Summary
Background
Vaginal dysbiosis covers imbalances in the vaginal microbiota, defined by altered composition of bacteria, viruses, and fungi and is associated with euploid pregnancy losses, premature birth, infertility, or bacterial vaginosis. A large proportion of women who have vaginal dysbiosis do not experience any symptoms. Antibiotics are the traditional treatment, recently combined with local probiotics in some cases. Vaginal Microbiota Transplantation (VMT) with eubiotic vaginal bacterial microbiota after antibiotic eradication of pathogens has successfully been performed in a case study with five patients, but no VMT has been performed without the use of antibiotics.
Methods
This is a proof of concept case study. The patient was found to have vaginal dysbiosis at the RPL clinic at Copenhagen University Hospital Hvidovre, Denmark on the 23rd of June 2021. She was offered and accepted to receive experimental treatment in the form of a VMT as a compassionate use case. VMT is the transfer of cervicovaginal secretions (CVS) from a healthy donor with a Lactobacillus-dominant vaginal microbiome to a recipient with a dysbiotic vaginal microbiome. CVS is a mixture of e.g., mucus, bacteria, metabolites present in the vaginal canal. Potential donors were thoroughly screened for the absence of STIs, and the most suitable donor sample for the specific patient in this study was determined via an in vitro microbiome competition assay.
Findings
A 30-year-old patient with one livebirth and a complicated pregnancy history of two stillbirths and 1 s trimester pregnancy loss in gestational weeks 27 (2019), 17 (2020) and 23 (2020) respectively with complaints of vaginal irritation and discharge that had aggravated in all her pregnancies. Her vaginal microbiome composition showed a 90% dominance of Gardnerella spp. After one VMT there was a complete shift in microbiome composition to 81.2% L. crispatus and 9% L. jensenii with a concurrent resolvement of vaginal symptoms. Single nucleotide polymorphism-analysis confirmed her microbiome to be of donor origin and it remain stable now 1.5 years after the VMT. Five months after the VMT she became pregnant and has successfully delivered a healthy baby at term.
Interpretation
Here we report a successful VMT with confirmed donor strain engraftment followed by a successful pregnancy and delivery after a series of late pregnancy losses/stillbirths. Findings suggest that VMT is a potential treatment for severe vaginal dysbiosis. Further, larger studies are required.
Funding
The study was partially funded (i.e., analysis costs) by Freya Biosciences Aps, Fruebjergvej, 2100 Copenhagen, Denmark.
Keywords: Vaginal microbiota transplantation, VMT, Vaginal microbiome, Shotgun metagenomics, Single nucleotide variant, Recurrent pregnancy loss, Miscarriage
Research in context.
Evidence before this study
A search of PubMed, MEDLINE, Embase, Google, Google Scholar and the Cochrane database was performed in February 2021 and again in May 2023. Search terms included “vaginal microbiota transplantation OR vaginal microbiome transplant”. Only one study from 2019 was found where Vaginal Microbiota Transplant (VMT) was performed in humans, with a pretreatment of antibiotics. The study reports 5 women with recurrent Bacterial Vaginosis, who had a pretreatment with either clindamycin or metronidazole followed by one or more VMT’s. Four out of five patients converted their vaginal microbiome from dysbiotic to eubiotic, but with no proof of donor microbiome engraftment. While the concept of VMT has been suggested and discussed in existing literature, limited evidence is available about the actual procedure. Here we provide valuable biological data regarding the potential benefits of VMT.
Added value of this study
Through this research, we have generated novel findings suggesting the potential of VMT as a treatment for both vaginal dysbiosis and pregnancy loss. Our study involved the application of VMT for the treatment of vaginal dysbiosis, without prior antibiotic pretreatment. The results demonstrate a complete shift in the composition of the microbiome, symptom relief, and documented donor strain engraftment.
Implications of all the available evidence
Supported by the results of this study, VMT without the use of antibiotics suggests a potential treatment for vaginal dysbiosis and pregnancy loss. Further studies are necessary.
Introduction
A healthy vaginal microbiome is typically dominated by a single or a few species of vaginal lactobacilli (L. crispatus, L. gasseri, L. iners, or L. jensenii)1, 2, 3 in contrast to other parts of the body where a healthy microbial community typically contains a much higher species diversity. The production of lactic acid by these lactobacilli lowers the pH, to values below pH 4.5, and this acidification of the vaginal milieu benefits the individual.4 A more diverse vaginal microbiome dominated by other bacteria such as Bifidobacterium vaginale (formerly Gardnerella vaginalis), Fannyhessea vaginae (formerly Atopobium vaginae), Gardnerella spp. and Prevotella spp. is described as vaginal dysbiosis (VD).1, 2, 3 VD is shown to occur in 18–29% of women5,6 and the condition is strongly associated with a lower chance of conceiving during In Vitro Fertilization (IVF), a higher risk of euploid pregnancy loss, and obstetric complications such as premature rupture of membranes (PPROM) and preterm birth.7, 8, 9, 10 Many women with VD report no vaginal symptoms such as odor or discharge and appear to be otherwise healthy even after a gynecological evaluation.11
Traditionally, symptomatic VD is treated with orally or vaginally administered antibiotics, such as metronidazole or clindamycin, with a cure rate of 80–90% one month after treatment.1,12,13 However, antibiotic treatment for symptomatic VD has some major drawbacks, including a recurrence rate of up to 60% within 12 months after treatment12 and a potential risk for the development of antibiotic resistance in vaginosis-related bacteria.14, 15, 16 Further, orally administered antibiotics may cause a disturbance in the gut microbiome,17 and local application of antibiotics often leads to subsequent vulvovaginal candidiasis.18 To minimize risks associated with antibiotic treatment of VD, a study from 2019 reported 5 women with recurrent VD who were treated with Vaginal Microbiota Transplantation (VMT) from healthy donors following an antibiotic regimen. The results were quite encouraging, showing that four women experienced long-term clinical remission after up to three combined antibiotic and VMT treatments.19
This is a report of a 30-year-old woman with recurrent VD for nine years and with significant clinical VD symptoms and a history of three late pregnancy losses after one successful pregnancy. As proof-of-concept, we outline the process of the first successful VMT without antibiotic pretreatment.
Methods
Patient
The patient was a 30-year-old woman with four previous pregnancies; the first uncomplicated pregnancy resulted in a liveborn boy (2017) followed by a complicated pregnancy history including two stillbirths and 1 s trimester pregnancy loss. She was referred to the Recurrent Pregnancy Loss Clinic at Copenhagen University Hospital Hvidovre, Denmark on the 23rd of June 2021. The first stillbirth in week 27 (2019) was due to placental abruption. The autopsy was reported as normal. The second pregnancy ended in week 17 (2020) and was due to cervical insufficiency. Therefore, the patient received a prophylactic cervical cerclage in gestational week 13 of the next pregnancy. Unfortunately, that pregnancy ended due to a PPROM with no clinical sign of infection and resulted in a stillbirth at gestational week 23 (2020). Following the first stillbirth, the patient was screened for antiphospholipid syndrome (APLS) and thrombophilia with negative results (Table 1). During all prior pregnancies, the patient reported suffering from VD symptoms described as heavy vaginal discharge with yellow/green color, smelly odor, itching and pain. She reported repeated treatments with antifungal medication followed by no effect at all or a recurrence in symptoms after a few weeks. The patient was diagnosed with APLS based on her clinical history and confirmed increased values of beta-2-glycoprotein 1-IgG in August 2021 despite negative results in 2019 after the stillbirth. Due to persisting vaginal symptoms, a vaginal swab was taken and analyzed with shotgun sequencing, confirmed the VD. Results of the Recurrent Pregnancy Loss (RPL) evaluation in 2021 are shown in Table 1. Taken together, these data indicated that an improved pregnancy outcome may be achieved through an intervention to replace the dysbiotic vaginal microbiota with a Lactobacillus-dominated microbiota. She was offered and accepted a compassionate use (CU) VMT as an experimental treatment for her VD. She signed an informed consent with Freya Biosciences Aps (Fruebjergvej 3, Copenhagen, Denmark) and the Department of Gynecology, Copenhagen University Hospital Hvidovre, Copenhagen, Denmark. Freya Biosciences Aps performed the donor cervicovaginal secretion (CVS) collection and the selection assay, and then provided the donor CVS material for the VMT procedure (see Supplementary Materials and Methods and Supplementary Table S1).
Table 1.
Results of the recurrent pregnancy loss (RPL) evaluation according to standardized guidelines (ESHRE-guideline).
| Normal/reference values | August 2019 | May 2021 | August 2021 | |
|---|---|---|---|---|
| Hematology | ||||
| Homocysteine | <15 umol/L | n/a | 17.8 umol/L | |
| Coagulation factor II + VII + X | <1.2 | <1.0 | 0.9 | |
| Antithrombin | 0.83–1.15 IU/L | 1.07 IU/L | 1.14 IU/L | |
| Lupus anticoagulant | 0 | 1.14 | 0 | |
| Protein C | 0.7–1.3 IU/L | 1.27 IU/L | 1.34 IU/L | |
| Protein S | 0.57–1.3 IU/L | 1.04 IU/L | 1.06 IU/L | |
| F2 mutation | Neg. | Wild type | Wild type | |
| FV Leiden mutation | Neg. | Wild type | Wild type | |
| Metabolism | ||||
| HbA1C | <48 mmol/mol | n/a | 35 mmol/mol | |
| Glucose | <7.7 mol/L | n/a | 5.8 mol/L | |
| Cholesterol HDL | >1 mmol/L | n/a | 1.5 mmol/L | |
| Cholesterol LDL | <3 mmol/L | n/a | 3.4 mmol/L | |
| Cholesterol VLDL | <0.9 mmol/L | n/a | 0.8 mmol/L | |
| Triglyceride | <2 mmol/L | n/a | 1.7 mmol/L | |
| Endocrinology | ||||
| Thyrotropin (TSH) | 0.4–4.8 IU/L | n/a | 1.85 IU/L | |
| Thyroxin (T4) | 12–22 IU/L | n/a | 15.8 IU/L | |
| Thyroid peroxidase-Ab | <35 IU/L | n/a | 159 IU/L | |
| Autoantibodies | ||||
| ANA | <0.7 | n/a | 0.2 | |
| PCNA-antibody | Neg. | n/a | Neg. | |
| CHD-4-antibody-IgG [Mi-2] | Neg. | n/a | Neg. | |
| Anti-dsDNA-IgG | Neg. | n/a | Neg. | |
| Anti-DNA topoisomerase 1-IgG | Neg. | n/a | Neg. | |
| Anti-EXOSC10-IgG | Neg. | n/a | Neg. | |
| Anti-fibrillarin-IgG | Neg. | n/a | Neg. | |
| Jo1-antibody-IgG | Neg. | n/a | Neg. | |
| Anti-major centromere B-IgG | Neg. | n/a | Neg. | |
| Anti-ribosomal protein-IgG | Neg. | n/a | Neg. | |
| RNA pol III RPC1-antibody-IgG | Neg | n/a | Neg | |
| Sjøgren syndrome [SSA]-IgG | Neg. | n/a | Neg. | |
| Sjøgren syndrome [SSB]-IgG | Neg. | n/a | Neg. | |
| Smiths-antibody-IgG | Neg. | n/a | Neg. | |
| U1 snRNP (70 kDa + A + C)-antibody-IgG | Neg. | n/a | Neg. | |
| Transglutaminase antibody-IgA [tTG] | n/a | <1 kU/L | ||
| Thyroglobulin-antibody | n/a | 85 units/L | ||
| Anti-beta-2-glycoprotein 1-IgM | <20 kU/La | n/a | <0.9 kU/L | |
| Anti-beta-2-glycoprotein 1-IgG | <20 kU/La | 12 kU/L | 34 kU/L | 31 kU/L |
| Cardiolipin antibody-IgG | <20 kU/La | <10 UI/L | 8.2 UI/L | |
| Cardiolipin antibody-IgM | <20 kU/La | <10 UI/L | 3.8 UI/L |
The patient was tested negative for antiphospholipid syndrome (APLS) after the first stillbirth in 2019. She was confirmed APLS positive in August 2021 before her 5th pregnancy. Numbers and letters in bold indicate increased levels.
According to David Garcia, MD, et al. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med 2018; 378:2010–21. https://doi.org/10.1056/NEJMra1705454.
Patient vaginal microbiome collection
Timepoints for vaginal microbiome sampling pre- and post-VMT are illustrated in Fig. 1. For clinical and home-sampling, Zymo Collection Swab, 80XX (Zymo research, California, US) swabs were used and stored in COPAN (Brescia, Italy) containers.
Fig. 1.
Overview of microbiome composition pre- and post-pregnancy. The first Vaginal Microbiota Transplantation (VMT) was administered in September 2021 and resulted in rapid resolution of dysbiosis and Lactobacillus crispatus-dominance (pre-pregnancy period from days 7–153 after VMT). In February 2022 the patient became pregnant. In gestational week six, the microbiome analysis revealed 41.8% of Gardnerella spp., at which time a second VMT with CVS from the same donor was planned two weeks later. Analysis of the vaginal sample taken on the day of the 2nd VMT (+224 days post-VMT, marked in bold letters) revealed that the patient had converted back to a L. crispatus-dominance already prior to the 2nd VMT.
Patient CVS sample processing and pH measurement
CVS samples were obtained through self-collection using a menstrual cup (Softdisc menstrual cup by The Flex Company, also sold under the name ‘Flex disc’) after thorough instructions. The patient wore sterile gloves to insert the menstrual cup longitudinally into the vagina, left it in place for 10 s, and then twisted the cup while removing it. The patient then placed the CVS-coated menstrual cup into a 50 mL Falcon tube (Corning, US) which was then further processed immediately. The 50 mL tube was centrifuged (Eppendorf Centrifuge 5810 R) for 5 min at 190g at 4 °C to collect the CVS. The menstrual cup was then removed, and the 50 mL tube was re-weighed. 2.5 μL of sterile saline was added per mg sample. The diluted sample was aliquoted into cryovials for biobanking and DNA extraction. An amount of 150 μL of diluted sample was placed in an Eppendorf tube for pH measurement and set aside at room temperature for 15 min. Using a pH meter (VWR pHenomenal pH 1100L with VWR pH electrode pHenomenal MIC 220) the pH was measured three times, and the mean pH was used for further analyses.
Ethical approval
The patient was a compassionate use case and signed an informed consent with both the Department of Obstetrics and Gynaecology, Copenhagen University Hospital - Hvidovre, Kettegaard allé 30, Hvidovre 2650, Denmark and Freya Biosciences Aps, Fruebjergvej, 2100 Copenhagen, Denmark. The setup for donor selection, the VMT product, in this case owned and named FB101 by Freya and is an internal donor program in Freya Biosciences. How to test and apply the product is part of a Randomized Controlled Trial approved by the Danish authorities, the Danish Ethical Committee (Journal-nr: H-20068230) and the Danish Data protection Agency (Pactius): P-2020-1080. The Randomized Controlled Trial is reported on ClinicalTrials.gov Identifier: NCT04855006.
Donor screening and donation setup
The donor was screened as part of a larger program at Freya Biosciences to identify women suitable for donating CVS for VMT. In brief, a vaginal sample was shotgun-sequenced (at Seqbiome, Cork, IE) to determine the microbiome composition following the methods described above. Individuals with >80% relative abundance of vaginal Lactobacillus spp. (i.e., L. crispatus, L. jensenii, L. gasseri, L. iners) and <5% selected vaginal dysbiosis-associated bacteria (i.e., Atopobium spp., Fannyhessea vaginae, Bifidobacterium vaginalis, Prevotella spp.) were subjected to a medical and gynecological exam, including vaginal inspection and a vaginal ultrasound (at TFP Stork fertility clinic, Copenhagen, DK), and tested for the following pathogens and conditions using standard diagnostic tests: Human immunodeficiency virus (HIV p24 antigen and/or antibody against HIV 1/2), Hepatitis B virus s-antigen, Hepatitis B virus c-antibody (total), Hepatitis B surface antibody, Hepatitis C virus antibody, cytomegalovirus (CMV) antibody (IgG and IgM) and Treponema pallidum antibody, human papillomavirus (HPV 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 66, 68, 69, 70, 73, 82), herpes simplex virus (HSV) 1 and 2 TMA amplified mRNA test, culture urinary tract infections, Chlamydia trachomatis, Neisseria gonorrhoeae DNA/RNA PCR, Trichomonas vaginalis culture, Mycoplasma genitalium DNA/RNA PCR, Streptococcus group A, B, C, and G culture and Candida spp. culture. The medical examination included a general health check including medical history and medication usage, demographics, heart rate and blood pressure measurements. Individuals that met the vaginal microbiome criteria mentioned above, had no positive pathogen test, and had no abnormal findings in the medical and gynecological exam were considered suitable donors. The approved donor then donated samples over a donation period of maximally 40 days, during which the following restrictions applied: abstinence from vaginal and anal sexual intercourse; no swimming; no use of intravaginal products (e.g., tampons, soap). At each visit, the donor filled out a questionnaire to affirm adherence to these restrictions together with general health questions. After the donation period, the same tests as in the initial screening were performed again, and the donor was fully approved when passing all. The donor samples were self-collected similar to patient CVS samples. The donor gave a total of 15 donations. Two of the CVS-samples were used in this study (see Supplementary Materials and Methods and Supplementary Table S1 for details on samples and sample processing, as well as on the in vitro assay for donor selection).
Vaginal microbiome analysis/extraction
One mL of each vaginal swab sample or 150 μL CVS was used for DNA extraction with MolYsis™ Complete5 DNA extraction kit (Cat# D-321-100; Molzym GmbH & Co. KG, Bremen, Germany). Samples were processed according to manufacturer’s instruction with following alterations: lysis of bacteria in a thermoshaker at 250 rpm for 30 min and 37 °C (β-mercaptoethanol not added), followed by Proteinase K treatment in a heating block at 56 °C for 10 min (samples were shortly vortexed and placed back after 3 min and 7 min). Eluted DNA was stored at 5 °C for a maximum of 24 h before initiation of library preparation. DNA libraries were prepared using the Nextera XT DNA Sample Preparation Kit (Illumina) according to the manufacturer’s instructions and sequenced on a MiSeq (Illumina Inc., San Diego, USA) using 2 × 150 bp paired-end reads.
Microbiome bioinformatics
The quality of the fastqc reads before and after quality trimming was assessed with FastQC v. 0.11.8.20 Reads were quality trimmed according to qualified_quality_phred of 20 and minimum read length of 50 using fastp v. 0.20.1.21 The reads were depleted of human sequences by aligning the trimmed reads to the human genome (hg38, University of California, Santa Cruz) using bowtie2 v. 2.3.4.122 with end-to-end alignment and a maximum fragment length for valid paired-end alignments (-X) of 2000. Microbial profiling of the human DNA depleted reads was performed with Kraken 2 v. 2.1.223 using the PlusPF database available from https://benlangmead.github.io/aws-indexes/k2 which includes RefSeq genomes of archaea, bacteria, viruses, plasmids, human, UniVec Core, protozoa, and fungi. Species and genus level abundances were estimated from the Kraken 2 reports using Bracken v. 2.6.2. The relative abundance of bacterial species and genera detected was calculated relative to the total number of reads classified as bacterial.
VMT procedure
The VMT product was stored in a cryovial in a −80 °C freezer. The thawing procedure consisted of: 1 h at room temperature followed by 30 min in a 37 °C heating cabin. The VMT product was then drawn up into a 10 mL syringe affixed with a 180 mm Intrauterine insemination catheter (Wallace, 180 mm Catheter, Cooper Surgical, CT, USA). The catheter was then introduced into the vagina (to the fornix) without the use of a speculum and the content of the syringe was applied. To ensure a complete emptying of the catheter, an additional 0.5 mL of air was drawn into the syringe and applied as well.
Single nucleotide variant (SNV)-analysis
Samples at all timepoints where the coverage was high enough to generate SNV profiles before and after the two VMTs, and before and after the patient became pregnant, were used for analysis. L. crispatus SNV profiles were generated using MetaSNV under default settings, using a publicly available NCBI L. crispatus genome to generate the reference database. Reads were first aligned to the database using Bowtie2 and filtered to exclude alignments smaller than 45 base pairs and with less than 97% identity. Filtered reads were then used to generate the SNV profile of each metagenomic sample and Manhattan distances were calculated between profiles.
Role of the funding source
The funder of the study had no role in study design and patient data collection but as specified below participated in donor selection and data analysis. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Pre-VMT
The first patient sample in July 2021 (Pre-Pregnancy, −89 days pre-VMT) showed a 91.3% dominance of Gardnerella spp. and a lack of Lactobacillus spp. (Fig. 1). At that time, the patient reported itching, odor, and heavy amounts of vaginal fluid that was green/yellow in color. On evaluation, irritation of the vaginal mucosa was noted, and the presence of odor and discoloration of the vaginal fluid was confirmed (Table 2). A sample taken shortly before the planned VMT confirmed the microbial dysbiosis, including sustained predominance of Gardnerella spp. (Fig. 1) and the presence of VD-associated symptoms (Table 2).
Table 2.
Overview of symptoms, pH measurements and cervical length (only in pregnancy) measurements before pregnancy and in pregnancy.
| Days pre-/post VMT | Pre VMT |
Post VMT |
Post VMT (pregnancy) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −26 | 0 | +14 | +35 | +68 | +153 | +209 GA: 5 + 2 | +245 GA: 10 + 3 | +273 GA: 15 + 3 | +301 GA: 19 + 2 | +322 GA: 22 + 4 | +367 GA: 25 + 2 | +392 GA: 32 + 2 | December 2022 GA: 37 + 5 | |
| Clinical observation (MD) | ||||||||||||||
| Odor (+/÷) | + | + | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ |
| Color of vaginal fluid | Green/yellow | Yellow | White | White | White | White | White lumps | White | White | White | White | White | White | White |
| Irritated mucous membranes | + | + | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ |
| pH in CVS | 4.25 | 3.87 | 4.5 | 4.26 | 3.83 | 3.78 | 3.81 | 3.74 | 3.86 | 3.86 | 4.49 | |||
| Cervical length (mm) | 50.3 | 40.5 | 46.72 | 39.08 | 30.6 | 48.11 | 44.4 | |||||||
| Patient reported symptoms | ||||||||||||||
| Itching/pain (+/÷) | + | + | ÷ | ÷ | + | ÷ | + | ÷ | ÷ | + | ÷ | ÷ | ÷ | ÷ |
| Odor (+/÷) | + | + | ÷ | ÷ | ÷ | ÷ | + | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ | ÷ |
| Color of vaginal fluid | Green/yellow | Green/yellow | White | White | Transparent | White | White | White | Transparent | White | White | White | White | White |
| Amount of vaginal fluid | Heavy | Heavy | Light | Light | Light | Light | Heavy | Light | Light | Light | Light | Light | Light | Light |
In vitro assay for donor selection
In order to “match” a donor sample with the highest likelihood of successful engraftment in the patient, an in vitro competition assay was performed (See Supplementary Materials and Methods). The aliquots of cervicovaginal secretions (CVS) obtained from four thoroughly screened healthy donors with Lactobacillus-dominated microbiota were evaluated for inhibition of the VD-associated bacteria in the patient CVS using a plate diffusion assay. After five days, the undiluted samples from donors one and two both showed equally large inhibition zones or “halos” around the donor CVS (3 mm diameter), indicating similar inhibition of the VD-associated bacteria (Table 3, Supplementary Figure S1). Subsequently, the donor CVS samples were then also diluted 5-fold prior to plating, which resulted in a slight reduction of the halo for donor one (1.5–2 mm) and a loss of the inhibitory effect for donor two. The CVS from donors three and four were not effective in inhibiting microbial growth at either concentration. Based on these results, CVS obtained from donor 1 was selected for administration to the patient.
Table 3.
Overview of donor sample properties and inhibition zones (halos) in the in vitro assay for donor selection.
| Donor | L. crispatus % | L. gasseri % | L. iners % | L. jensenii % | Other lactobacilli % | Selected vaginal dysbiosis-associated bacteria % | pH | Viable cells or CFU/dose | Halo (mm) un-diluted | Halo (mm) 5× diluted |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 88.7 | <0.05 | 4.0 | 7.2 | <0.05 | 0 | 4.1 | 3.1E08 | 3 | 1.5–2 |
| 2 | 97.6 | 0 | 0.3 | 2.0 | 0.1 | 0 | 3.8 | 9.9E08 | 3 | 0 |
| 3 | 97.3 | 2.2 | 0 | 0.1 | 0.2 | 0 | 4.0 | 3.4E08 | 0 | 0 |
| 4 | 0 | 0 | 99.6 | 0 | 0 | 0.1 | 3.9 | 1.0E11 | 0 | 0 |
The percentages for each species are relative abundances as determined by whole metagenome shotgun sequencing of the donor sample used in the assay. The pH and CFU are the pH and CFU of the donor sample used in the assay. The viable cells or CFU was measured in the fresh sample; see Table 2 for CFU in the stored sample of the selected donor sample.
Transplantation, VMT
A VMT was performed without antibiotic pretreatment on menstrual cycle day ten in September 2021. On the day of VMT, the vaginal microbiome was confirmed to be dysbiotic with 80.4% Gardnerella spp. and 14% F. vaginae (Fig. 1), and the CVS pH was 4.25 (Table 2). The composition of the donor CVS used for VMT was 88.7% L. crispatus, 7.2% L. jensenii and 4% L. iners with a pH of 4.1 (Supplementary Table S1). At 14 days post VMT, the patient’s microbiome had shifted to 81.2% L. crispatus and 9% L. jensenii (Fig. 1) and the CVS pH was 3.87 (Table 2). The patient remained Lactobacillus dominant, mainly driven by L. crispatus at six sampling times during a four-month follow-up period, except for one time 139 days post-VMT where L. crispatus was mixed with L. iners and L. jensenii that were also present in the donor CVS (Fig. 1).
The patient experienced a significant improvement of VD-symptoms with a reduction in amount of vaginal discharge, change of CVS color to transparent/light white, and disappearance of symptoms such as itching and pain only two days post VMT and throughout the following four months after VMT (Table 2).
Pregnancy post VMT
In February 2022, by natural conception, the patient became pregnant. Due to the newly diagnosed antiphospholipid syndrome, she was prescribed acetylsalicylic-acid 75 mg daily from the time of a positive pregnancy test until gestational week 38 + 0, and low-molecular-weight heparin 4500 IE daily from the time of a positive pregnancy test until six weeks postpartum. Throughout her pregnancy, she was examined at the hospital every three-four weeks. A vaginal ultrasound to measure cervical length, a gynecological examination, a vaginal swab for microbiome analysis, CVS collection for pH measurements, and an interview regarding vaginal symptoms were performed during these visits (Table 2).
At one timepoint 209 days post-VMT, at gestational age 5 + 2, the microbiome analysis revealed 41.8% of Gardnerella spp., at which time a second VMT with CVS from the same donor was planned two weeks later. However, analysis of the vaginal sample taken on the day of the 2nd VMT revealed that the patient had in the meantime spontaneously converted back to a L. crispatus-dominance of donor origin (see next section). Fig. 1 shows the stable L. crispatus dominated vaginal microbiome throughout the remainder of her pregnancy.
The cervical length remained stable and above three cm throughout pregnancy and vaginal pH measurements were all well below 4.5 in all samples (Table 2). In contrast to her previous pregnancies, the patient did not suffer from VD-symptoms throughout this pregnancy.
The patient underwent a planned, uncomplicated cesarean section at gestational age 37 + 5 resulting in the livebirth of a boy.
Confirmation of donor engraftment via SNV analysis
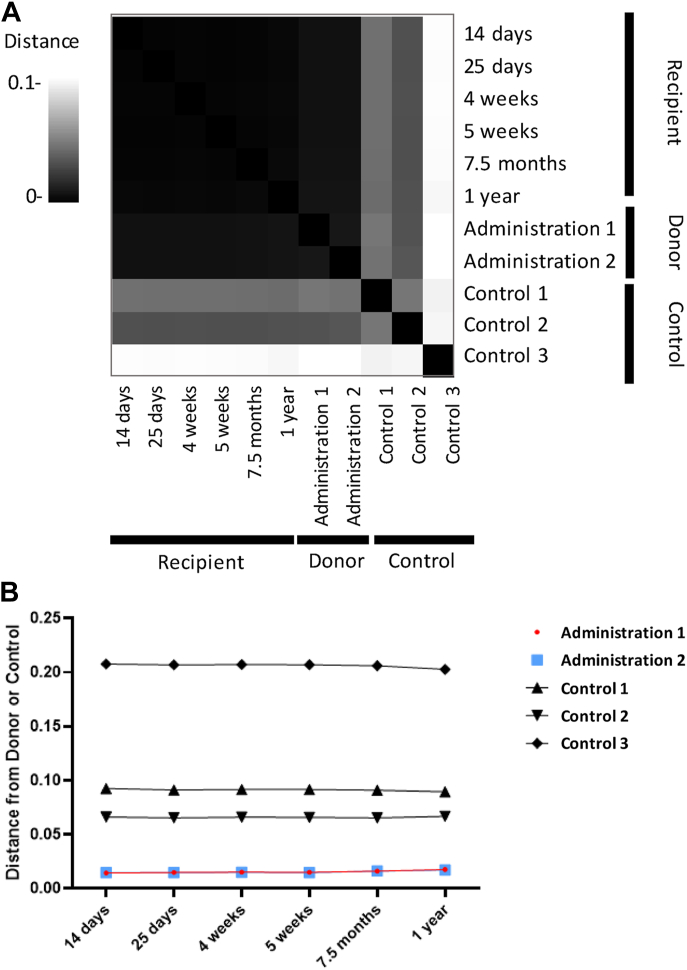
To confirm the engraftment of the donor L. crispatus strain(s), we performed a SNV-analysis to compare the genomic profiles of the L. crispatus sequenced in the CVS used for VMT, CVS obtained from other donors that was not used for VMT (Control 1–3), and in the patient after VMT. The L. crispatus SNV profiles of the patient after VMT were consistently similar to the two CVS samples used for VMT (Donor 1A and Donor 1B) (Fig. 2A and B). The SNV profiles of the donor samples were also very similar to each other, reflecting the stable presence of the L. crispatus used for VMT. Moreover, when compared to the L. crispatus genomes in CVS from other donors that were not used to treat the patient, we show that the L. crispatus in the patient samples after VMT were more similar to the VMT donor than to other healthy controls. This strongly supports that the L. crispatus observed in the patient after the VMT comprises the same L. crispatus variant that was present in the donor CVS material and remained the dominant variant throughout pregnancy for up to one year after the first VMT (Figs. 1 and 2A).
Fig. 2.
Comparison of donor and control microbiome. (A) Heatmap illustrating pairwise Manhattan distances between Single Nucleotide Variant (SNV) profiles generated from metagenomic sequencing of CVS samples. The distance scale indicates close to distant profiles from black to white respectively. The times on the recipient samples refer to the time they were collected since the initial VMT. The donor samples include two samples obtained from the same individual donor and were used for VMT at two different time points (see text), Administration 1 and 2, as well as three other samples from three separate individuals that were not used for VMT, Control 1, 2 and 3. The heatmap was performed in https://metasnv.embl.de. (B) Line graph illustrating specific longitudinal trends of the same pairwise Manhattan distances between SNV profiles used to generate the heatmap (Fig. 2A). The horizontal axis labels refer to the time since the first VMT administration, at which samples were collected for metagenomic sequencing. The vertical axis indicates close to distant profiles from low to high respectively. Each line illustrates the pairwise distance between the recipient samples and different individual donor samples over time. Samples obtained from the same individual that were used for VMT are labeled Administration 1 and 2, and three other samples from three separate individuals that were not used for VMT are labeled as Control 1, 2 and 3.
Discussion
Here we report that a successful VMT appeared to eradicate VD with a sustained long-term effect and without the use of pretreatment with antibiotics. Furthermore, we present the first VMT with strain-level confirmed engraftment of the donor microbiome.
VD is a severe challenge worldwide in women’s health leading to infertility, pregnancy loss and major obstetrical complications such as PPROM and preterm labor.7,8,24 Moreover, due to high recurrence rates and increasing resistance to antibiotics, there is a need to develop alternative and durable treatments for this condition.1,12,13 Ideally, a therapy without the use of antibiotics could prove to be a better and potentially more sustainable solution.25,26
The concept of applying VMT as a treatment for VD was introduced in 2019 reporting data from five patients suffering from recurrent symptomatic VD, who were treated with a VMT after an antibiotic regimen. Four of five patients had long-term remission after VMT.19 Although the study showed promising results, it must be noted that due to the antibiotic pretreatment, the positive effect observed in four out of five patients could be due to the pretreatment alone. Moreover, three of five patients underwent 2–3 VMT’s before resolution in symptoms, in one patient change of donor was needed. This underlines the power of a donor selection based on other methods, like performed in this study. In the mentioned previous study, although both recipient and donor samples underwent 16S rDNA and shotgun sequencing, except for a PCA analysis, the authors did not provide proof that the Lactobacillus variant found in the recipients originated from the donor. Furthermore, the diagnosis of the remission of VD was based on clinical criteria, such as the disappearance of clinical symptoms and a light microscopy-based diagnosis of a Lactobacillus-dominated microbiota.
Here, we used shotgun metagenomic sequencing to differentiate the bacterial species for single nucleotide polymorphisms indicative of distinct variants. Using SNV analysis, we observed high similarity between the L. crispatus in the donor CVS and the L. crispatus in the patient after VMT, which were then both dissimilar to other L. crispatus in CVS from other potential donors whose samples were not used for VMT. Thus, it seems reasonable to conclude that the success of the VMT was due to engraftment of the donor L. crispatus variant, and not from spontaneous conversion originating from already present low-abundance variants or from external sources. This approach to confirm donor engraftment is novel compared to the five previously published VMT cases.19
Results of studies using single-strain vaginal or oral probiotics with or without antibiotic pretreatment for vaginal dysbiosis indicate no or only a slight improvement.27,28 Depending on the study, this could be due to the use of non-vaginal species, the delivery mode, or the single strain composition. Data from the gastrointestinal tract suggest that the efficacy of fecal microbiota transplantation (FMT) for the management of recurrent Clostridium difficile colitis is based on the concept that the community and not one single strain needs to be present for the establishment of a healthy and sustainable microbiome.29, 30, 31, 32 VMT contains the whole community-system that has a history of stable colonization: bacteria, including multiple strains of lactobacilli, bacteriophages, proteins, cytokines, lipids, antimicrobial peptides. Their interaction can possibly be the key to a lasting engraftment and lower risk of recurrence. The mechanisms of how a VMT could improve the engraftment and have a sustained effect on the entirety of the vaginal microbial community, as well as many other variables that might impact a successful VMT-procedure remain unknown.33, 34, 35
Whether her last pregnancy was successful due to the VMT, the therapy of the APLS or the combination of both remains unclear. The patient was tested for APLS in 2019 with negative results (Table 1). APLS is an acquired thrombophilia36 and we would argue that the thrombophilia was indeed not present at that timepoint but developed from there onwards and was diagnosed after the third loss. VD symptoms were present in all her pregnancies. We therefore cannot determine if the successful pregnancy was solely due to APLS therapy. However, the patient experience clinical relief of symptoms and microbiological VD-resolution. Future, randomized controlled trials will provide insight into the possibilities of using VMT as a treatment for women with VD and pregnancy complications such as RPL or infertility.
Herein we report for the first time a successful VMT without antibiotic pre-treatment leading to immediate and sustained relief of clinical VD symptoms and confirmed donor strain engraftment, followed by a successful pregnancy and delivery in a woman with a series of late pregnancy losses/stillbirths. This indicates the need to further study the potential role of VMT as a future antibiotic-sparing treatment in cases of recurring vaginal dysbiosis as well as in cases of possible VD-associated pregnancy/IVF complications.
Contributors
Patient recruitment, clinical performance, and sampling: Henriette Svarre Nielsen, Kilian Vomstein, and Tine Wrønding. Donor program, in vitro assay design: Brynjulf Mortensen, Laura M. Ensign, Elleke F. Bosma, Tine Wrønding, and Henriette Svarre Nielsen. Wrote first version of the manuscript: Tine Wrønding, Kilian Vomstein, Elleke F. Bosma, and Henriette Svarre Nielsen. Revising the manuscript critically for important intellectual content: Tine Wrønding, Kilian Vomstein, Henriette Svarre Nielsen, Elleke F. Bosma, Brynjulf Mortensen, Henrik Westh, Julie Elm Heintz, Sara Mollerup, Laura M. Ensign, Kevin DeLong, Johan van Hylckama Vlieg, Anne Bloch Thomsen, and Andreas Munk Petersen. Performed the vaginal microbiome analysis and bioinformatics: Julie Elm Heintz, Sarah Mollerup, Kevin DeLong, Henrik Westh, and Andreas Munk Petersen. Tine Wrønding, Henriette Svarre Nielsen, Kilian Vomstein Sarah Mollerup, and Kevin DeLong have verified the underlying data. All authors made substantial contributions to the study set-up, data interpretation and manuscript revision. All authors approved the final manuscript before publication.
Data sharing statement
The datasets generated and analyzed for this study are available from the corresponding author after publication upon reasonable request.
Declaration of interests
Elleke F. Bosma, Brynjulf Mortensen, Kevin DeLong and Johan van Hylckama Vlieg are employees of Freya Biosciences. Johan van Hylckama Vlieg, Anne Bloch Thomsen and Laura Ensign are shareholders of Freya Biosciences. Elleke F. Bosma and Brynjulf Mortensen are listed as inventors on a patent application filed on the use of VMT for RPL. Laura Ensign is the Chair of the Scientific Advisory Board for Freya Biosciences, receives consulting payments from Freya Biosciences, and is an inventor on patent applications related to VMT that are owned by Johns Hopkins University. Anne Bloch Thomsen is a full-time employee of Pfizer as Country Medical Director, CMO of Pfizer Denmark and Iceland and is on the Board of Pfizer Denmark, and is a board member of EMPROS Pharma Sweden. Henriette Svarre Nielsen has received scientific grants from Freya Biosciences, Ferring Pharmaceuticals, BioInnovation Institute, Ministry of Education, Novo Nordisk Foundation, Augustinus Fonden, Oda og Hans Svenningsens Fond, Demant Fonden, and Ole Kirks Fond. HSN received personal payment or honoraria for lectures and presentations from Ferring Pharmaceuticals, Merck, Astra Zeneca, Cook Medical, and IBSA Nordic. Henrik Westh, Andreas Munk Petersen Sarah Mollerup received materials for the study funded by Freya Biosciences and paid to institution. Tine Wrønding, Kilian Vomstein and Julie Elm Heinz do not have any conflicts of interest regarding this publication.
Acknowledgements
The study was partially funded (i.e., analysis costs) by Freya Biosciences Aps, Fruebjergvej, 2100 Copenhagen, Denmark. We thank Freya Biosciences for funding in this research field. Further we thank the woman and her family in this case to participate in providing new and important knowledge.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102070.
Appendix A. Supplementary data
References
- 1.Coudray M.S., Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020;245:143–148. doi: 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano M.G., Parikh H.I., Brooks J.P., et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat Med. 2019;25(6):1001–1011. doi: 10.1038/s41591-019-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng L., Norenhag J., Hu Y.O.O., et al. Vaginal microbiota and human papillomavirus infection among young Swedish women. NPJ Biofilms Microbiomes. 2020;6(1):39. doi: 10.1038/s41522-020-00146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danielsson D., Teigen P.K., Moi H. The genital econiche: focus on microbiota and bacterial vaginosis. Ann N Y Acad Sci. 2011;1230(1):48–58. doi: 10.1111/j.1749-6632.2011.06041.x. [DOI] [PubMed] [Google Scholar]
- 5.Haahr T., Zacho J., Bräuner M., Shathmigha K., Skov Jensen J., Humaidan P. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. BJOG. 2019;126(2):200–207. doi: 10.1111/1471-0528.15178. https://onlinelibrary.wiley.com/doi/abs/10.1111/1471-0528.15178 Available from: [DOI] [PubMed] [Google Scholar]
- 6.Peebles K., Velloza J., Balkus J.E., McClelland R.S., Barnabas R.V. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis. 2019;46(5):304–311. doi: 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 7.Juliana N.C.A., Suiters M.J.M., Al-Nasiry S., Morré S.A., Peters R.P.H., Ambrosino E. The association between vaginal microbiota dysbiosis, bacterial vaginosis, and aerobic vaginitis, and adverse pregnancy outcomes of women living in Sub-Saharan Africa: a systematic review. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.567885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haahr T., Zacho J., Bräuner M., Shathmigha K., Skov Jensen J., Humaidan P. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. BJOG. 2019;126(2):200–207. doi: 10.1111/1471-0528.15178. [DOI] [PubMed] [Google Scholar]
- 9.Grewal K., Lee Y.S., Smith A., et al. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022;20(1):38. doi: 10.1186/s12916-021-02227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fettweis J.M., Serrano M.G., Brooks J.P., et al. The vaginal microbiome and preterm birth. Nat Med. 2019;25(6):1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koumans E.H., Sternberg M., Bruce C., et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw C.S., Morton A.N., Hocking J., et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 13.Joesoef M.R., Schmid G.P., Hillier S.L. Bacterial vaginosis: review of treatment options and potential clinical indications for therapy. Clin Infect Dis. 1999;28(Suppl 1):S57–S65. doi: 10.1086/514725. [DOI] [PubMed] [Google Scholar]
- 14.Schuyler J.A., Mordechai E., Adelson M.E., Sobel J.D., Gygax S.E., Hilbert D.W. Identification of intrinsically metronidazole-resistant clades of Gardnerella vaginalis. Diagn Microbiol Infect Dis. 2016;84(1):1–3. doi: 10.1016/j.diagmicrobio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Faught B.M., Reyes S. Characterization and treatment of recurrent bacterial vaginosis. J Womens Health. 2019;28(9):1218–1226. doi: 10.1089/jwh.2018.7383. [DOI] [PubMed] [Google Scholar]
- 16.Ferris M.J., Masztal A., Aldridge K.E., Fortenberry J.D., Fidel P.L., Martin D.H. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis. 2004;4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann P., Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79(6):471–489. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Shukla A., Sobel J.D. Vulvovaginitis caused by Candida species following antibiotic exposure. Curr Infect Dis Rep. 2019;21(11):44. doi: 10.1007/s11908-019-0700-y. [DOI] [PubMed] [Google Scholar]
- 19.Lev-Sagie A., Goldman-Wohl D., Cohen Y., et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25(10):1500–1504. doi: 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]
- 20.McDermott M.F., Frenkel J. Hereditary periodic fever syndromes. Neth J Med. 2001;59(3):118–125. doi: 10.1016/s0300-2977(01)00149-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood D.E., Lu J., Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20(1):257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulavi E., Mwendwa F., Atandi D.O., et al. Vaginal microbiota in women with spontaneous preterm labor versus those with term labor in Kenya: a case control study. BMC Microbiol. 2022;22(1):270. doi: 10.1186/s12866-022-02681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin M.N., Beigi R.H., Meyn L.A., Hillier S.L. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol. 2005;43(9):4492–4497. doi: 10.1128/JCM.43.9.4492-4497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beigi R.H., Austin M.N., Meyn L.A., Krohn M.A., Hillier S.L. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004;191(4):1124–1129. doi: 10.1016/j.ajog.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Cohen C.R., Wierzbicki M.R., French A.L., et al. Randomized trial of Lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382(20):1906–1915. doi: 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jepsen I.E., Saxtorph M.H., Englund A.L.M., et al. Probiotic treatment with specific lactobacilli does not improve an unfavorable vaginal microbiota prior to fertility treatment—a randomized, double-blinded, placebo-controlled trial. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1057022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerardin Y., Timberlake S., Allegretti J.R., Smith M.B., Kassam Z. Beyond fecal microbiota transplantation: developing drugs from the microbiome. J Infect Dis. 2021;223(Supplement_3):S276–S282. doi: 10.1093/infdis/jiaa700. [DOI] [PubMed] [Google Scholar]
- 30.Draper L.A., Ryan F.J., Smith M.K., et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6(1):220. doi: 10.1186/s40168-018-0598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianotti R.J., Moss A.C. Fecal microbiota transplantation: from Clostridium difficile to inflammatory bowel disease. Gastroenterol Hepatol. 2017;13(4):209–213. [PMC free article] [PubMed] [Google Scholar]
- 32.van Nood E., Vrieze A., Nieuwdorp M., et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 33.Antonio M.A.D., Meyn L.A., Murray P.J., Busse B., Hillier S.L. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous lactobacilli. J Infect Dis. 2009;199(10):1506–1513. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 34.Mirmonsef P., Hotton A.L., Gilbert D., et al. Free glycogen in vaginal fluids is associated with lactobacillus colonization and low vaginal pH. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farage M.A., Miller K.W., Sobel J.D. Dynamics of the vaginal ecosystem—hormonal influences. Infect Dis Res Treat. 2010;3 [Google Scholar]
- 36.Öztürk M.A., Haznedaroğlu I.C., Turgut M., Göker H. Current debates in antiphospholipid syndrome: the acquired antibody-mediated thrombophilia. Clin Appl Thromb. 2004;10(2):89–126. doi: 10.1177/107602960401000201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.