Abstract
Borna disease virus (BDV), a negative nonsegmented single-stranded RNA virus, has not been fully characterized morphologically. Here we present what is to our knowledge the first data on the fine ultrastructure and morphogenesis of BDV. The supernatant of MDCK cells persistently infected with BDV treated with n-butyrate contained many virus-like particles and more BDV-specific RNA than that of untreated samples. The particles were spherical, enveloped, and approximately 130 nm in diameter; had spikes 7 nm in length; and reacted with BDV p40 antibody. A thin nucleocapsid, 4 nm in width, was present peripherally in contrast to the thick nucleocapsid of hemagglutinating virus of Japan. The BDV particles reproduced by budding on the cell surface.
Borna disease virus (BDV) is the etiological agent for Borna disease, which is a central nervous system disease characterized by profound behavioral abnormalities, inflammatory cell infiltrates in the central nervous system, and accumulation of BDV-specific proteins in the limbic system neurons (for reviews, see references 21, 23 and 28). Though natural infection has only been confirmed to occur in horses and sheep, the potential host range of BDV includes other mammals and birds (4, 24, 25). Demonstration of specific viral antigens in patients with affective disorders suggests that BDV or a related virus may be pathogenic to humans as well (1–3, 5, 30, 31). More recently, BDV RNA has been frequently detected in peripheral blood mononuclear cells from patients with neuropsychiatric disorders (6, 18, 19, 33) and in the brain tissue of schizophrenics and depressives (32). The broad spectrum of animal hosts for BDV indicates that transmission of the virus most likely occurs via extracellular virus particles.
BDV is a negative nonsegmented single-stranded (NNS) RNA virus with a genomic organization similar to that of other members of the order Mononegavirales (8, 11), including the families Paramyxoviridae, Rhabdoviridae, and Filoviridae. However, in contrast to those of other animal NNS RNA viruses, BDV replication and transcription occur in the nucleus (7, 8, 10, 37), and BDV uses RNA splicing for the regulation of its gene expression (12, 34, 35). These findings have led to the classification of a new family of animal NNS RNA viruses, the Bornaviridae (27).
Many attempts to identify BDV particles by electron microscopy have been made. To identify the rare virus particles in vitro as well as in vivo, several investigators have used techniques for high virus yields (15, 22, 29, 37) and purification. In the purification techniques (22, 29), BDV particles were obtained by affinity chromatography purification or lipid extraction with Freon. Therefore, these particles were condensed to 50 to 60 nm in diameter during extraction with high salt concentrations or were reduced in diameter due to removal of the envelope. Pauli and Ludwig (26) developed high-yield virus infectivity systems by the addition of salts or n-butyrate. Zimmermann et al. (37) used a system with a higher concentration of salts to identify the virion of BDV as an enveloped particle ranging from 50 to 100 nm in diameter, by electron microscopic negative staining. The trials were also extended to hypertonic treatment to promote the release of cell-bound viruses (7, 15). They confirmed the presence of particles ranging from 80 to 130 nm in diameter by negative staining. However, they did not determine the morphology, especially the inner structure, in detail. Compans et al. (9) observed intracytoplasmic virus particles in ultrathin sections, but the fine structure and morphogenesis of the extracellular virus have not been well characterized.
The development of a method for higher BDV yields by treatment with n-butyrate in Madin-Darby canine kidney (MDCK) cells persistently infected with BDV (MDCK/BDV cells) (26) prompted us to attempt visualization of the virus particles by electron microscopy. Here we present the first data, to our knowledge, on the fine ultrastructure and morphogenesis of BDV cultured in cells persistently infected with BDV.
The MDCK/BDV cell line (16) was kindly provided by R. Rott, Justus-Liebig-Universität Giessen, Giessen, Germany. The cells were cultured in Dulbecco’s minimum essential medium (Dainippon Pharmaceutical Co. Ltd., Osaka, Japan) at 37°C for 24 h in a plastic culture flask. In order to induce the production of BDV particles with n-butyrate, the cells were further incubated in the same medium containing 8 mM n-butyric acid sodium salt (Sigma, St. Louis, Mo.) at 37°C for 48 h as previously described (26). The n-butyrate-induced MDCK/BDV (referred to as induced MDCK/BDV) cell culture and its supernatant were compared with those of uninduced MDCK/BDV cells and non-BDV-infected MDCK cells cultured under the same conditions. For comparison, Molt-4 or MDCK cells were inoculated with human immunodeficiency virus type 1 (HIV-1, strain BRU) and a paramyxovirus, hemagglutinating virus of Japan (HVJ) (17), and cultured in RPMI 1640 and Dulbecco’s minimal essential medium, respectively. All cell culture media were supplemented with 5% fetal bovine serum and 100 IU of penicillin and 100 μg of streptomycin per ml (GIBCO BRL, Grand Island, N.Y.).
Detection of BDV-specific RNA in cultured supernatant.
Thirty-milliliter aliquots of the culture supernatants of induced and uninduced MDCK/BDV cells were clarified by centrifugation at 2,200 × g for 5 min prior to filtration through a 0.45-μm-pore-size membrane (Millipore, Bedford, Mass.). The filtrates were ultracentrifuged at 100,000 × g for 120 min, and the pellets were resuspended in 200 μl of 0.15 M phosphate-buffered saline (pH 7.2) as a virus suspension for reverse transcriptase PCR (RT-PCR) and negative staining. For RT-PCR assay, RNAs were purified from the mixture of 100 μl of virus suspension and 5 μl of RNA suspension containing 105 copies of pAW109 RNA (Perkin-Elmer Corp., Branchburg, N.J.), using an RNA purification kit (Invisorb RNA kit; ID Labs Biotechnology, London, Ontario, Canada). The purified RNA was amplified by RT-PCR with the EZ rTth RNA kit (Perkin-Elmer) and a primer pair for the BDV p40 gene. In brief, the reverse transcription step was performed in a thermal cycler (2400-k; Perkin-Elmer) at 60°C for 30 min, followed by 94°C for 2 min. The reverse transcripts were subsequently subjected to amplification consisting of 35 cycles of denaturation at 94°C for 1 min and annealing and polymerization at 60°C for 1.5 min, followed by a final polymerization step for 10 min at 60°C. The primers were designed to detect PCR products of BDV p40 (36). The sequences of the primers were as follows: 5′-GATGACGATCCTATCACAACC-3′ (bp 339 to 359) and 5′-GTCACGGCGCGATATGTTTC-3′ (bp 590 to 609).
The products of RT-PCR were analyzed by 10% polyacrylamide gel electrophoresis with ethidium bromide staining. The intensity of each band from induced and uninduced MDCK/BDV cell cultures and the standard size marker of 300 bp were measured with the microcomputer imaging device system of Imaging Research Inc. (St. Catharines, Ontario, Canada). The quantities of BDV-specific RNA were expressed as relative intensities on the basis of a standard size marker. The relative intensities of the bands at 271 bp from induced and uninduced MDCK/BDV cell cultures were semiquantitatively determined to be 180 and 26%, respectively, by densitometric analysis in which a 300-bp marker band was used as an intensity standard on the electrophoresis gel (data not shown). The supernatant of the induced MDCK/BDV cell culture was shown to contain more BDV-specific RNA than that of the uninduced MDCK/BDV cell culture, though it remains to be clarified whether the BDV-specific RNA is a part of viral RNA or mRNA which coded for BDV p40, reported to be nucleoprotein (11). This increment of BDV-specific RNA might indicate the induced production of the virus. We speculated that there are more BDV particles in the supernatant of induced MDCK/BDV cell cultures than in that of uninduced MDCK/BDV cell cultures. Therefore, we subjected the supernatants from induced MDCK/BDV cell cultures to negative staining.
Detection of virus-like particles in culture supernatant.
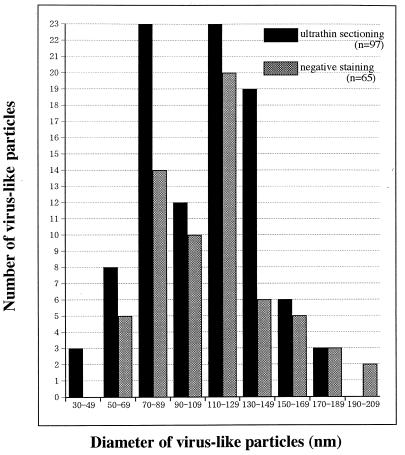
The virus suspensions from induced MDCK/BDV cell cultures were mixed with 0.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2). The mixture was poured on 300-mesh copper grids supported by hydrophilic carbon-coated collodion films and left for 2 min at room temperature; the excess suspension was removed by absorption with filter paper. The grid was stained with 0.5% aqueous uranyl acetate solution for 1 min and observed under an electron microscope (H-300; Hitachi, Tokyo, Japan) at an accelerating voltage of 75 kV. In the suspension from the induced MDCK/BDV cell culture, many spherical virus-like particles were observed by negative staining (data not shown). Figure 1 shows the frequency distribution graph for the diameters of the particles, indicating a bimodal distribution, with two peaks in the ranges of 70 to 89 nm and 110 to 129 nm with wide tails. The bimodal frequency distribution may suggest the existence of two kinds of particles in the supernatant, which has also been reported previously by Zimmermann et al. (37). To visualize the fine structure and to confirm whether these particles were BDV, we examined induced MDCK/BDV cells by ultrathin sectioning and immunoelectron microscopy using anti-BDV p40 serum.
FIG. 1.
Distribution of the diameters of virus-like particles observed by negative staining and ultrathin sectioning.
Visualization of the virus.
Cells were fixed with 0.2% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2) for 3 min at room temperature and collected with a plastic scraper. The cells were suspended in 2% glutaraldehyde in the same buffer at 4°C for 60 min and washed five times with the buffer. The fixed cells were further fixed with 1% osmium tetroxide (OsO4) in the same buffer at 4°C for 60 min, washed with the buffer, dehydrated in a graded ethanol series, and embedded in epoxy resin. Ultrathin sections were made with an ultramicrotome (Porter-Blum MT-5000; Sorvall, DuPont Medical Products, Wilmington, Del.), stained doubly with uranyl acetate and lead citrate, and observed under an electron microscope (H-7100; Hitachi) at an accelerating voltage of 100 kV. For immunoelectron microscopy, the collected cells were fixed in 1% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2) at 4°C for 60 min, dehydrated in a graded ethanol series, and embedded in Lowicryl K4M resin. Ultrathin sections were made in the same manner as that described above and mounted on 300-mesh nickel grids supported by carbon-coated collodion films. The ultrathin sections on the grids were treated with 5% normal goat serum in phosphate-buffered saline to block nonspecific reactions. The sections were reacted with a drop of rabbit antiserum against recombinant BDV p40 (33) at room temperature for 60 min and washed with 50 mM Tris-HCl buffer (pH 8.2). The sections were then reacted again with anti-rabbit immunoglobulin G goat serum which had been labeled with 10-nm-diameter colloidal gold (Amersham, Little Chalfont, England) at room temperature for 60 min and were then washed with the same buffer. The immunostained sections were fixed with 1% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2) at room temperature, washed with distilled water, immersed in a mixture of 0.01% ruthenium red and 0.5% OsO4 in 0.05 M cacodylate buffer (20), washed with distilled water, doubly stained with uranyl acetate and lead citrate, and observed under an electron microscope.
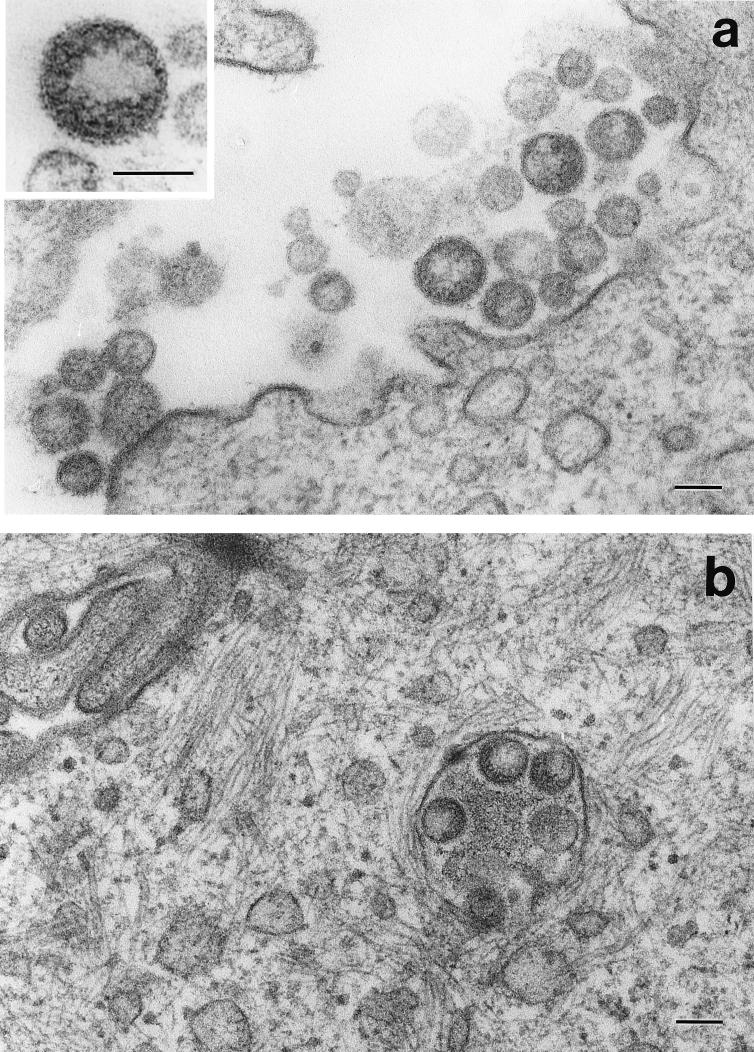
Ultrathin sections of the induced MDCK/BDV cells embedded in epoxy resin were examined to determine the fine structure and morphogenesis of the particles. The virus-like particles were located in clusters in the vicinity of the surfaces of the induced MDCK/BDV cells (Fig. 2a). Particles were rarely observed in cytoplasmic vacuoles (Fig. 2b). The diameters of the particles, including those observed by negative staining, were distributed bimodally and ranged from 50 to 190 nm (Fig. 1). Each particle was covered by an envelope which possessed spikes approximately 7.0 ± 0.7 nm long (mean ± standard deviation) (Fig. 2a, inset). In the particle, we observed a crescent-shaped area which contained some electron-dense granules (Fig. 2a, inset).
FIG. 2.
Ultrathin section of induced MDCK/BDV cells embedded in epoxy resin. (a) Extracellular virus-like particles. (Inset) Extracellular virus-like particles at a high magnification. (b) Virus-like particles in cytoplasmic vacuoles. Bars, 100 nm.
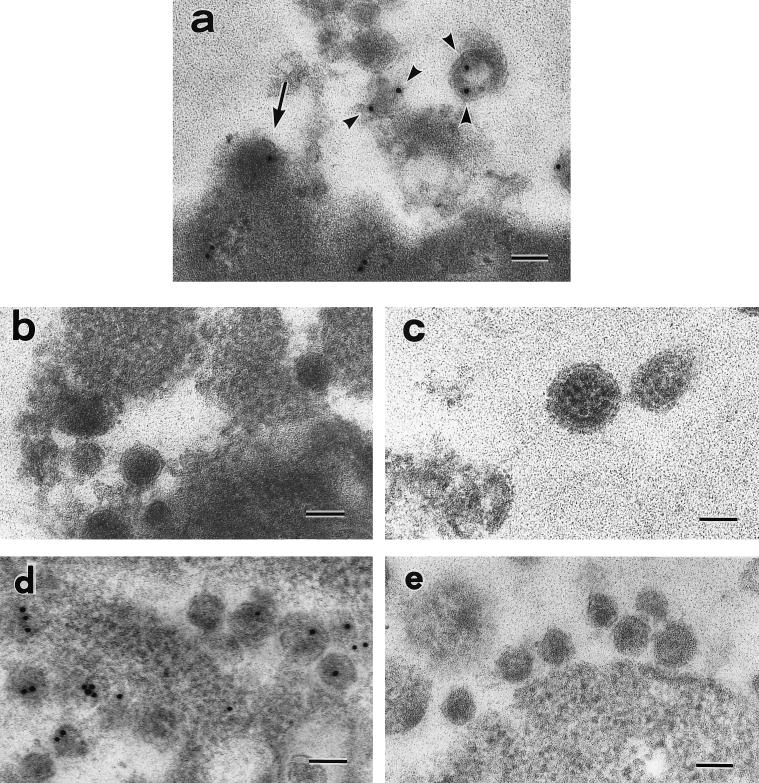
Virus-like particles embedded in Lowicryl K4M resin were also examined by immunoelectron microscopy to determine whether they contained BDV antigens. The enveloped virus-like particles in the vicinity of the induced MDCK/BDV cells reacted with anti-BDV p40 antibody (Fig. 3a); large particles ranging from 90 to 130 nm in diameter were labeled with anti-BDV p40 antibody, but small particles, less than 90 nm in diameter, were not labeled (Fig. 3a). Immunoelectron microscopic labeling of induced MDCK/BDV cells with normal rabbit serum (Fig. 3b) and MDCK/HVJ cells with anti-BDV p40 antibody (Fig. 3c) did not reveal any positive results. The labeling of anti-BDV p40 antibody was mainly localized in electron-dense areas in the particles (Fig. 3a). These areas in some particles were crescent shaped or they lined the envelope, as observed in the preparations embedded in epoxy resin. This immunolabeling procedure was also confirmed for HIV-1-infected cells with a polyclonal antibody for HIV-1 RT. HIV-1 particles reacted with anti-HIV-1 RT rabbit antibody when the same procedure was used (Fig. 3d) but did not react with normal rabbit serum (Fig. 3e).
FIG. 3.
Antibody reactivity of BDV-, HVJ-, and HIV-infected cells as detected by immunoelectron microscopy. (a) Induced MDCK/BDV cells reacted with anti-BDV p40 antiserum. The arrow shows the budding of BDV, and the arrowheads show particles that have reacted with the antiserum. (b) Induced MDCK/BDV cells did not react with normal rabbit serum. (c) MDCK/HVJ cells did not react with anti-BDV p40 antiserum. (d) Molt-4/HIV-1 cells reacted with anti-HIV-1 RT rabbit serum. (e) Molt-4/HIV-1 cells did not react with normal rabbit serum. Bars, 100 nm.
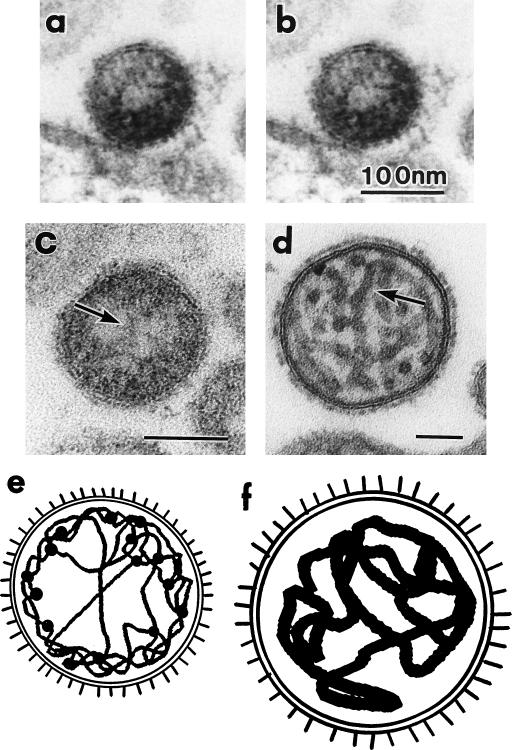
To confirm whether the granules existed inside the particles or in the envelope spikes, sections were tilted and examined, and stereoscopic observation of electron micrographs (Fig. 4a and b) revealed that most granules were present inside the particles. We compared the inner structures of BDV and HVJ (Fig. 4c and d). The nucleocapsids of HVJ were approximately 20.8 ± 2.9 nm in width (Fig. 4d and f), while those of BDV were approximately 4.0 ± 1.0 nm (Fig. 4c and e). The total lengths of the nucleocapsids could not be determined in these sections.
FIG. 4.
Inner structures of BDV and HVJ. Stereoscopic observation of electron micrographs of virus-like particles observed in ultrathin sections of induced MDCK/BDV cells embedded in epoxy resin. The ultrathin sections were observed at tilts of +5° (a) and −5° (b). (c) Ultrathin section of BDV particles, with an arrow showing the thin nucleocapsid. (d) Ultrathin section of HVJ particles, with an arrow showing the thick nucleocapsid. (e and f) Schematic diagrams of BDV and HVJ, respectively.
The electron-dense granules in the BDV particle (Fig. 2a, inset) were approximately 5.9 ± 2.8 nm in diameter, which was not significantly different from the width of BDV nucleocapsids (P = 0.056). However, the electron-dense granules were a little greater in mean size than the nucleocapsid. This increment in the granules may depend on the attachments of some associated proteins, such as phosphoproteins or L proteins, which are suggested by the nucleic acid sequences (8, 11). Thin nucleocapsids probably tend to gather in the peripheral region of the virion because the nucleocapsid did not fill the entire space of the virion, unlike that of HVJ. Therefore, the centers of most BDV particles appear to be less electron dense. Elucidation of the detailed structure of the nucleocapsid of BDV must await the isolation of the nucleocapsids and information on the three-dimensional volume and shape of nucleoproteins (p40 proteins).
Morphogenesis of BDV.
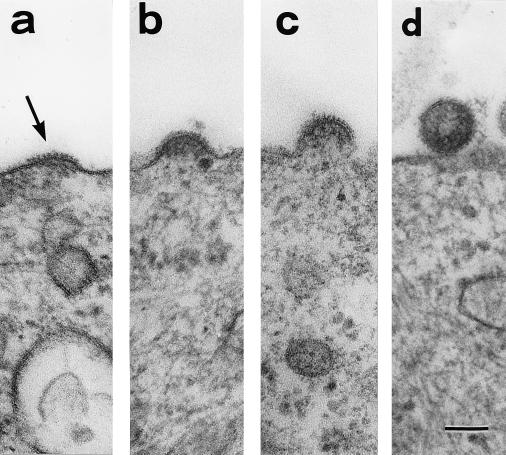
Spike-like structures were found on parts of the surfaces of induced MDCK/BDV cells (Fig. 5a). The spiked membrane area was first seen to be expanding, and the electron-dense fibrils were in line with the area (Fig. 5b). The spiked membrane then grew into a hemisphere, at which time the electron-dense granules were seen beneath the membrane (Fig. 5c). Finally, the spiked membrane closed and became a separate particle. The particle in Fig. 5d had probably just been released from the cell surface. These findings suggest that the virus-like particles reproduce by budding at the cell surface. It was confirmed by immunoelectron microscopy that the buds react with the anti-BDV p40 antibody (Fig. 3a).
FIG. 5.
Sequential images of the budding process in induced MDCK/BDV cells. The spiked membrane area (arrow in panel a) becomes an extracellular particle (d). (b and c) Intermediate stages of the budding. Bar, 100 nm.
Conclusions.
In the present study, we identified many extracellular particles in the supernatants of induced MDCK/BDV cell cultures according to the method of Pauli and Ludwig (26). The particles were confirmed to be BDV by immunoelectron microscopy. Zimmermann et al. (37) previously identified BDV as enveloped particles in two sizes, 50 to 60 nm and 90 nm in diameter, by negative staining after induction of a BDV-infected human oligodendroglial cell line with high concentrations of salt. They also demonstrated that both the large and small particles carried BDV antigens, by immunoelectron microscopic negative staining with antisera against BDV surface antigens. In this study, we used antiserum against BDV inner antigens (p40) and found that the large particles carried them but the small particles did not. Danner and Mayr (13) reported that infectious BDV did not pass through a membrane with a pore size of less than 80 nm, showing that the small particles are probably a noninfectious incomplete form of BDV. However, it remains to be clarified whether the small particles are defective or immature, as described by Zimmermann et al. (37). Compans et al. (9) found intracytoplasmic virus-like particles in MDCK/BDV cells. We also identified such particles in the cytoplasm (data not shown). However, we have not yet observed any interactions, such as membrane fusion, between the cytoplasmic particles and the cell membrane or any intracytoplasmic particles attached to the cell surface. The pathway of the viral constituents from the nucleus to the surface of the cell membrane could not be determined in the present study.
The order Mononegavirales, to which the family Bornaviridae belongs, also includes Rhabdoviridae, Filoviridae, and Paramyxoviridae. Rhabdoviridae and Filoviridae are filamentous and Paramyxoviridae are spherical. Molecular biological analyses suggest that BDV resembles rhabdovirus (11, 14). However, on the basis of particle shape, BDV is probably more similar to paramyxovirus than to rhabdovirus or filovirus because BDV is spherical. With regard to the inner structure of the virion of BDV, the nucleocapsids are observed to be very thin (approximately 4 nm) compared with those of HVJ (approximately 21 nm). The subunits of nucleocapsids in HVJ were estimated to be 5 nm by helical-pitch analysis (17). Therefore, we may only see the nucleocapsid as a fibril of RNA, or probably the p40 nucleoproteins do not always cover the whole RNA genome. Such features will be clarified when we can directly observe BDV nucleocapsids isolated from a large number of extracellular viruses.
Consequently, the characteristics of the particles that were observed are as follows: (i) they are about 100 to 130 nm in diameter, (ii) they are covered by an envelope with approximately 7-nm-long spikes, (iii) they have a crescent-like inner structure with a nucleocapsid approximately 4 nm in width, (iv) they reproduce by budding at the cell surface, and (v) they are associated with incomplete small particles.
Acknowledgments
We thank R. Rott, Justus-Liebig-Universität Giessen, for supplying the MDCK/BDV cell line; Juan Carlos de la Torre, The Scripps Research Institute, La Jolla, Calif., for supplying valuable antibody; and Luc Montagnier, Institut Pasteur, Paris, France, for supplying HIV strain BRU. We also thank Yoshihiko Fujioka and Akie Hanada for expert technical assistance.
REFERENCES
- 1.Bode L, Riegel S, Ludwig H, Amsterdam J D, Lange W, Koprowski H. Borna disease virus-specific antibodies in patients with HIV infection and with mental disorders. Lancet. 1988;ii:689. doi: 10.1016/s0140-6736(88)90505-3. [DOI] [PubMed] [Google Scholar]
- 2.Bode L, Riegel S, Lange W, Ludwig H. Human infections with Borna disease virus: seroprevalence in patients with chronic diseases and healthy individuals. J Med Virol. 1992;36:309–315. doi: 10.1002/jmv.1890360414. [DOI] [PubMed] [Google Scholar]
- 3.Bode L, Ferszt R, Czech G. Borna disease virus infection and affective disorders in man. Arch Virol Suppl. 1993;7:159–167. doi: 10.1007/978-3-7091-9300-6_13. [DOI] [PubMed] [Google Scholar]
- 4.Bode L, Durrwald R, Ludwig H. Borna virus infections in cattle associated with fatal neurological disease. Vet Rec. 1994;135:283–284. doi: 10.1136/vr.135.12.283. [DOI] [PubMed] [Google Scholar]
- 5.Bode L, Steinbach F, Ludwig H. A novel marker for Borna disease virus infection. Lancet. 1994;343:297–298. doi: 10.1016/s0140-6736(94)91147-9. [DOI] [PubMed] [Google Scholar]
- 6.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 7.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of the infected cell. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briese T, Schneemann A, Lewis A, Park Y-S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compans R W, Melsen L R, de la Torre J C. Virus-like particles in MDCK cells persistently infected with Borna disease virus. Virus Res. 1994;33:261–268. doi: 10.1016/0168-1702(94)90107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubitt B, Oldstone C, Valcarcel J, de la Torre J C. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a non-segmented negative strand RNA virus. Virus Res. 1994;34:69–79. doi: 10.1016/0168-1702(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 13.Danner K, Mayr A. In vitro studies on Borna virus. II. Properties of the virus. Arch Virol. 1979;61:261–271. doi: 10.1007/BF01315012. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Dunia D, Cubitt B, Grasser F A, de la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 17.Hosaka Y, Hosoi J. Study of negatively stained images of Sendai virus nucleocapsids using minimum-dose system. J Ultrastruct Res. 1983;84:140–150. doi: 10.1016/s0022-5320(83)90125-9. [DOI] [PubMed] [Google Scholar]
- 18.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 19.Kishi M, Arimura Y, Ikuta K, Shoya Y, Lai P K, Kakinuma M. Sequence variability of Borna disease virus open reading frame II found in human peripheral blood mononuclear cells. J Virol. 1996;70:635–640. doi: 10.1128/jvi.70.1.635-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno T, Fujioka Y, Goto T, Morimatsu S, Morita C, Nakano T, Sano K. Contrast-enhancement for the image of human immunodeficiency virus from ultrathin section by immunoelectron microscopy. J Virol Methods. 1998;72:137–143. doi: 10.1016/s0166-0934(98)00022-6. [DOI] [PubMed] [Google Scholar]
- 21.Lipkin W I, Briese T, de la Torre J C. Borna disease virus: molecular analysis of a neurotropic infectious agent. Microb Pathog. 1992;13:167–170. doi: 10.1016/0882-4010(92)90017-i. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig H, Becht H. Borna disease—a summary of our present knowledge. In: ter Meulen V, Katz M, editors. Slow virus infections of the central nervous system. Investigational approaches to etiology and pathogenesis of these diseases. New York, N.Y: Springer-Verlag; 1977. pp. 75–83. [Google Scholar]
- 23.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 24.Lundgren A L, Czech G, Bode L, Ludwig H. Natural Borna disease in domestic animals other than horses and sheep. J Vet Med. 1993;40:298–303. doi: 10.1111/j.1439-0450.1993.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 25.Malkinson M, Weisman Y, Ashash E, Bode L, Ludwig H. Borna disease in ostriches. Vet Rec. 1993;133:304. doi: 10.1136/vr.133.12.304-b. [DOI] [PubMed] [Google Scholar]
- 26.Pauli G, Ludwig H. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 1985;2:29–33. doi: 10.1016/0168-1702(85)90057-7. [DOI] [PubMed] [Google Scholar]
- 27.Pringle C R. The universal system of virus taxonomy of the International Committee on Virus Taxonomy (ICTV), including new proposals ratified since publication of the sixth ICTV report in 1995. Arch Virol. 1998;143:203–209. doi: 10.1007/s007050050280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richt J A, Vande Woude S, Zink M C, Clements J E, Herzog S, Stitz L, Rott R, Narayan O. Infection with Borna disease virus: molecular and immunobiological characterization of the agent. Clin Infect Dis. 1992;14:1240–1250. doi: 10.1093/clinids/14.6.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richt J A, Clements J E, Herzog S, Pyper J, Wahn K, Becht H. Analysis of virus-specific RNA species and proteins in Freon-113 preparations of the Borna disease virus. Med Microbiol Immunol. 1993;182:271–280. doi: 10.1007/BF00579625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richt J A, Herzog S, Pyper J, Clements J E, Narayan O, Bechter K, Rott R. Borna disease virus: nature of the etiologic agent and significance of infection in man. Arch Virol Suppl. 1993;7:101–109. doi: 10.1007/978-3-7091-9300-6_9. [DOI] [PubMed] [Google Scholar]
- 31.Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 32.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I the Bornavirus Study Group. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 33.Sauder C, Muller A, Cubitt B, Mayer J, Steinmetz J, Trabert W, Ziegler B, Wanke K, Lantzsch N M, de la Torre J C, Grasser F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 35.Schneider P A, Schneemann A, Lipkin W I. RNA splicing in Borna disease virus, a nonsegmented, negative-strand RNA virus. J Virol. 1994;68:5007–5012. doi: 10.1128/jvi.68.8.5007-5012.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sierra-Honigmann A M, Rubin S A, Estafanous M G, Yolken R H, Carbone K M. Borna disease virus in peripheral blood mononuclear and bone marrow cells of neonatally and chronically infected rats. J Neuroimmunol. 1993;45:31–36. doi: 10.1016/0165-5728(93)90160-z. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann W, Breter H, Rudolph M, Ludwig H. Borna disease virus: immunoelectron microscopic characterization of cell-free virus and further information about the genome. J Virol. 1994;68:6755–6758. doi: 10.1128/jvi.68.10.6755-6758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]