Abstract
T-cell immunoreceptor with Ig and ITIM domains (TIGIT) is an inhibitory checkpoint receptor that negatively regulates T-cell responses. CD226 competes with TIGIT for binding to the CD155 ligand, delivering a positive signal to the T cell. Here we studied the expression of TIGIT and CD226 in a cohort of 115 patients with chronic lymphocytic leukemia (CLL) and report expression of TIGIT and CD226 by leukemic cells. By devising a TIGIT/CD226 ratio, we showed that CLL cells favoring TIGIT over CD226 are typical of a more indolent disease, while those favoring CD226 are characterized by a shorter time to first treatment and shorter progression-free survival after first treatment. TIGIT expression was inversely correlated to the B-cell receptor (BCR) signaling capacity, as determined by studying BTK phosphorylation, cell proliferation and interleukin-10 production. In CLL cells treated with ibrutinib, in which surface IgM and BCR signaling capacity are temporarily increased, TIGIT expression was downmodulated, in line with data indicating transient recovery from anergy. Lastly, cells from patients with Richter syndrome were characterized by high levels of CD226, with low to undetectable TIGIT, in keeping with their high proliferative drive. Together, these data suggest that TIGIT contributes to CLL anergy by down-regulating BCR signaling, identifying novel and actionable molecular circuits regulating anergy and modulating CLL cell functions.
Introduction
Chronic lymphocytic leukemia (CLL) is a B-cell malignancy characterized by clinical and molecular heterogeneity.1 The leukemic niche is critical to CLL development and progression in that it provides signals that influence CLL cell behavior,2,3 among which those channeled through the B-cell receptor (BCR) regulate key biological programs such as proliferation, metabolic adaptation and chemokine/cytokine secretion.4 BCR signaling capacity varies according to somatic hypermutation of the variable region of the immunoglobulin heavy chain region gene (IGHV). CLL samples harboring unmutated (UM) IGHV have stronger signaling capacity compared to mutated (M) cases that display a more anergic phenotype.5
CLL typically shows remarkable perturbations of both the innate and the adaptive immune responses, which are already evident from early stages of the disease and become severe in advanced/relapsed or therapy-resistant cases.6-10 Notably, leukemic cells play a pivotal role in shaping the microenvironment towards tolerance through multiple mechanisms.11 For example, circulating CLL cells share phenotypic features of regulatory B cells and secrete interleukin (IL)-10, which in turn affects T-cell responses.12 Interestingly, it was observed that IL-10 production is enhanced in anergic IGHV-M compared to IGHV-UM CLL cases, which are characterized by a more aggressive clinical course.13
The hypothesis behind this work is that the immunomodulatory molecule T-cell immunoreceptor with Ig and ITIM domains (TIGIT) can contribute to promote B-cell anergy and to shape the environment towards tolerance. TIGIT is an inhibitory receptor expressed on T, NK and NKT cells, sharing structural and mechanistic similarities with PD-1 and CTLA-4.14 The cytoplasmic tail contains an immunoglobulin tail tyrosine (ITT)-like phosphorylation motif and an ITIM domain, like PD-1, through which TIGIT recruits the phosphatase SHIP1 to inhibit downstream activation of NFκB, PI3K and MAPK pathways.15 TIGIT has a competing receptor, CD226/DNAM-1 (DNAX accessory molecule-1), resulting in opposite signaling outcomes upon binding to the same set of ligands, similar to what has been described for the CTLA-4/CD28 pair.16 The TIGIT/CD226 ligands belong to the nectin-family member poliovirus receptor (PVR), the best known of which is CD155. Signaling triggered upon CD155 binding to CD226 potentiates T-cell receptor (TCR) signaling and CD8+ T-cell cytotoxicity against tumor cells (positive signaling).17 In contrast, concomitant TIGIT expression on the cell surface prevents CD226 activation either by sequestering CD155 or by impeding CD226 homodimerization and phosphorylation, resulting in negative signaling.18 Whether TIGIT triggers a full inhibitory cascade or functions by preventing the CD226-mediated positive co-stimulatory signal remains unclear. Since the two receptors are co-expressed on the cell surface, a TIGIT/CD226 ratio is often preferred to highlight the imbalance towards a positive or a negative signaling outcome.19 Even though few data are available regarding TIGIT expression in the B-cell compartment, a recent paper has described the molecule on the surface of normal human memory B cells, where it directly suppresses T-cell responses.20
In CLL patients, TIGIT expression was shown to be progressively increased in the CD4+ T-cell compartment, reaching the highest levels in advanced stages of the disease. Functionally, TIGIT+/CD4+ T lymphocytes sustain CLL cell viability more efficiently than the TIGIT- counterpart and TIGIT inhibition interferes with the production of prosurvival cytokines by CD4+ T cells.21 However, no information is available on TIGIT expression in the leukemic cell compartment. The present study was undertaken to investigate expression of the TIGIT/CD226/CD155 axis in CLL, with a specific focus on leukemic B cells, and to explore the role of this axis in BCR activation.
Methods
Sample cohort
Peripheral blood samples from CLL patients were obtained after informed consent, in accordance with Institutional Guidelines and the Declaration of Helsinki. The study was approved by the Institutional Review Board of each recruiting center. We examined a retrospective cohort of 115 CLL samples and 11 buffy coats from age- and sex-matched healthy subjects. The patients’ characteristics are summarized in Online Supplementary Table S1. Serial samples collected before treatment initiation and after 2 and 24 weeks of ibrutinib treatment were obtained from 14 additional patients (Online Supplementary Table S2). Clinical and molecular characteristics of CLL samples used in histological studies on lymph node biopsies are reported in Online Supplementary Table S3.
Xenografts derived from patients with Richter syndrome (RS-PDX) were obtained as described previously.22,23 Where indicated, primary CLL cells were cultured in RPMI 10% fetal calf serum in the presence or absence of ibrutinib used at 1 and 5 μM for 48 h.
Antibodies and reagents
A list of the antibodies used in flow cytometry and of the specific reagents used in functional assays is provided in Online Supplementary Table S4.
Flow cytometry
Surface expression of TIGIT, CD226 and CD155 was evaluated by flow cytometry on CLL peripheral blood mononuclear cells performing multiparametric staining to identify B or T lymphocytes and monocytes (Online Supplementary Table S5). Samples were acquired with a FACSCelesta cytometer (BD Biosciences) and data were analyzed with FlowJo v10 software (FlowJo).
Modulation of the TIGIT/CD226/CD155 axis
Modulation of the signaling triggered by CD155 binding either TIGIT or CD226 was performed both in the short term, to evaluate its inference on αIgM-mediated pBTK induction, and in the long term, to investigate the impact on CpG/IL-15-induced CLL proliferation. For the short-term experiments, cells were pre-treated in ice for 1 h with 5 µg/mL recombinant human (rh)TIGIT-Fc or with αTIGIT or αCD226 blocking monoclonal antibodies (5 µg/106 cells) for 30 min and then with rhCD155-Fc (5 µg/mL) for 1 h, before αIgM stimulation. For the long-term experiments, to prevent internalization, αTIGIT and αCD226 blocking monoclonal antibodies were coated onto magnetic beads and rhTIGIT-Fc and rhCD155-Fc chimeras were immobilized onto a cell culture plate. Briefly, 10x106 Dynabeads Sheep anti-Mouse IgG (Invitrogen, Thermofisher) were washed twice with phosphate-buffered saline, 0.1% bovine serum albumin and then coated with 1.5 μg of either antibody, by incubating overnight at 4°C on a rotating wheel, following the manufacturer’s instructions. Coated beads were used to treat CLL cells by pre-mixing them at a 2:1 bead:cell ratio immediately before plating the cells in a 96-well plate. In parallel, 96-well plates were coated overnight at 4°C with 1 μg/well of rhTIGIT-Fc or rhCD155-Fc chimeras.
Statistical analyses
Statistical analyses were performed with GraphPad v7 (GraphPad Software Inc, La Jolla, CA, USA). A Mann-Whitney or Wilcoxon matched-pairs signed rank test was used to determine statistical significance. Contingency tests were performing using the Fisher test.
Methods for RNA extraction and quantitative real-time polymerase reaction, confocal microscopy, the phosflow assay, CpG/IL-15 stimulation and IL-10 production are entirely described in the Online Supplementary Material.
Results
TIGIT axis expression in peripheral blood mononuclear cells patients with chronic lymphocytic leukemia
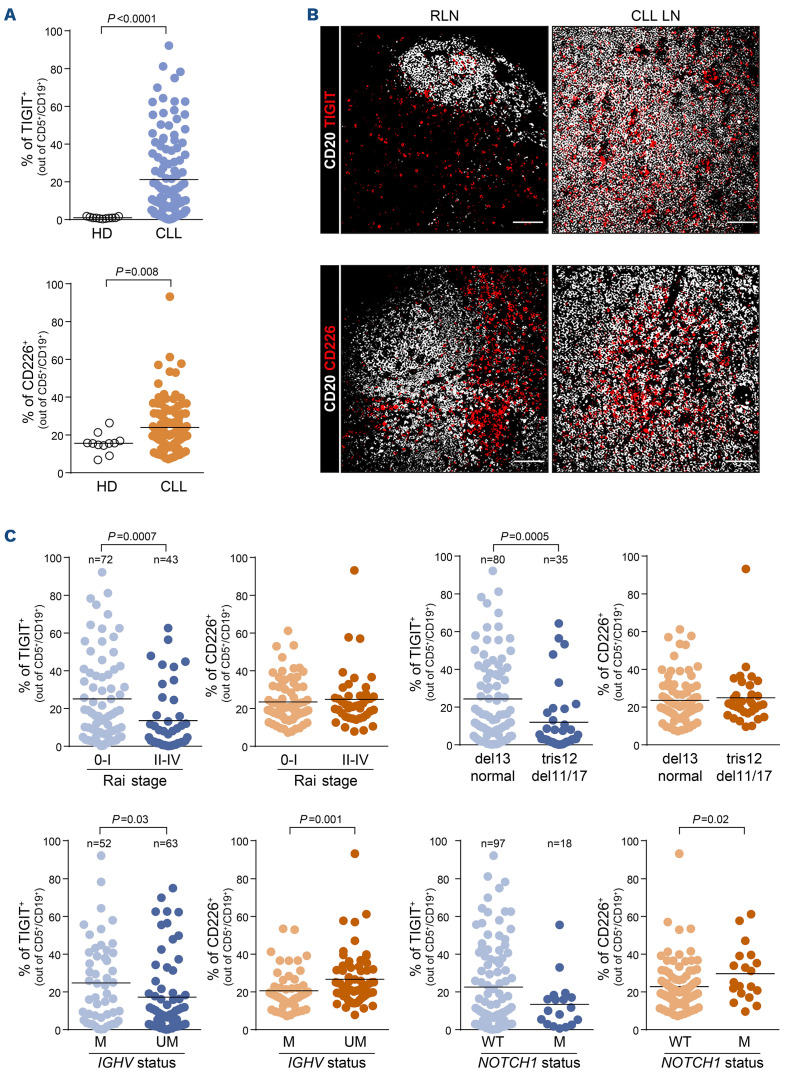
Peripheral blood mononuclear cell preparations from 115 patients with a confirmed diagnosis of CLL (Online Supplementary Table S1) were tested for expression of TIGIT, CD226 and of the CD155 ligand and compared to preparations from a small cohort (n=11) of sex-matched healthy subjects of a comparable age. To this aim, we set up a multiparametric staining protocol for flow cytometry to analyze expression of the three molecules simultaneously on B and T lymphocytes and monocytes (Online Supplementary Table S5; Online Supplementary Figures S1 and S2). Leukemic B lymphocytes variably expressed TIGIT on the cell surface, whereas normal CD19+ B cells were uniformly negative (mean levels of expression were 21.22±21.97% of TIGIT+ cells in CLL samples vs. 0.97±0.47% in healthy subjects). CD226 was also expressed at significantly higher levels in CLL samples than in normal B cells (mean 24±12.9% in CLL vs. 15.6±5.2% in healthy subjects) (Figure 1A).
Histological analyses of lymph nodes confirmed low germinal center B-cell-associated TIGIT expression in reactive lymph node samples, while CLL lymph nodes showed higher levels of expression. Similar results were obtained when examining the co-staining of CD226 with CD20+ B cells (Figure 1B).
To complete the picture, we evaluated TIGIT axis expression on T lymphocytes and monocytes from CLL patients. The results indicate that TIGIT was variably expressed on CD4+ T lymphocytes with a significant increase in advanced stages (Rai II-IV) and IGHV-UM CLL, in line with previous data.21 In addition, CD8+ T cells were highly TIGIT+, marking an exhausted phenotype (Online Supplementary Figures S3A and S4A). Accordingly, CD226 expression on CD8+ T lymphocytes decreased with advanced CLL stages, concurrent with the acquisition of further exhaustion (Online Supplementary Figures S3B and S4B). Lastly, monocytes showed the highest CD155 levels, suggesting that this cell lineage provides the ligand to engage either TIGIT or CD226. In line with a picture of progressive immune cell dysfunction, CD155 expression on monocytes decreased in Rai II-IV CLL patients (Online Supplementary Figures S3C and S5A-C).
We then correlated TIGIT expression on leukemic B cells with clinical and molecular features of the disease. We found that samples bearing markers of indolent disease or good prognosis (including Rai stage 0-I, normal karyotype or deletion 13 and IGHV-M genes) expressed TIGIT at significantly higher levels than the counterparts (Rai stage II-IV, trisomy 12, deletion 11 or deletion 17, and IGHV-UM genes) (Figure 1C). Lower TIGIT levels were also observed in NOTCH1-mutated cases, although this finding did not reach statistical significance. No differences were observed according to CD38 or CD49d levels (Online Supplementary Figure S6A).
CD226 had an opposite trend of expression, being associated with features of more aggressive disease, such as absence of somatic hypermutations in the IGHV genes, presence of NOTCH1 mutation and surface expression of CD38 and CD49, suggesting higher CD226 expression in CLL subsets that have a greater BCR signaling capacity compared to their counterparts (Figure 1C, Online Supplementary Figure S6A).
CD155 was generally present at low levels in CLL cells, without significant differences across disease subsets (Online Supplementary Figure S7).
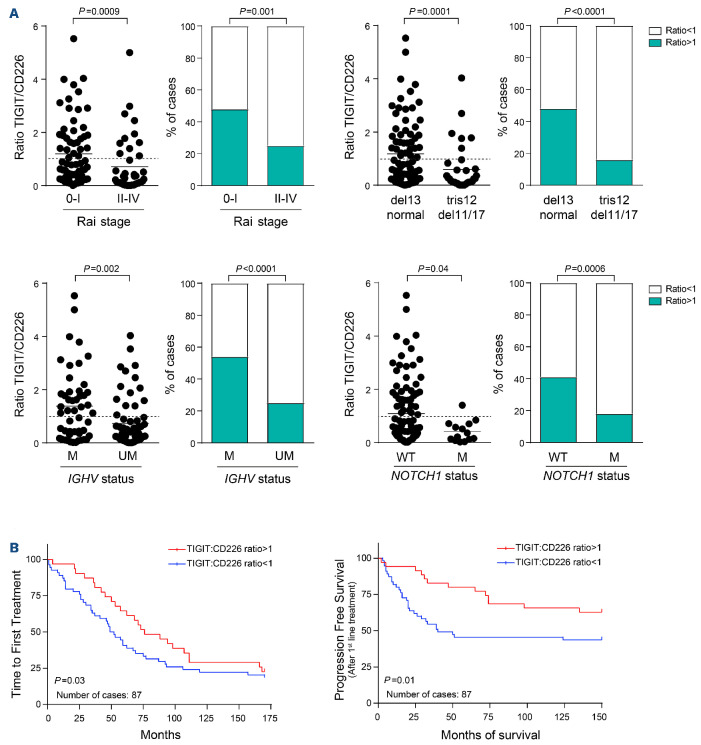
Definition of an operational TIGIT:CD226 ratio
Considering that TIGIT and CD226 have opposing roles on the cell surface and compete for binding to the same ligand, we determined a TIGIT:CD226 ratio, based on percentage of expression. A TIGIT:CD226 ratio ≥1 indicates prevalence of TIGIT-expressing cells and consequently a predominance of negative signaling, whereas a TIGIT:CD226 ratio <1 indicates prevalence of CD226-expressing cells and therefore positive signaling. In line with this reasoning, CLL samples with a TIGIT:CD226 ratio ≥1 were enriched in the good prognosis subsets, while samples with a ratio <1 were more frequent in the presence of adverse prognostic markers (e.g., advanced stages, IGHV-UM, unfavorable cytogenetics, NOTCH1 mutations), confirming the validity of this approach (Figure 2A, Online Supplementary Figure S6B). Interestingly, prevalence of CD226 signaling, as defined by a TIGIT:CD226 ratio <1, correlates with significantly earlier time to first treatment and shorter progression-free survival after first-line therapy (Figure 2B).
TIGIT expression is associated with chronic lymphocytic leukemia anergy
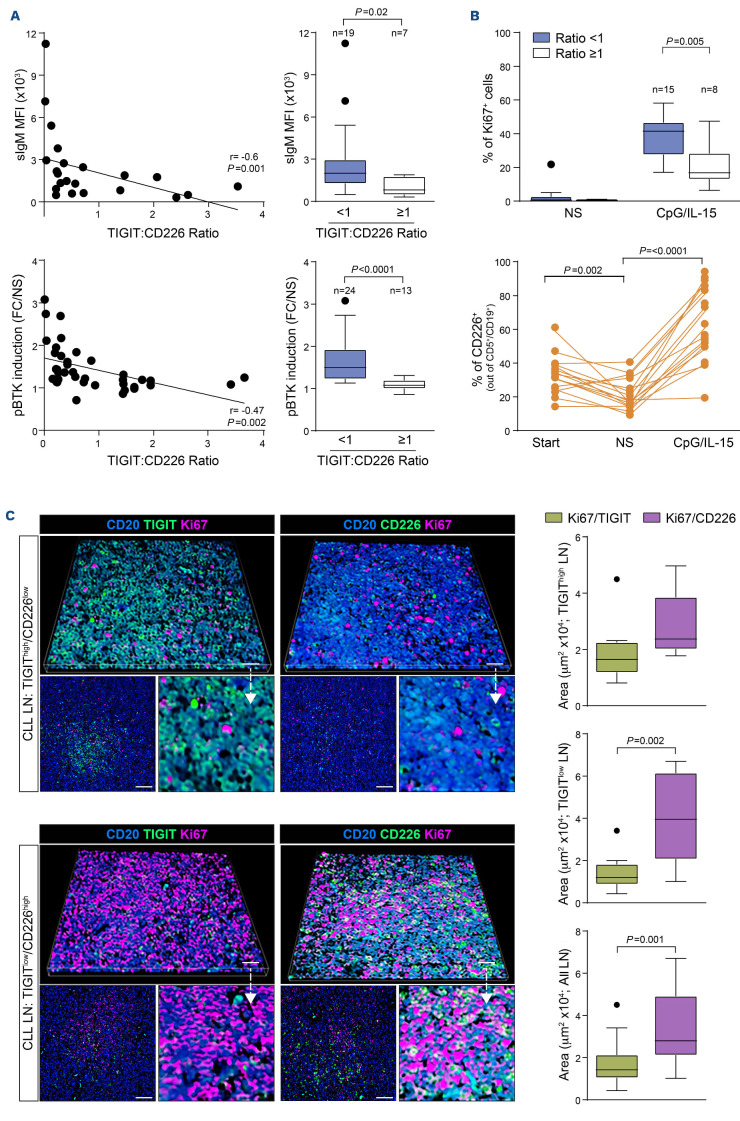
To investigate the interplay between TIGIT and BCR signaling capacity, we selected a homogeneous subset of samples carrying IGHV-UM, with a normal fluorescence in situ hybridization profile or deletion 13q as a sole abnormality and without NOTCH1 mutation, to avoid experimental biases.24 We found an inverse correlation between TIGIT expression and BCR signaling capacity, evaluated analyzing baseline surface IgM levels and BTK phosphorylation in response to IgM crosslinking as read outs (Figure 3A, left panels). Moreover, when splitting CLL samples according to TIGIT:CD226 ratio, we confirmed that samples with a ratio <1 (TIGIT-) had significantly higher surface IgM levels and stronger phospho-BTK induction than cases with a ratio ≥1 (TIGIT+) (Figure 3A, right panels).
Figure 1.
TIGIT and CD226 are deregulated in chronic lymphocytic leukemia and differentially expressed among subsets of patients. (A) Percentages of TIGIT+ and CD226+ cells in 115 chronic lymphocytic leukemia (CLL) samples and 11 age- and sex-matched healthy donors. Statistical analysis: Student t test. (B) Representative multispectral immunofluorescence confocal images of non-malignant reactive (n=4) or CLL (n=6) lymph node formalin-fixed paraffin-embedded biopsy tissues for TIGIT or CD226 (red) expression in the lymph node microenvironment (CD20, white). Original magnification, x20, scale bar: 50 μm. (C) From left to right: percentages of TIGIT+ and CD226+ cells in samples stratified according to Rai stage and cytogenetic profile (top panels); percentages of TIGIT+ and CD226+ cells in samples stratified according to IGHV mutational status and to the presence of NOTCH1 mutations (bottom panels). Statistical analyses: Student t test. HD: healthy donor; RLN: reactive lymph node; CLL LN: CLL lymph node; M: mutated; UM: unmutated; WT: wildtype.
Figure 2.
Ratio between TIGIT+ and CD226+ cells in chronic lymphocytic leukemia samples. (A) We calculated a ratio between the percentage of TIGIT+ and CD226+ cells in our cohort of chronic lymphocytic leukemia samples. A ratio ≥1 indicates prevalence of TIGIT+ cells and predominant negative signaling; a ratio <1 indicates prevalence of CD226+ cells and predominant positive signaling. For each clinical or molecular marker (Rai stage, IGHV mutational status, cytogenetics, NOTCH1 mutations) there is a dot plot showing ratio values for each sample (left) and a contingency plot indicating the enrichment of samples with a ratio ≥1 or <1 in either prognostic category. The dashed line at y=1 indicates the threshold discriminating between negative signaling (TIGIT:CD226 ratio ≥1, prevalence of TIGIT) and positive signaling (TIGIT:CD226 ratio <1, prevalence of CD226). Statistical analysis: Student t test. (B) Kaplan-Meier curves comparing the time to first treatment and progression-free survival (before and after treatment, respectively) of CLL patients divided according to TIGIT:CD226 ratio. Statistical analysis: Mantel-Cox test. M: mutated; UM: unmutated; WT: wildtype.
We next examined whether high TIGIT levels were associated with weaker responses of CLL cells to activation/proliferation signals, such as CpG/IL-15, analyzed in the same CLL subset used to test BCR signaling. In line with the results obtained in the BCR signaling studies, when dividing samples according to TIGIT:CD226 ratio, samples with prevalence of TIGIT showed a lower proliferative response to CpG/IL-15 compared to samples with a ratio <1 (Figure 3B, upper panel).
Analysis of TIGIT and CD226 expression in these cells, after 6 days of exposure to CpG/IL-15, showed a marked upregulation of CD226, with a concomitant slight down-modulation of TIGIT (Online Supplementary Figure S8A). Unstimulated cells showed a high level of spontaneous apoptosis; in the remaining live cells, CD226 expression was downregulated compared to the expression levels before starting in vitro culture (Figure 3B, lower panel). Modulation of CD226 and TIGIT levels could explain, at least in part, the observation that TIGIT+ CLL samples showed a productive proliferative response, albeit weaker than TIGIT- samples. Accordingly, the TIGIT:CD226 ratio in stimulated cells was <1 in all samples, in line with a prevalence of “positive” signaling (Online Supplementary Figure S8B).
In line with these findings, we found that CLL lymph node biopsies with higher TIGIT expression showed lower CD226 levels (Figure 3C). These tissues samples had a significantly lower expression of the proliferation marker Ki67, when compared to samples showing low TIGIT and high CD226. Consistently, we found that CD226+ CLL cells were mainly associated with Ki67 expression regardless of TIGIT levels, which was significant in TIGITlow, CD226high lymph nodes as revealed by Ki67/TIGIT versus Ki67/CD226 co-localization image analysis (Figure 3C).
TIGIT+ chronic lymphocytic leukemia cells produce more interleukin-10
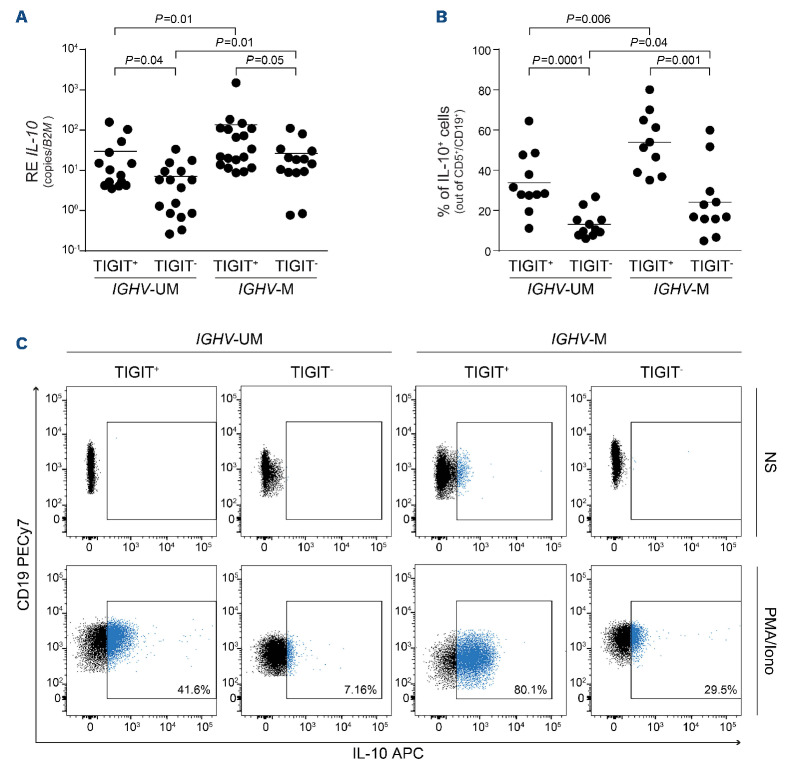
Considering that IGHV-M anergic CLL cells produce and secrete more IL-10 than IGHV-UM reactive cells,13 we investigated whether high TIGIT expression correlated with IL-10 production. We found that samples with a TIGIT:CD226 ratio ≥1 had significantly higher IL10 mRNA levels, both in the IGHV-UM and the IGHV-M CLL groups (Figure 4A). Similar results were obtained when measuring IL-10 production by flow cytometry, with TIGIT+ CLL cells staining more positive than TIGIT- cells for IL-10 after 5 h stimulation with phorbol 12-myristate 13-acetate and ionomycin, even in IGHV-UM cases (Figure 4B, C). The observation that IGHV-M cells showed higher IL10 expression and production, at the mRNA and at the protein levels, respectively, both in the TIGIT+ and in the TIGIT- subsets, suggests that TIGIT is associated with different IL-10 profiles but also that other regulatory mechanisms exist.
Figure 3.
High surface TIGIT expression is associated with chronic lymphocytic leukemia cell anergy. (A) Top panels. Inverse correlation between surface IgM levels and the percentage of TIGIT-expressing cells in chronic lymphocytic leukemia (CLL) samples harboring unmutated IGHV and with a normal karyotype or deletion 13 as the sole abnormality (left), and surface IgM levels in CLL samples divided according to TIGIT:CD226 ratio (right). Bottom panels. Inverse correlation between the induction of BTK phosphorylation (pBTK) upon B-cell receptor stimulation and the TIGIT:CD226 ratio in CLL samples (left), and fold changes of αIgM-mediated pBTK induction in CLL samples divided according to TIGIT:CD226 ratio (right). (B) Ki67 staining of TIGIT+ and TIGITCLL samples (top) and flow cytometry analysis of surface CD226 upregulation (bottom) in response to CpG/IL-15 culture. (C) Representative multispectral immunofluorescence and three-dimensional volume rendered confocal images of TIGIThigh/CD226low (n=3) or TIGITlow/CD226high (n=3) CLL lymph node formalin-fixed paraffin-embedded biopsy tissues stained for CD20 (blue), Ki67 (magenta) and TIGIT or CD226 (green). Original magnification, x20, scale bars of the larger image: 100 μm, of the lower image: 50 μm. The images on the right represent a magnification of the top image, as indicated by the arrow. Quantification of the co-localization of Ki67 and TIGIT or Ki67 and CD226 limited to CD20+ cells from CLL lymph node tissues. Graphs relative to the quantification in TIGIThigh/CD226low lymph nodes (top), TIGITlow/CD226high lymph nodes (middle) or all the lymph node samples together (bottom) are shown. Statistical analyses: Student t test. MFI: mean fluorescent intensity; FC/NS: fold-change compared to unstimulated sample; LN: lymph nodes.
TIGIT axis expression during disease follow-up
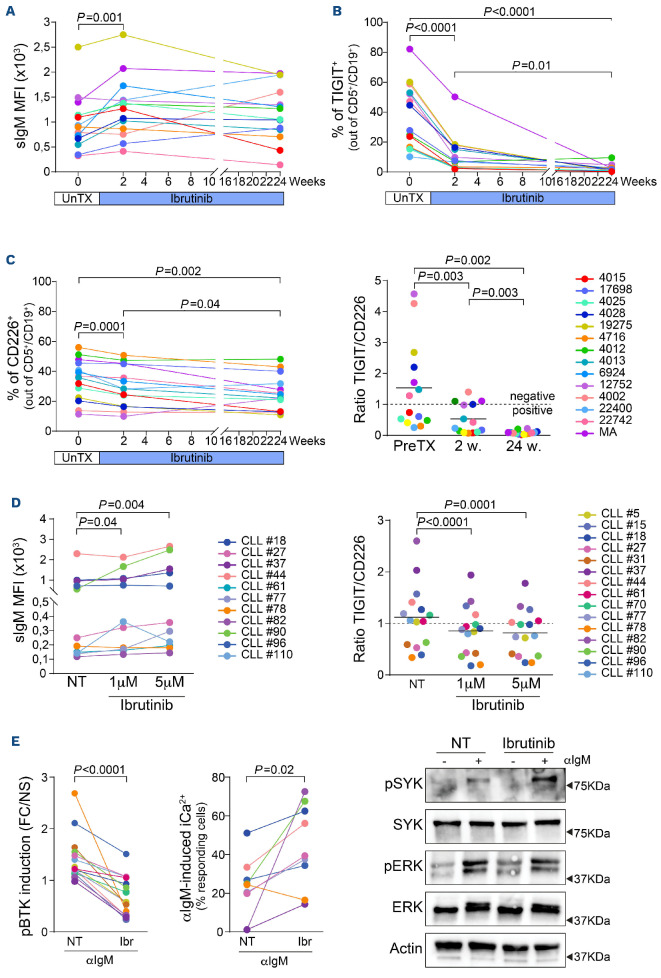
We next studied modulation of surface TIGIT, CD226 and CD155 over time, focusing specifically on the effects exerted by BTK inhibitors, since they are known to modulate BCR signaling. To this aim, we took advantage of a cohort of 14 samples collected systematically before ibrutinib initiation, after 2 weeks and after 24 weeks of treatment. The characterization of these samples is reported in Online Supplementary Table S2. Previous studies have shown that BTK blockade is followed by upregulation of surface IgM levels, evident already after 1 week on therapy and maintained for at least 3 months.25 This apparently paradoxical behavior was also observed in our cohort, in which sIgM levels were increased after 2 weeks of treatment and remained higher than the baseline at 24 weeks (Figure 5A). In line with heightened BCR signaling activity, surface TIGIT, which was expressed before therapy initiation by all samples, invariably decreased upon ibrutinib treatment, starting within the first 2 weeks of treatment and reaching minimal levels at the 24-week time-point (Figure 5B). TIGIT mRNA levels showed the same behavior (Online Supplementary Figure S9A). In contrast, mRNA levels of CD226 increased markedly (Online Supplementary Figure S9A), while surface levels were minimally, but significantly decreased (Figure 5C, left panel), raising the question of whether the molecule can reach the cell surface. However, given the relative changes of TIGIT and CD226, their ratio dropped to <1 in all samples examined, including those with a ratio ≥1 be fore treatment, suggesting a switch towards “positive” CD226-mediated signaling (Figure 5C, right panel). TIGIT downregulation appeared specific to B cells, as we found that ibrutinib treatment did not alter its expression on CD4+ or CD8+ T-cell subsets (Online Supplementary Figure S9C, D). In contrast, CD155 surface expression decreased minimally along the patients’ follow-up (Online Supplementary Figure S9B).
Figure 4.
TIGIT+ cells produce more IL-10. (A) Quantitative real-time polymerase chain reaction analysis of IL10 baseline expression of chronic lymphocytic leukemia (CLL) samples divided according to IGHV mutational status and TIGIT surface levels. (B) FACS analysis of IL-10 intracellular staining after 5 h stimulation with phorbol 12-myristate 13-acetate (50 ng/mL) and 1 μM ionomycin in CLL samples divided according to IGHV mutational status and TIGIT surface levels. (C) Representative flow cytometry plots of IL-10 production in unstimulated and stimulated CLL cells. Statistical analyses: Student t test. RE: relative expression; IL-10: interleukin-10: B2M: β2-microglobulin; UM: unmutated: M: mutated; NS: unstimulated; PMA/Iono: stimulated with phorbol 12-myristate 13-acetate/ionomycin.
This response to ibrutinib was also documented in vitro, by exposing primary CLL cells from untreated patients to ibrutinib (1 μM and 5 μM for 48 h). In these cells, we observed increased sIgM levels, with a concomitant decrease of the TIGIT:CD226 ratio (Figure 5D, Online Supplementary Figure S9E). Previous investigators have reported that upon BTK inhibition, the BCR retains the capability to mobilize Ca2+ in response to antibody ligation, as well as the capability of tyrosine phosphorylating SYK and ERK1/2.26 In our samples, despite inhibition of tyrosine phosphorylation of BTK, a marked increase in intracellular Ca2+ mobilization upon BCR ligation was observed, comparing untreated versus ibrutinib-treated primary CLL cells. The same cells showed prominent tyrosine phosphorylation of SYK and ERK1/2, clearly indicating that the BCR pathway is bypassing the signaling block imposed by ibrutinib (Figure 5E).
TIGIT and CD226 are expressed on Richter syndrome samples
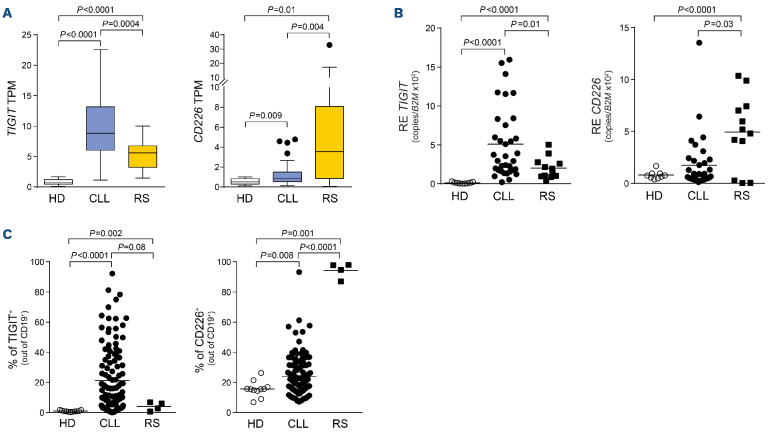
Stemming from these observations, we evaluated expression of TIGIT and CD226 in cases of Richter syndrome (RS), a rare but often fatal complication of CLL characterized by transformation of the leukemia into an aggressive lymphoma.27-29 To this purpose, we exploited RNA-sequencing analysis performed on primary formalin-fixed paraffin-embedded RS lymph nodes and compared it to that of CLL samples and matched healthy subjects, from previously published datasets (EGA accession numbers EGAD00001004046 and EGAD00001000258).30,31 RS samples showed lower TIGIT expression compared to CLL samples, in line with our observation of TIGIT marking a more indolent disease. Accordingly, CD226 expression in RS cells was higher than in either cells from healthy donors or CLL samples (Figure 6A). Results were substantiated by using RS-patient-derived xenograft models recently established in our laboratory.22,23 Both quantitative polymerase reaction performed on four RS-PDX at different passages and flow cytometry analyses confirmed the RNA-sequencing results, with TIGIT being expressed at lower levels than in CLL cases and CD226 showing the highest expression (Figure 6B, C). Interestingly, all these four models show a highly active BCR signaling pathway.32
Figure 5.
TIGIT and CD226 expression during the follow-up of chronic lymphocytic leukemia patients treated with ibrutinib. (A) Surface IgM levels before and during ibrutinib therapy. (B) Surface expression of TIGIT before treatment initiation, and after 2 and 24 weeks of ibrutinib therapy (C) (Left panel) Surface expression of CD226 and (Right panel) TIGIT:CD226 ratio before treatment initiation, and after 2 and 24 weeks of ibrutinib therapy. The dashed line at y=1 indicates the threshold discriminating between negative signaling (TIGIT:CD226 ratio ≥1, prevalence of TIGIT) and positive signaling (TIGIT:CD226 ratio <1, prevalence of CD226). Statistical analyses (A-C): one-way analysis of variance. (D) (Left panel) Surface IgM levels of chronic lymphocytic leukemia (CLL) cells treated in vitro with 1 and 5 μM ibrutinib for 48 h. (D) (Right panel) TIGIT:CD226 ratio measured in primary CLL cells in the presence or in the absence of ibrutinib used at 1 and 5 μM for 48 h. The dashed line at y=1 indicates the threshold discriminating between negative and positive signaling. (E) Phospho-BTK MFI levels in response to anti-IgM ligation in primary CLL cells left untreated or exposed to 1 μM ibrutinib for 48 h (left panel). In the same cells, intracellular Ca2+ levels were monitored by flow cytometry (middle panel) and SYK and ERK1/2 phosphorylation by western blot (right panel). (D, E) Statistical analyses: Student t test. MFI: mean fluorescence intensity; UnTX: before treatment; PreTX: before treatment; w: weeks; NT: untreated; FC/NS: fold change compared to unstimulated samples; Ibr: ibrutinib.
CD155 transcript levels were barely detectable in RS samples, although the molecule was expressed on the cell surface (Online Supplementary Figure S10).
Modulation of TIGIT and CD226 signaling
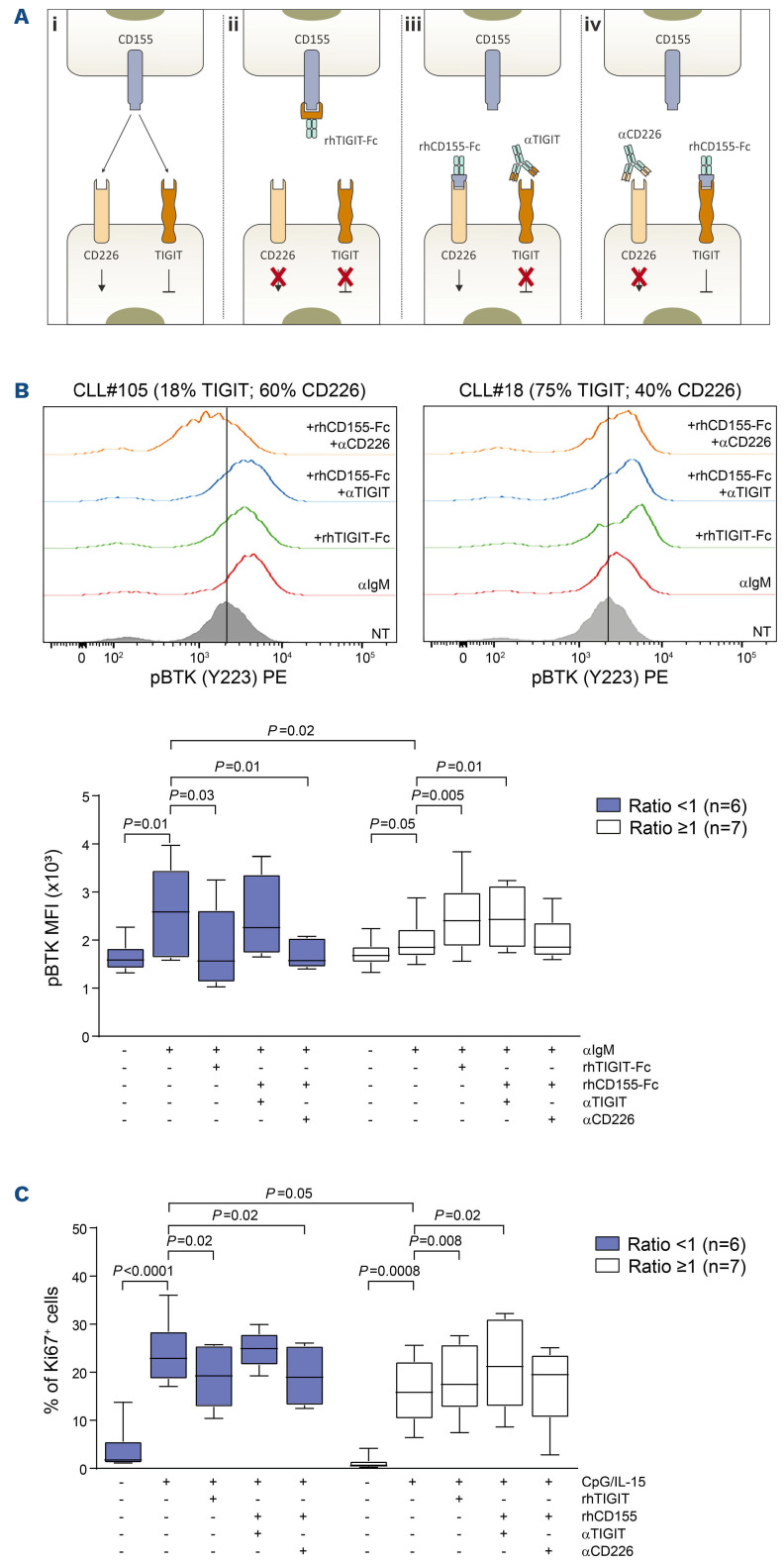
To understand the functional role of TIGIT and CD226 in altering BCR signaling capacity and cell proliferation, we selectively interfered with either receptor/ligand interaction, taking advantage of specific rhFc chimeras or blocking monoclonal antibodies. Read-outs were BTK phosphorylation in response to αIgM-mediated BCR crosslinking or Ki67 staining following CpG/IL-15 culture, respectively. A schematic representation of the experiments and reagents is depicted in Figure 7A. Specifically, we used: (i) a rhTIGIT-Fc chimera that sequesters CD155 expressed on the cell surface and prevents it from binding either TIGIT or CD226, blocking downstream signaling of the prevalent receptor in that CLL population; (ii) a rhCD155-Fc chimera that works as an artificial ligand and can bind and activate both TIGIT and CD226, depending on which receptor is prevalent; and (iii) the rhCD155-Fc chimera in combination with blocking monoclonal antibodies directed against either TIGIT or CD226 (αTIGIT or αCD226) in order to discriminate between the activation of one receptor and the other.
Experiments were carried out in the same IGHV-UM CLL cases with a normal fluorescence in situ hybridization profile or deletion 13 used in previous experiments. Pretreatment of TIGIT- CLL cells with rhTIGIT-Fc affects mostly CD226 signaling, which is more expressed than TIGIT in these samples. We found that the BCR signaling capacity upon receptor engagement in the presence of rhTIGIT-Fc was significantly downregulated in TIGIT-samples compared to when αIgM was given alone, and phospho-BTK induction was similar to that of TIGIT+ samples. In contrast, pre-treatment of TIGIT+ CLL cells with rhTIGIT-Fc mostly prevents CD155 binding to TIGIT, likely abrogating its negative regulation of the BCR. Accordingly, in these samples, αIgM-induced BTK phosphorylation was significantly increased in the presence of rhTIGIT-Fc, and was more similar to that of TIGIT- samples. Furthermore, stimulation of the BCR in the presence of rhCD155-Fc and of αTIGIT blocking antibody enhanced BTK phosphorylation in TIGIT+ samples, while this combination had no effects on TIGIT- samples, which already had maximal phospho-BTK induction. Lastly, pre-treatment of CLL cells with rhCD155-Fc and αCD226 blocking antibody downmodulated the BCR signaling capacity of TIGIT- samples, in which CD226 is prevalent and could help to improve BCR responses, to levels comparable to those of TIGIT+ CLL samples, whose phospho-BTK induction was unaffected in the presence of agents preventing CD226 signaling (Figure 7B).
Similar results were obtained when examining the proliferative response to CpG/IL-15 stimulation in the same conditions (Figure 7C).
These results suggest that CD155 binding to TIGIT triggers an inhibitory signaling that decreases responsiveness of CLL cells to the antigen, and that, if CD155-TIGIT interactions are interrupted, we can boost CLL cell responses. On the other hand, CD155 binding to CD226 exerts an opposite “positive” effect on intracellular signaling and interrupting this axis might induce CLL cell anergy.
Discussion
This work shows that the immunomodulatory molecule TIGIT is expressed by CLL cells where it is a marker of anergy. TIGIT was identified nearly a decade ago and shown to be part of an axis including CD226 and CD155 which shares similarities with other checkpoint inhibitors.33 In the current view, TIGIT and CD226 are expressed by T cells and can negatively (TIGIT) or positively (CD226) affect TCR signaling, once engaged by the common CD155 ligand. Coherent with this view, T cells expressing high levels of TIGIT are reported in different cancers where they define a subset of exhausted and dysfunctional T lymphocytes.21,34-36 In CLL, high TIGIT expression is found on T lymphocytes from patients with advanced disease, co-expressing exhaustion markers21 (Online Supplementary Figure S6).
The recent finding of TIGIT expression on normal memory B cells, where it directly contributes to suppress T-cell responses,20 prompted us to extend these observations to CLL cells. Here, we show for the first time that circulating and resident leukemic B cells express TIGIT and CD226, at variance with the normal CD19+ subset. CD155, in contrast, was mostly expressed on the monocyte compartment. When dividing our cohort of 115 patients according to specific prognostic markers, we observed that high TIGIT was associated with features of indolent disease while high CD226 was more frequent in subsets characterized by elevated BCR signaling capacity (e.g., IGHV-UM, NOTCH1-M or CD38+ and CD49d+ cases). Since TIGIT and CD226 are concomitantly present on the cell surface, we devised a ratio between the two markers: a ratio ≥1 indicates predominance of TIGIT and hence of inhibitory effects (negative signaling), while a ratio in favor of CD226 prompts for a co-stimulatory effect (positive signaling). Accordingly, aggressive cases of CLL were enriched with samples showing a ratio in favor of CD226. It is therefore likely that this axis might modulate signaling of CLL cells, similarly to what is observed in T lymphocytes.
Figure 6.
TIGIT and CD226 expression in Richter syndrome. (A) Values of TIGIT and CD226 from RNA-sequencing experiments carried out in normal B cells, chronic lymphocytic leukemia (CLL) samples and primary formalin-fixed paraffin-embedded lymph nodes from Richter syndrome (RS) samples. (B) Quantitative real-time polymerase chain reaction analysis of TIGIT and CD226 expression in our cohort of healthy subjects, CLL samples and RS-patient-derived xenograft (PDX) models at different passages. (C) Flow cytometry analysis of TIGIT and CD226 surface expression in healthy subjects, CLL and RS-PDX. Statistical analyses: Student t test. TPM: transcripts per million; HD: healthy donors; RE: relative expression; B2M: (32-microglobulin.
Figure 7.
Modulation of TIGIT and CD226 interactions with CD155. (A) Schematic representation of the mechanisms of action of rhTIGIT-Fc and rhCD155-Fc chimeras and of αTIGIT and αCD226 blocking monoclonal antibodies: (i) CD155 can bind either TIGIT or CD226, triggering opposite signaling outcomes; (ii) rhTIGIT-Fc chimera prevents CD155 binding and can therefore inhibit both TIGIT and CD226 signaling, thus affecting the signaling through the prevalent receptor on the cell surface. rhCD155-Fc chimera works as an artificial ligand and can bind to either receptor; therefore, (iii) when giving it in combination with the αTIGIT blocking monoclonal antibody it is possible to induce signaling through CD226; while (iv) in combination with αCD226 monoclonal antibody, signaling through TIGIT is preserved. (B) Flow cytometry analysis of pBTK induction in response to αIgM-mediated B-cell receptor crosslinking in the presence of modulators of TIGIT and CD226 activity: top panels show plots of two representative TIGIT+ and TIGIT- samples, bottom panel shows cumulative results of pBTK induction. (C) Cumulative results of Ki67 staining upon CpG/IL-15 culture in the presence of modulators of TIGIT and CD226 activity. Statistical analyses: Student t test. MFI: mean fluorescent intensity.
To determine a possible role for TIGIT in CLL homeostasis, we first explored the effects on BCR signaling capacity, specifically focusing on IGHV-UM samples, selected to harbor high or low surface TIGIT. We found an inverse correlation between TIGIT expression and baseline sIgM levels or the induction of BTK phosphorylation in response to receptor engagement, suggesting that surface TIGIT is associated with a more anergic CLL behavior. In a cohort of samples collected systematically after ibrutinib therapy, we observed a sharp decrease in surface TIGIT following treatment. This is in line with the recent observation that leukemic cells, released from the lymph nodes by ibrutinib, upregulate sIgM and SYK because they no longer receive persistent antigen stimulation, as if they turned less anergic despite downstream inhibition of BTK.25 The mechanism behind TIGIT downregulation remains to be determined. Speculatively, it could rely on the inhibition of transcription factors downstream to the BCR signaling, including NFATC1,37 FOXP138 and NFKB,39 which have putative binding sites on the TIGIT promoter (not shown, prediction made using the CiiiDER online tool at http://www.ciiider.org/).40
TIGIT downregulation with concomitant surface IgM upregulation were also confirmed by in vitro exposure of primary CLL cells to ibrutinib. While BTK tyrosine phosphorylation was invariably inhibited in these cells, BCR ligation was followed by SYK and ERK1/2 tyrosine phosphorylation and mobilization of intracellular Ca2+ to levels higher than those observed in untreated cells, indicating recovery from anergy. This behavior was previously attributed to the interruption of chronic antigen stimulation due to release of CLL cells exposed to ibrutinib from the lymph node niche, at the same time making them more dependent on BCR engagement and consequently more susceptible to apoptosis if the ligand is not present, as is the case for peripheral circulation.25
A formal demonstration of the effects of TIGIT and CD226 on BCR signaling capacity comes from experiments in which interactions of these receptors with the CD155 ligand were interrupted using specific recombinant chimeras and monoclonal antibodies. rhTIGIT-Fc chimera sequesters CD155 and prevents its binding to either receptor, thus affecting the signaling through the prevalent receptor on the cell surface (Figure 7Aii). Therefore, in TIGIT- samples, blocking CD155 mostly affects signaling through CD226, removing its positive contribution to BCR signaling and, consistently, we observed a reduced αIgMinduced BTK phosphorylation. In contrast, in TIGIT+ samples, TIGIT signaling is affected by CD155 sequestering, removing its inhibitory effect and increasing BTK phosphorylation. Comparable results were achieved when selectively activating CD226 or TIGIT by providing chimeric CD155 ligand together with a monoclonal antibody blocking the unintended receptor (Figure 7Aiii-iv). Using the same experimental set-up, we examined CLL cell proliferation in response to CpG/IL-15 stimulation and observed significant differences between TIGIT+ and TIGIT- samples, with the latter showing a proliferative advantage over the former. Again, when interrupting TIGIT/CD226 interactions with CD155 we could modulate responses to CpG/IL-15 with different outcomes in TIGIT+ and TIGIT- samples.
Lastly, when analyzing IL-10 secretion in our sample cohort, we found that TIGIT+ cases of CLL produce more IL-10 than do TIGIT- ones, both in the IGHV-M and in the IGHV-UM subsets. This finding is in line with previous observations that TIGIT+ normal memory B cells suppress T-cell responses more efficiently than the TIGIT- counterpart, possibly via IL-10,20 and also with existing literature showing that IL-10 production is enhanced in more anergic CLL and is associated with a less aggressive clinical phenotype.13,41
Our results indicate that TIGIT and CD226 are aberrantly expressed on leukemic B cells, and this is the first time that deregulation of this axis has been described on tumor cells and not only in the T-cell compartment. In addition, this work provides evidence of an association between TIGIT expression and an anergic phenotype of the CLL cell. The mechanism behind TIGIT upregulation in CLL is still not understood. However, a recent paper reported a signature of aberrantly expressed immune regulatory molecules, including TIGIT, in CLL cells with a peculiar methylation pattern compared to that of healthy B lymphocytes.42
The translational implications of these results remain to be determined. Since therapeutic anti-TIGIT antibodies are in clinical trials for cancer patients, it would be tempting to determine whether, in CLL patients, they may revert anergy, increasing BCR signaling capacity, and therefore making leukemic cells more susceptible to targeted inhibitors. Further research will tell us more about this immunoregulatory pathway and its possible clinical implications.
Supplementary Material
Acknowledgments
The authors thank the Nikon Imaging Facility at King’s College London.
Funding Statement
Funding: This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC IG-23095 to SD; My First AIRC grant MFAG-23107 to TV; AIRC 5x1000 #21198 to GG), by the ITN INTEGRATA program (grant agreement 813284 to SD), by the Italian Ministry of Health (GR-2016-02364298 to TV), by the Ministry of Education, University and Research-MIUR and “Progetto Strategico di Eccellenza Dipartimentale” (D15D18000410001 to SD as part of the Department of Medical Sciences, University of Turin).
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804-815. [DOI] [PubMed] [Google Scholar]
- 2.Puente XS, Jares P, Campo E. Chronic lymphocytic leukemia and mantle cell lymphoma: crossroads of genetic and microenvironment interactions. Blood. 2018;131(21):2283-2296. [DOI] [PubMed] [Google Scholar]
- 3.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313-4320. [DOI] [PubMed] [Google Scholar]
- 5.Packham G, Krysov S, Allen A, et al. The outcome of B-cell receptor signaling in chronic lymphocytic leukemia: proliferation or anergy. Haematologica. 2014;99(7):1138-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573-581. [DOI] [PubMed] [Google Scholar]
- 7.Peters FS, Strefford JC, Eldering E, Kater AP. T-cell dysfunction in chronic lymphocytic leukemia from an epigenetic perspective. Haematologica. 2021;106(5):1234-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachonikola E, Stamatopoulos K, Chatzidimitriou A. T cells in chronic lymphocytic leukemia: a two-edged sword. Front Immunol. 2020;11:612244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorcari S, Maffei R, Atene CG, Potenza L, Luppi M, Marasca R. Nurse-like cells and chronic lymphocytic leukemia B cells: a mutualistic crosstalk inside tissue microenvironments. Cells. 2021;10(2):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griggio V, Perutelli F, Salvetti C, et al. Immune dysfunctions and immune-based therapeutic interventions in chronic lymphocytic leukemia. Front Immunol. 2020;11:594556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arruga F, Gyau BB, Iannello A, Vitale N, Vaisitti T, Deaglio S. Immune response dysfunction in chronic lymphocytic leukemia: dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci. 2020;21(5):1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiLillo DJ, Weinberg JB, Yoshizaki A, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27(1):170-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drennan S, D'Avola A, Gao Y, et al. IL-10 production by CLL cells is enhanced in the anergic IGHV mutated subset and associates with reduced DNA methylation of the IL10 locus. Leukemia. 2017;31(8):1686-1694. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48-57. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Zhang H, Li M, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20(3):456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol. 2015;6:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CJ, Andrews DM, McLaughlin NM, et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184(2):902-911. [DOI] [PubMed] [Google Scholar]
- 18.Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923-937. [DOI] [PubMed] [Google Scholar]
- 19.Deng C, Li W, Fei Y, et al. Imbalance of the CD226/TIGIT immune checkpoint is involved in the pathogenesis of primary biliary cholangitis. Front Immunol. 2020;11:1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan MM, Nair SS, O'Leary JG, et al. Implication of TIGIT(+) human memory B cells in immune regulation. Nat Commun. 2021;12(1):1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catakovic K, Gassner FJ, Ratswohl C, et al. TIGIT expressing CD4+ T cells represent a tumor-supportive T cell subset in chronic lymphocytic leukemia. Oncoimmunology. 2017;7(1):e1371399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaisitti T, Braggio E, Allan JN, et al. Novel Richter syndrome xenograft models to study genetic architecture, biology, and therapy responses. Cancer Res. 2018;78(13):3413-3420. [DOI] [PubMed] [Google Scholar]
- 23.Vaisitti T, Arruga F, Vitale N, et al. ROR1 targeting with the antibody-drug conjugate VLS-101 is effective in Richter syndrome patient-derived xenograft mouse models. Blood. 2021;137(24):3365-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arruga F, Bracciama V, Vitale N, et al. Bidirectional linkage between the B-cell receptor and NOTCH1 in chronic lymphocytic leukemia and in Richter's syndrome: therapeutic implications. Leukemia. 2020;34(2):462-477. [DOI] [PubMed] [Google Scholar]
- 25.Drennan S, Chiodin G, D'Avola A, et al. Ibrutinib therapy releases leukemic surface IgM from antigen drive in chronic lymphocytic leukemia patients. Clin Cancer Res. 2019;25(8):2503-2512. [DOI] [PubMed] [Google Scholar]
- 26.Chiodin G, Drennan S, Martino EA, et al. High surface IgM levels associate with shorter response to ibrutinib and BTK bypass in patients with CLL. Blood Adv. 2022;6(18):5494-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadmor T, Levy I. Richter transformation in chronic lymphocytic leukemia: update in the era of novel agents. Cancers (Basel). 2021;13(20):5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condoluci A, Rossi D. Richter syndrome. Curr Oncol Rep. 2021;23(3):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131(25):2761-2772. [DOI] [PubMed] [Google Scholar]
- 30.Beekman R, Chapaprieta V, Russinol N, et al. The reference epigenome and regulatory chromatin landscape of chronic lymphocytic leukemia. Nat Med. 2018;24(6):868-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira PG, Jares P, Rico D, et al. Transcriptome characterization by RNA sequencing identifies a major molecular and clinical subdivision in chronic lymphocytic leukemia. Genome Res. 2014;24(2):212-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iannello A, Vitale N, Coma S, et al. Synergistic efficacy of the dual PI3K-delta/gamma inhibitor duvelisib with the Bcl-2 inhibitor venetoclax in Richter syndrome PDX models. Blood. 2021;137(24):3378-3389. [DOI] [PubMed] [Google Scholar]
- 33.Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. TIGIT, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol. 2021;12:699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freed-Pastor WA, Lambert LJ, Ely ZA, et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell. 2021;39(10):1342-1360.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauvin JM, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125(5):2046-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao Q, Wang L, Yuan M, Jin X, Chen Z, Wu C. TIGIT induces (CD3+) T cell dysfunction in colorectal cancer by inhibiting glucose metabolism. Front Immunol. 2021;12:688961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf C, Garding A, Filarsky K, et al. NFATC1 activation by DNA hypomethylation in chronic lymphocytic leukemia correlates with clinical staging and can be inhibited by ibrutinib. Int J Cancer. 2018;142(2):322-333. [DOI] [PubMed] [Google Scholar]
- 38.Cerna K, Oppelt J, Chochola V, et al. MicroRNA miR-34a downregulates FOXP1 during DNA damage response to limit BCR signalling in chronic lymphocytic leukaemia B cells. Leukemia. 2019;33(2):403-414. [DOI] [PubMed] [Google Scholar]
- 39.Rozovski U, Harris DM, Li P, et al. Activation of the B-cell receptor successively activates NF-kappaB and STAT3 in chronic lymphocytic leukemia cells. Int J Cancer. 2017;141(10):2076-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gearing LJ, Cumming HE, Chapman R, et al. CiiiDER: a tool for predicting and analysing transcription factor binding sites. PLoS One. 2019;14(9):e0215495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanna BS, Llao-Cid L, Iskar M, et al. Interleukin-10 receptor signaling promotes the maintenance of a PD-1(int) TCF-1(+) CD8(+) T cell population that sustains anti-tumor immunity. Immunity. 2021;54(12):2825-2841.e10. [DOI] [PubMed] [Google Scholar]
- 42.Wierzbinska JA, Toth R, Ishaque N, et al. Methylome-based cell-of-origin modeling (Methyl-COOM) identifies aberrant expression of immune regulatory molecules in CLL. Genome Med. 2020;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.