V” shape and show little contact between the LBDs and DBDs. C: Charge-surface representation of the complex showing basic region of USP LBD (blue arrow) that could interact with acidic DNA. D: Amino acid side chains (pink atom-colored sticks and blue arrows) of R360, K394, R404 and R405 residues in helix 9 of USP LBD are close enough to make direct contact with an extended portion of the DNA phosphate backbone (72).
LRH-1, liver receptor Homolog-1
Liver receptor homolog-1 (LRH-1; NR5A2) is a monomeric nuclear receptor with broad expression particularly high in the liver, pancreas, breast, and intestine. LRH-1 is a well-validated drug target for non-alcoholic steatohepatitis (NASH), type-2 diabetes (74), and pancreatic cancer (75). LRH-1 is the only monomeric nuclear receptor with a published full-length model, so the structure of LRH-1 bound to a DNA revealed previously unknown mechanisms for interdomain communication between the DBD and LBD, and effects on LRH-1 function (16). The integrated model of full-length LRH-1 was determined in the presence DNA oligo, coactivator peptide, and bacterial phosphatidylglycerol (PG) (18:1/16:1) from the recombinant expression system using solution-based biophysical techniques and computational modeling (Fig. 12A), although a wide variety of different phospholipid species and acyl chain lengths were found associated with the full-length LRH-1 (16).
Fig. 12.
Integrated structural model of full-length LRH-1 monomer complexed with co-purifying bacterial PG(18:1/16:1) phospholipid. A: Overall architecture of the complete LRH-1 monomer (blue LBD and green DBD) structure on DNA oligo (grey), with PGC1α co-activator peptide (black ribbon), and P6L phosphatidylglycerol PG(18:1,16:1) phospholipid ligand (black atom-colored sticks). B: Close-up highlighting the FTZ-F1 helix of the NR5A DBDs, which extends beyond the C-terminal extension (CTE) of more typical nuclear receptor DBDs. A charge clamp inter-domain interaction (red circle) between R174 in the DBD FTZ-F1 helix and D314 in helix 2 of the LBD is highlighted. C: The DBD residue S148 (red circle) lies in the putative DBD-LBD interface and was identified as a human polymorphism in the gnomAD patient database (S148R). The S148R mutation disrupts LRH-1 structure as determined by SAXS and LRH-1 function in cellular assays (16).
One of the most prominent features of LRH-1 is the FTZ-F1 DBD helix, which is on the C-terminal side of the DBD, adjacent to but distinct from the C-terminal extension (CTE). Previous studies have shown that mutations in the DBD FTZ-F1 region decrease transcriptional activity, but do not alter DNA binding (76). Further, full-length LRH-1 has a better affinity for DNA compared to the isolated DBD (77), suggesting potential interactions between the various domains of LRH-1. The full-length structure (16) confirmed several interdomain interactions between the LBD and DBD, including a novel charge clamp between DBD FTZ-F1 helix (R174) and helix 2 of the LBD (D314) (Fig. 12B). The full-length LRH-1 structure also revealed an extensive contact surface between the LBD and DBD outside the FTZ-F1 helix (Fig. 12C). At the nexus of this interface is the DBD residue S148 in the DBD, S148R is a human polymorphism that has not been formally associated with any disease in humans (Fig. 12C). The S148R mutation in full-length LRH-1 protein has decreased affinity for PGC1α coactivator peptide and decreased transcriptional activation (16), suggesting a mutation in the DBD can alter function of the LBD. Further, cross-linking mass spectrometry (XL-MS) suggest the physical proximity of the DBD and LBD which can facilitate this communication in full-length LRH-1 (Fig. 12B, C). MD simulations and network analyses (78, 79) of these interfaces suggested a significant role of these interdomain contact sites in regulating allosteric communication between the LBD and DBD, confirmed by extensive structural and functional analyses (16). These genetic, structural, and functional tests indicate strong interdomain communication between the LBD and DBD in LRH-1, yet how interdomain communication might be regulated by phospholipid binding remains to be determined. Previous HDX studies of the isolated LRH-1 LBD suggested high-affinity ligands induce ligand-dependent changes to HDX solvent accessibility in helix 2 (78, 79), suggesting high-affinity ligands might induce changes to the interdomain interface in LRH-1. HDX pattern of full-length LRH-1 is distinct from that of the isolated domains (16, 18) further suggesting the domains of LRH-1 do not behave as “beads-on-a-string,” but communicate with each other (Fig. 13). Thus, it remains to be determined how ligands regulate full-length LRH-1 structure.
Fig. 13.
Integrated structural model of full-length LRH-1 monomer complexed with co-purifying bacterial PG(18:1/16:1) phospholipid with mapped HDX-MS data. Helix 2 of LRH-1 LBD shows a distinct HDX pattern when the isolated LBD HDX is compared to full-length LRH-1 HDX (red-colored region), suggesting differences in structural dynamics that are unique to full-length receptor. On the other hand, helix 12 demonstrates HDX that are similar in both isolated LBD and the full-length LRH-1 protein (blue-colored region). Further, multiple lines of evidence suggest helix 2 exists in the interface between the LBD and DBD, indicative of communication between the two domains.
Discussion
The structures of isolated LBDs of nuclear receptors have established structure-function relationships that were critical in bringing FDA-approved drugs to the clinic while providing a deeper understanding of the allosteric mechanisms endogenous lipids use to control transcription and physiology. The structural studies outlined here suggest events occurring in one domain of a nuclear receptor can be allosterically translated to other domains and that this type of communication occurs more often than not among the nuclear receptors where full-length structural information is available. Although the changes induced by lipids and synthetic drugs to full-length nuclear receptor structure are unlikely to ever be as easily measured as changes to the isolated LBD, they may be no less important to fully understand the lipid-mediated regulation of nuclear receptors. New modes of interdomain communication have the potential to reveal untapped potential in nuclear receptor pharmacology and to better understand the basic science of how endogenous lipids regulate nuclear receptor function. The field looks towards cryo-EM, crystal structures, and a wider acceptance of solution-based integrated approaches to reveal the structure–function relationships describing how lipids regulate interdomain interfaces. By exploring how lipids regulate interdomain communication in nuclear receptors, we can reveal fundamentally new aspects of how fatty acids, phospholipids, cholesterol, and heme-based metabolites go about regulating these important transcription factors.
Data availability
All data in the manuscript can be acquired by contacting the corresponding author (ray.blind@vanderbilt.edu).
Conflicts of interest
The authors declare they have no conflicts of interest.
Acknowledgments
The authors would like to thank M. Merced Malabanan, Katrina T. Poon and Sophia K. Danzeisen for critically evaluating the manuscript.
Author contributions
W. J. C., Z. H.: investigation; W. J. C.: data analysis; W. J. C., Z. H., and R. B. D.: writing–original draft preparation; R. D. B.: supervision; R. D. B.: conceptualization; R. B. D.: writing–reviewing and editing.
Funding and additional information
This work was supported by the National Institutes of Health [GM138873, HG008341]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Gustafsson J.A. Historical overview of nuclear receptors. J. Steroid Biochem. Mol. Biol. 2016;157:3–6. doi: 10.1016/j.jsbmb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Evans R.M., Mangelsdorf D.J. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla A. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 4.Howard K.J., Holley S.J., Yamamoto K.R., Distelhorst C.W. Mapping the HSP90 binding region of the glucocorticoid receptor. J. Biol. Chem. 1990;265:11928–11935. [PubMed] [Google Scholar]
- 5.Oppenheimer J.H. Thyroid hormone action at the cellular level. Science. 1979;203:971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- 6.Göttlicher M., Widmark E., Li Q., Gustafsson J.Å. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auwerx J. Regulation of gene expression by fatty acids and fibric acid derivatives: an integrative role for peroxisome proliferator activated receptors: the belgian endocrine society lecture 1992. Horm. Res. 1992;38:269–277. doi: 10.1159/000182557. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A., Endo N., Rutledge S.J., Vogel R., Shinar D., Rodan G.A. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol. Endocrinol. 1992;6:1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- 9.Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowder M.K., Seacrist C.D., Blind R.D. Phospholipid regulation of the nuclear receptor superfamily. Adv. Biol. Regul. 2017;63:6–14. doi: 10.1016/j.jbior.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musille P.M., Kohn J.A., Ortlund E.A. Phospholipid--driven gene regulation. FEBS Lett. 2013;587:1238–1246. doi: 10.1016/j.febslet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruse M.D., Privalsky M.L., Sladek F.M. Competitive cofactor recruitment by orphan receptor hepatocyte nuclear factor 4α1: modulation by the F domain. Mol. Cell. Biol. 2002;22:1626–1638. doi: 10.1128/MCB.22.6.1626-1638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeyakumar M., Tanen M.R., Bagchi M.K. Analysis of the functional role of steroid receptor coactivator-1 in ligand-induced transactivation by thyroid hormone receptor. Mol. Endocrinol. 1997;11:755–767. doi: 10.1210/mend.11.6.0003. [DOI] [PubMed] [Google Scholar]
- 14.Aranda A., Pascual A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 15.Plaster N., Sonntag C., Schilling T.F., Hammerschmidt M. REREa/Atrophin-2 interacts with histone deacetylase and Fgf8 signaling to regulate multiple processes of zebrafish development. Dev. Dyn. 2007;236:1891–1904. doi: 10.1002/dvdy.21196. [DOI] [PubMed] [Google Scholar]
- 16.Seacrist C.D., Kuenze G., Hoffmann R.M., Moeller B.E., Burke J.E., Meiler J., et al. Integrated structural modeling of full-length LRH-1 reveals inter-domain interactions contribute to receptor structure and function. Structure. 2020;28:830–846.e9. doi: 10.1016/j.str.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastinejad F., Ollendorff V., Polikarpov I. Nuclear receptor full-length architectures: confronting myth and illusion with high resolution. Trends Biochem. Sci. 2015;40:16–24. doi: 10.1016/j.tibs.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T.P., et al. Structure of the intact PPAR-γ-RXR-α nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bougarne N., Weyers B., Desmet S.J., Deckers J., Ray D.W., Staels B., et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018;39:760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 21.Maréchal L., Laviolette M., Rodrigue-Way A., Sow B., Brochu M., Caron V., et al. The CD36-PPARγ pathway in metabolic disorders. Int. J. Mol. Sci. 2018;19:1529. doi: 10.3390/ijms19051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrke M., Lazar M.A. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Berger J., Wagner J.A. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol. Ther. 2002;4:163–174. doi: 10.1089/15209150260007381. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 25.Olefsky J., Sltiel A. PPARγ and the treatment of insulin resistance. Trends Endocrinol. Metab. 2000;11:362–368. doi: 10.1016/s1043-2760(00)00306-4. [DOI] [PubMed] [Google Scholar]
- 26.Staels B. PPAR agonists and the metabolic syndrome. Therapie. 2007;62:319–326. doi: 10.2515/therapie:2007051. [DOI] [PubMed] [Google Scholar]
- 27.Gampe R.T.J., Montana V.G., Lambert M.H., Miller A.B., Bledsoe R.K., Milburn M.V., et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol. Cell. 2000;5:545–555. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 28.Kroker A.J., Bruning J.B. Review of the structural and dynamic mechanisms of PPARγ partial agonism. PPAR Res. 2015;2015 doi: 10.1155/2015/816856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vera I.M.S., Zheng J., Novick S., Shang J., Hughes T.S., Brust R., et al. Synergistic regulation of coregulator/nuclear receptor interaction by ligand and DNA. Structure. 2017;25:1506–1518.e4. doi: 10.1016/j.str.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes T.S., Chalmers M.J., Novick S., Kuruvilla D.S., Chang M.R., Kamenecka T.M., et al. Ligand and receptor dynamics contribute to the mechanism of graded PPARγ agonism. Structure. 2012;20:139–150. doi: 10.1016/j.str.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repa J.J., Liang G., Ou J., Bashmakov Y., a Lobaccaro J., Shimomura I., et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teboul M., Enmark E., Li Q., Wikstrom A.C., Pelto-Huikko M., Gustafsson J.A. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2096–2100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Repa J.J., Mangelsdorf D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 34.Gerin I., Dolinsky V.W., Shackman J.G., Kennedy R.T., Chiang S.H., Burant C.F., et al. LXRβ is required for adipocyte growth, glucose homeostasis, and β cell function. J. Biol. Chem. 2005;280:23024–23031. doi: 10.1074/jbc.M412564200. [DOI] [PubMed] [Google Scholar]
- 35.Huang P., Chandra V., Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu. Rev. Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lou X., Toresson G., Benod C., Suh J.H., Philips K.J., Webb P., et al. Structure of the retinoid X receptor α-liver X receptor β (RXRα-LXRβ) heterodimer on DNA. Nat. Struct. Mol. Biol. 2014;21:277–281. doi: 10.1038/nsmb.2778. [DOI] [PubMed] [Google Scholar]
- 37.Kliewer S.A. Orphan nuclear receptors: shifting endocrinology into reverse. Science. 1999;284:757–760. doi: 10.1126/science.284.5415.757. [DOI] [PubMed] [Google Scholar]
- 38.Evans R.M. The nuclear receptor superfamily: a rosetta stone for physiology. Mol. Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- 39.Belorusova A.Y., Evertsson E., Hovdal D., Sandmark J., Bratt E., Maxvall I., et al. Structural analysis identifies an escape route from the adverse lipogenic effects of liver X receptor ligands. Commun. Biol. 2019;2:431. doi: 10.1038/s42003-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rastinejad F., Perlmann T., Evans R.M., Sigler P.B. Structural determinants of nuclear receptor assembly on DNA direct repeats. Nature. 1995;375:203–211. doi: 10.1038/375203a0. [DOI] [PubMed] [Google Scholar]
- 41.Khorasanizadeh S., Rastinejad F. Nuclear-receptor interactions on DNA-response elements. Trends Biochem. Sci. 2001;26:384–390. doi: 10.1016/s0968-0004(01)01800-x. [DOI] [PubMed] [Google Scholar]
- 42.Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem. Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 43.Chandra V., Wu D., Li S., Potluri N., Kim Y., Rastinejad F. The quaternary architecture of RARβ-RXRα heterodimer facilitates domain-domain signal transmission. Nat. Commun. 2017;8:1–9. doi: 10.1038/s41467-017-00981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allenby G., Bocquel M.T., Saunders M., Kazmer S., Speck J., Rosenberger M., et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. U. S. A. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altucci L., Leibowitz M.D., Ogilvie K.M., de Lera A.R., Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 46.Rastinejad F., Wagner T., Zhao Q., Khorasanizadeh S. Structure of the RXR–RAR DNA-binding complex on the retinoic acid response element DR1. EMBO J. 2000;19:1045–1054. doi: 10.1093/emboj/19.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogenberg V., Guichou J.-F., Vivat-Hannah V., Kammerer S., Pérez E., Germain P., et al. Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J. Biol. Chem. 2005;280:1625–1633. doi: 10.1074/jbc.M409302200. [DOI] [PubMed] [Google Scholar]
- 48.Orlov I., Rochel N., Moras D., Klaholz B.P. Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J. 2012;31:291–300. doi: 10.1038/emboj.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banerjee P., Chatterjee M. Antiproliferative role of vitamin D and its analogs - A brief overview. Mol. Cell. Biochem. 2003;253:247–254. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 50.Pinette K., Yee Y., Amegadzie B., Nagpal S. Vitamin D receptor as a drug discovery target. Mini Rev. Med. Chem. 2003;3:193–204. doi: 10.2174/1389557033488204. [DOI] [PubMed] [Google Scholar]
- 51.Bouillon R., Eelen G., Verlinden L., Mathieu C., Carmeliet G., Verstuyf A. Vitamin D and cancer. J. Steroid Biochem. Mol. Biol. 2006;102:156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Friedrich M., Axt-Fliedner R., Villena-Heinsen C., Tilgen W., Schmidt W., Reichrath J. Analysis of vitamin D-receptor (VDR) and retinoid X-receptor α in breast cancer. Histochem. J. 2002;34:35–40. doi: 10.1023/a:1021343825552. [DOI] [PubMed] [Google Scholar]
- 53.Rochel N., Ciesielski F., Godet J., Moman E., Roessle M., Peluso-Iltis C., et al. Common architecture of nuclear receptor heterodimers on DNA direct repeat elements with different spacings. Nat. Struct. Mol. Biol. 2011;18:564–570. doi: 10.1038/nsmb.2054. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Chalmers M.J., Stayrook K.R., Burris L.L., Wang Y., Busby S.A., et al. DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat. Struct. Mol. Biol. 2011;18:556–563. doi: 10.1038/nsmb.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sladek F.M., Zhong W., Lai E., Darnell J.E. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- 56.Chandra V., Huang P., Potluri N., Wu D., Kim Y., Rastinejad F. Multidomain integration in the structure of the HNF-4α nuclear receptor complex. Nature. 2013;495:394–398. doi: 10.1038/nature11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryffel G.U. Mutations in the human genes encoding the transcription factors of the hepatocyte nuclear factor (HNF)1 and HNF4 families: functional and pathological consequences. J. Mol. Endocrinol. 2001;27:11–29. doi: 10.1677/jme.0.0270011. [DOI] [PubMed] [Google Scholar]
- 58.Flanagan S.E., Kapoor R.R., Mali G., Cody D., Murphy N., Schwahn B., et al. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur. J. Endocrinol. 2010;162:987–992. doi: 10.1530/EJE-09-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wisely G.B., Miller A.B., Davis R.G., Thornquest A.D., Johnson R., Spitzer T., et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure. 2002;10:1225–1234. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 60.Hertz R., Magenheim J., Berman I., Bar-tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 61.Yuan X., Ta T.C., Lin M., Evans J.R., Dong Y., Bolotin E., et al. Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang W., Peng Y., Kiselar J., Zhao X., Albaqami A., Mendez D., et al. Multidomain architecture of estrogen receptor reveals interfacial cross-talk between its DNA-binding and ligand-binding domains. Nat. Commun. 2018;9:3520. doi: 10.1038/s41467-018-06034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang W., Greene G.L., Ravikumar K.M., Yang S. Cross-talk between the ligand- and DNA-binding domains of estrogen receptor. Proteins. 2013;81:1900–1909. doi: 10.1002/prot.24331. [DOI] [PubMed] [Google Scholar]
- 64.Deroo B.J., Korach K.S. Estrogen receptors and human disease Find the latest version : review series Estrogen receptors and human disease. J. Clin. Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang W., Ravikumar K.M., Chance M.R., Yang S. Quantitative mapping of protein structure by hydroxyl radical footprinting-mediated structural mass spectrometry: a protection factor analysis. Biophys. J. 2015;108:107–115. doi: 10.1016/j.bpj.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sobolev V., Sorokine A., Prilusky J., Abola E., Edelman M. Automated analysis of interatomic contacts in proteins. Bioinformatics. 1999;15:327–332. doi: 10.1093/bioinformatics/15.4.327. [DOI] [PubMed] [Google Scholar]
- 67.Dai S.Y., Chalmers M.J., Bruning J., Bramlett K.S., Osborne H.E., Montrose-Rafizadeh C., et al. Prediction of the tissue-specificity of selective estrogen receptor modulators by using a single biochemical method. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7171–7176. doi: 10.1073/pnas.0710802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yi P., Wang Z., Feng Q., Pintilie G.D., Foulds C.E., Lanz R.B., et al. Structure of a biologically active estrogen receptor-coactivator complex on DNA. Mol. Cell. 2015;57:1047–1058. doi: 10.1016/j.molcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perera S.C., Zheng S., Feng Q.L., Krell P.J., Retnakaran A., Palli S.R. Heterodimerization of ecdysone receptor and ultraspiracle on symmetric and asymmetric response elements. Arch. Insect Biochem. Physiol. 2005;60:55–70. doi: 10.1002/arch.20081. [DOI] [PubMed] [Google Scholar]
- 70.Oro A.E., McKeown M., Evans R.M. The Drosophila retinoid X receptor homolog ultraspiracle functions in both female reproduction and eye morphogenesis. Development. 1992;115:449–462. doi: 10.1242/dev.115.2.449. [DOI] [PubMed] [Google Scholar]
- 71.Jakób M., Kołodziejczyk R., Orłowski M.M., Krzywda S., Kowalska A., Dutko-Gwóźdź J., et al. Novel DNA-binding element within the C-terminal extension of the nuclear receptor DNA-binding domain. Nucleic Acids Res. 2007;35:2705–2718. doi: 10.1093/nar/gkm162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maletta M., Orlov I., Roblin P., Beck Y., Moras D., Billas I.M.L., et al. The palindromic DNA-bound USP/EcR nuclear receptor adopts an asymmetric organization with allosteric domain positioning. Nat. Commun. 2014;5:1–7. doi: 10.1038/ncomms5139. [DOI] [PubMed] [Google Scholar]
- 73.Rochel N., Wurtz J.M., Mitschler A., Klaholz B., Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell. 2000;5:173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]
- 74.Lee J.M., Lee Y.K., Mamrosh J.L., Busby S.A., Griffin P.R., Pathak M.C., et al. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–511. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corzo C.A., Mari Y., Chang M.R., Khan T., Kuruvilla D., Nuhant P., et al. Antiproliferation activity of a small molecule repressor of liver receptor homolog 1. Mol. Pharmacol. 2015;87:296–304. doi: 10.1124/mol.114.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solomon I.H., Hager J.M., Safi R., McDonnell D.P., Redinbo M.R., Ortlund E.A. Crystal structure of the human LRH-1 DBD-DNA complex reveals Ftz-F1 domain positioning is required for receptor activity. J. Mol. Biol. 2005;354:1091–1102. doi: 10.1016/j.jmb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Weikum E.R., Tuntland M.L., Murphy M.N., Ortlund E.A. A structural investigation into Oct4 regulation by orphan nuclear receptors, germ cell nuclear factor (GCNF), and liver receptor homolog-1 (LRH-1) J. Mol. Biol. 2016;428:4981–4992. doi: 10.1016/j.jmb.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eargle J., Luthey-Schulten Z. NetworkView: 3D display and analysis of protein·RNA interaction networks. Bioinformatics. 2012;28:3000–3001. doi: 10.1093/bioinformatics/bts546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sethi A., Eargle J., Black A.A., Luthey-Schulten Z. Dynamical networks in tRNA:protein complexes. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6620–6625. doi: 10.1073/pnas.0810961106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in the manuscript can be acquired by contacting the corresponding author (ray.blind@vanderbilt.edu).