Abstract
Closed fractures of distal radius and ulna are one of the most common skeletal injuries, occurring at all ages. Temporary arm immobilization through cast is part of the standard treatments. However, traditional casting procedures are time consuming, operator's skill dependent and do not always guarantee a satisfactory outcome. From a clinical perspective, casts are often considered uncomfortable and can be associated to skin lesions. To overcome these limitations, the recent growth of 3D technologies has enabled new standardized casting procedures: additive manufacturing (AM) is a technique that creates highly customized cast models from anatomical 3D data by using digitally controlled and operated material laying tools. Compared with conventional casts, those produced with AM technique could potentially reduce skin complications and satisfy both mechanical and clinical requirements of functionality, comfort, and aesthetics.
The objective of this study is to describe the new practical methodology to produce a 3D printable cast for upper arm immobilization. The parametric modelling tool, employed to develop a semi-automatic design system for generating the printable cast model, reduces the complex process of orthosis design to a few minutes and all the manufacturing operations remain unaffected by CAD skills of the operator. Specific hardware and software tools (3D scanner, modelling software and FDM technology) were chosen to mitigate design and production costs while guaranteeing suitable levels of data accuracy, process efficiency and design versatility. To highlight the effectiveness of the proposed solution, a finite element analysis simulation was performed on models with different geometry, highlighting the mechanical strength of generated structures.
The final result is a personalized 3D printed cast with a highly ventilated structure that is lightweight but still maintains a high level of strength and provides hygienic benefits, reducing the risk of cutaneous complications, potentially improving treatment efficacy and increasing patient satisfaction.
Keywords: Fused filament fabrication, Orthopedic cast, Forearm fracture, Topological optimization
1. Introduction
Fractures of distal radius and ulna are one of the most common skeletal injuries and occur at all ages of the general population. Closed reduction and cast immobilization remain a current treatment option in most cases, indeed it is effective and more cost-effective than other treatment methods.1,2 The standard cast, known as plaster of Paris, allows fracture immobilization and bone alignment to promote inner healing, diminish pain, protect the injury, and help compensate for surrounding muscular weakness. Use of plaster of Paris bandages for fracture casts became widespread worldwide; their clinical properties and advantages are well known and have not greatly changed since their first use in the 19th century.3 However, casting procedure is not without drawbacks: it is time-consuming and requires a skilled technician, moreover traditional plaster casts are perceived by patients as heavy and uncomfortable, and they can lead to different complications ranging from skin injuries (skin irritation, soreness, injuries, pressure sores) to compartment syndrome.3 More recently, several alternative materials to plaster of Paris have been proposed, such as fiberglass casts that are about three times stronger and one third in weight; however, even in this case, some disadvantages have been reported. Due to the higher rigidity of the material, the cast seems to be less accommodating to swelling, moreover the fiberglass casting sets very quickly and this could be a problem for less experienced medical providers who may not have enough time to properly wrap the injured extremity before the cast sets.4 Generally, regardless of the type of material used for casting, bacterial and fungal infections or pruritic dermatitis can spread because of the poor ventilation beneath the dense cast enclosure.5
Casts fabricated via 3D printing or rapid prototyping (RP), in particularly fused filament fabrication (FFF), have the potential to overcome these issues thanks to a porous design that accounts for ventilation while retaining the required mechanical strength for limb protection and immobilization.6, 7, 8 Moreover, it is possible to conceive custom-made designs that can be more effective as they are tailored to each patient, whose anatomy can be digitally reconstructed via reverse engineering (RE) from a 3D scan.6, 7, 8
The three main steps for the fabrication of a cast from RE/RP can be outlined as follows:
-
●
acquisition of the 3D geometry of the interested anatomy using a 3D scanner;
-
●
processing of the acquired data through dedicated software;
-
●
fabrication of the orthosis using a 3D printer.
In the first phase, the patient's anthropometric data must be gathered and processed, usually manually; this can generate inaccuracies such as flaws and distortions in the anatomical model. Direct modelling of the anatomy and of the cast design requires proficiency of Computer Aided Design (CAD) software, a skill that is not normally expected from a clinician. For this reason, over the last years new approaches for standardized procedures in the medical field have been attempted, and several studies proposed modelling procedures for patient-specific 3D-printed casts for wrist orthoses that exploit parametric modelling as opposed to direct modelling.8, 9, 10, 11, 12, 13
The authors describe how parametric modelling can be exploited to develop a semi-automatic design procedure to generate a topologically optimized, printable cast orthosis from 3D scanned anatomical data through the use of a programmable modelling tool. The cast models generated through this new procedure could find indication in the immobilization of distal fractures of the radius or ulna, or any other pathology requiring the use of a cast.
2. Methods
The study involved designing the desired cast orthosis by scanning a healthy volunteer limb, generating printable data through a programmable modelling tool, comparing the strength and geometry of different possible models by finite element analysis (FEA), and finally assessing the casts fitting on the previously scanned limb.
Scanning was performed using a Structure Sensor Mark II (Occipital) 3D scanning device. This scanner is based on active stereo vision, a system formed by a depth camera that adopts active infrared stereo vision technology to measure the depth of the environment. The sensor is made up of two image sensors, an IR projector, and a RGB sensor. The infrared projector projects non-visible structured IR pattern to improve depth accuracy in scenes. The depth image processor obtains the scene data by the two image sensors, and the depth values for each pixel can be calculated by correlating the points on the left image to the right image. Scanning devices based on this principle have a limited range of some meters, but their accuracy is relatively high. The accuracy of triangulation range finders is about tens of micrometers, which ensures an optimal scan result.14 When attached to a mobile device, the sensor allows scanning of the upper limb by a single technician. The technician should keep, during all scanning procedure, a constant distance from the limb, ensuring a higher quality scan.
The person's scanning posture must be agreed with an orthopedic doctor in order to put the fracture site in a neutral position, block all movements and restore normal functionality of the wrist joint. During all the procedure, the upper arm is held in place using supports for the elbow and hand to avoid any movement during the scan acquisition.
At the end of the scanning procedure, the technician measures the circumference of thumb, knuckles, wrist (scaphoid is used as reference point), middle and end of forearm, in the area where the cast should finish. These measurements are essential for subsequently comparing the actual measures to the values provided by the specific CAD software. The 3D scanning process helps turn an actual object, in this case upper arm, into a point cloud or digital mesh – an array of points or triangles approximating the surface of the object. Through image processing and triangulation algorithms, the forearm is converted into 3D information; at this early stage, the 3D mesh (most of the time in STL format) is processed with Autodesk MeshMixer.
The orthosis was designed by using Rhinoceros 3D. The process of designing the orthosis is described through the usage of Rhinoceros 3D version 7.0 and its parametric algorithms were constructed using Grasshopper 3D. Rhinoceros 3D's modelling tools feature Grasshopper, a graphical algorithm editor that is integrated with Rhinoceros 3D's modelling tools. This is used to design algorithms that then automate tasks in Rhinoceros. All the steps included in the algorithm are performed automatically; the technician needs only to input parameters according to the doctor indications, such as:
-
●
measurement of palm, wrist and end of the forearm circumference
-
●
density of the pattern
-
●
inner thickness of the pattern
-
●
overall thickness of the pattern and the edges.
3D printed cast were made of PETg (polyethylene terephtalate glycol). PETg is a transparent and completely recyclable thermoplastic resin, characterized by a high mechanical resistance that allows to obtain robust and long-lasting prints. Thanks to the low shrinkage coefficient, this material is an excellent choice for 3D prints that require large flat surfaces and for objects that must be resistant but more flexible than those made of ABS. In addition, it is suitable for contact with food, it satisfies the biocompatibility standards required for medical applications and orthopedic devices (ISO 10993–1), it is water-repellent, does not absorb water and is 100% recyclable. This makes 3D printing more sustainable without having to make compromises on material properties – and yet keep it affordable.
Finite element analysis (FEA) was performed through the software Abaqus/CAE; physical and material properties can be assigned to the geometry of 3D printed cast, together with loads and boundary conditions.
The analysis was carried out on three cast meshes having different pattern distributions but an identical overall shape: the first two were the result of parameters alteration in the parametric algorithm, whereas in the third the triangles distribution was manually manipulated. Prior to the analysis, the mesh was converted to a solid model (step file).
The first mesh (“para1”) was generated by the parametric algorithm with the following input parameters:
-
●
density: 3
-
●
thickness: 4 mm
-
●
structure thickness: 30%
The second mesh (“para2”) was characterized by:
-
●
density: 2
-
●
thickness: 6 mm
-
●
structure thickness: 40%
The third mesh (“manual”) was produced by manipulating the triangle distribution into an inhomogeneous one, with a slight increase in density at the wrist end and a decrease at the other end. This distribution is expected to increase the strength of the cast in one specific area and lighten it in another; in clinical practice this could result in increased material strength at the fracture area (e.g., the wrist), and lightning at the other part (e.g., the forearm). All the other parameters are exactly the same as the first mesh.
The three models were examined under the same load and boundary conditions in separated simulations. The two halves of each model (“thumb” and “logo”) were individually imported, and material properties were assigned based on the PETg filament technical datasheet (E = 1.94 GPa; ν = 0.43); the material was classified as elastic and isotropic. The two parts were transformed into an assembly of independent instances and positioned in a global coordinate system. A static analysis was performed, with automatic incrementation and direct resolution using the full Newton solution technique. Free meshing technique with tetrahedral units was selected, and the element type was defined as C3D4 (a 4-node linear tetrahedron with linear shape function). Approximate element size was set at 1.1 mm and 1.2 mm for the “thumb” and “logo” halves, respectively; maximum curvature deviation factor was set as 0.1, and minimum size factor was set at 0.1 of the element size. The interaction between the two halves was set as follows: hard contact condition as normal behavior (to avoid interpenetration), and penalty condition (friction coefficient = 0.22) as tangential behavior (to limit sliding). Moreover, a local tie condition was added to impose a perfect attachment in the four portions where plastic strips are placed, in order to avoid any type of movement in these areas; finally, an effective distance of 0.4 mm between the parts was imposed.
Boundary conditions were applied to lock the model in a defined space. A load F = −100 N along the y-axis was applied to a reference point that locked the displacement and rotation of an area of about 120 mm2 through a rigid beam multi-point constraint (MPC). A different area was chosen in the three models, to simulate an impulsive load in different, random situations. The simulation returned values of Von Mises equivalent tensile stress σv and maximum displacement throughout the models.
A second set of simulations was run only on the “thumb” halves, to better highlight the contribution of the different geometries to the stress distribution; in this case, the same reference point (260, −200, 55) placed above the tubular edges at the end of forearm was chosen for all models and locked to an area of about 30 mm2; a load F = −10 N along the y-axis was applied.
Models were printed through ATLAS TECHNIK 4070 (the 3D-printer was modified to allow to obtain a resistant product in less than 2 h), and their fitting was assessed visually after placing them on the volunteer limb.
3. Results and discussion
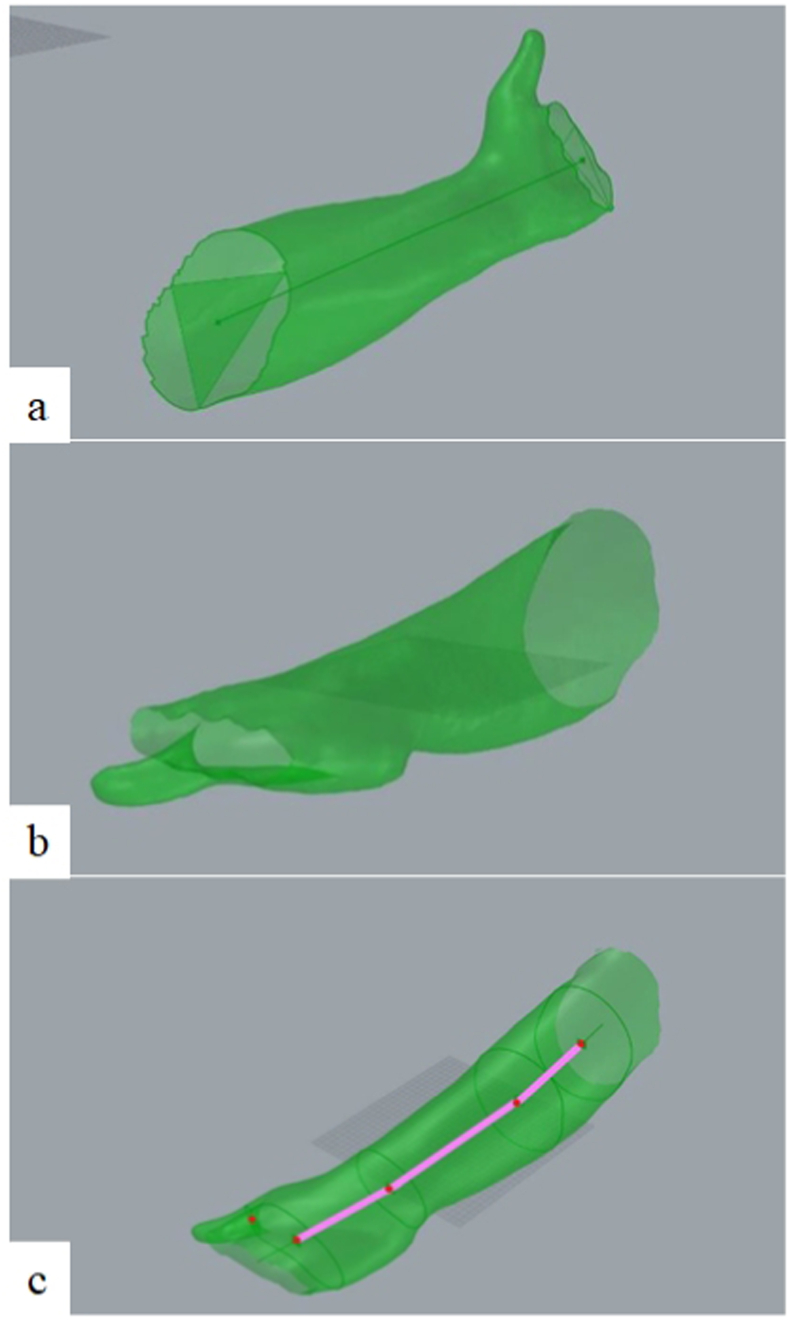
Fig. 1 shows the result of a scanning procedure performed on a volunteer's limb. The uploaded mesh (Fig. 1a) presents only few distortions, which can be eliminated through simple smoothing and hole filling operations. First of all, the support structures and unwanted portions of the arm are removed (Fig. 1b). Holes and spikes can be present in the 3D scan as a result of some dark areas of the limb or of non-uniform capture by the scanner. In this case, the area around the defect is selected (Fig. 1c), the hole filled and the surface smoothened (Fig. 1d). The whole mesh can be smoothened as well before proceeding with the cast design (Fig. 1e).
Fig. 1.
From scan to initial mesh of a limb.
After this first smoothing, the mesh of the scanned limb is imported in Rhinoceros (Fig. 2a). The algorithm identifies the curves that describe the two openings in correspondence of palm and the end of forearm and then, exploiting the repeatability of some geometric correlations on the upper arm, the parametric algorithm adjusts the position and the orientation of the input mesh to always obtain a correct positioning to continue the processing. Finally, the algorithm checks that the hand palm is facing downwards (based on the position of the thumb) and, if not, it rotates the mesh by additional 180° (Fig. 2a–c). Once the mesh is positioned, the cutting planes on the palm, thumb and forearm are identified and positioned, based on medical indications.
Fig. 2.
Geometries definition on the limb mesh.
At this point, the mesh is ready for the final processing phase. It is important to highlight that the mesh is partially deformed to respect the real measurement performed by the technician in order to obtain a mesh that faithfully reproduces the volunteer's limb.
Therefore, it is necessary to perform an additional homogeneous offset of 0.5 mm or more, according to the doctor's instructions, in order to build a cast that fits on the person without any pain.
A fundamental aspect that can be pursued by 3D-printed casts is transpiration and the possibility of a regular inspection of the injury site. Opposed to traditional plaster casts, additive manufactured casts can be perforated to increase ventilation of the treated area, reducing the risk of irritation and the overall device weight. A correct ventilation pattern, while being as light as possible, must preserve the mechanical properties of the device. On average, compared to a traditional plaster cast that completely covers the injured limb, with this type of structure, the covering area can be reduced by 50% or more, also ensuring the resistance of the design (as we will see in the FEA analysis), potentially allowing all patients to spend the healing period in complete safety.
The generation of ventilation holes is the most time-consuming step in a direct modelling approach, since it is manually performed by an expert CAD designer; here, a pattern is created based on Voronoi tessellation built on the scanned mesh. Fig. 3 highlights the procedure for generating the final cast mesh. First, the number of triangles is topologically reduced (therefore preserving the mesh shape) by setting a desired density of faces (Fig. 3a); then, the algorithm calculates the barycenter of each triangular element and then connects them to form a network (Fig. 3b). The network is offset internally to generate a solid Voronoi pattern (Fig. 3c). The mesh is cut by the planes identified by the thumb, palm and end forearm curves. Tubular edges are generated on the three curves and the mesh is separated in two halves which can be held together through an interlocking system. Finally, the 3D cast is rotated and positioned at the center of the work plane; each half is exported as a distinct STL file (Fig. 3d).
Fig. 3.
Generation of the Voronoi pattern and of the final cast mesh.
The FFF process, the resulting cast halves and the delivery on the person's limb are shown in Fig. 4a–c; the whole process usually takes less than 2 h. Fitting with the previously 3D scanned limb is confirmed (Fig. 4-c).
Fig. 4.
3D printed cast.
Finite element analysis (FEA) has been used in AM processes to predict and optimize mechanical characteristics and functional performance. The three models subject to the analysis (para1, para2 and manual) are shown in Fig. 5, together with the boundary conditions and the load application on the MPC reference point highlighted on the “thumb” halves (in the second set of simulations).
Fig. 5.
FEA analysis setup: a-c) para1, para2 and manual models, with respective d-f) boundary conditions and g-i) load application on the MPC reference points (in the second set of simulations).
This first set of simulations was performed in order to validate the three geometries subject to a predefined load; the distribution of the Von Mises stress σv and the magnitude displacement are reported in Fig. 6, and in Table 1.
Fig. 6.
1st FEA analysis: a-c) Von Mises stress and d-f) magnitude displacement as a result of the same load applied to the para1, para2 and manual models.
Table 1.
Maximum stress and displacement in the three models (1st analysis).
| Model | para1 | para2 | manual |
|---|---|---|---|
| Maximum stress (MPa) | 29.56 | 9.80 | 32.82 |
| Maximum displacement (mm) | 0.90 | 0.41 | 0.90 |
All models show a perfectly elastic behavior, with a maximum σv well below the tensile strength of the material (50 MPa) and a maximum displacement <1 mm. The impulsive load seems to affect only a small portion of the model, leaving the remaining structure almost unaffected; however, in the locking points of the cast, the stress was slightly higher.
The second set of simulations focused on the differences between the three geometries of the models; results are reported in Fig. 7, with emphasis on the maximum stress values (between 1 and 2 MPa).
Fig. 7.
2nd FEA analysis: a-c) Von Mises stress (values between 1 and 2 MPa) and d-f) magnitude displacement as a result of the same load applied to the para1, para2 and manual models.
As highlighted in Fig. 7a–c, stress values remained below 1 MPa in most of the structure for all models (grey color). Higher values are detected at the loading area, yet its stress values remained low in its immediate proximity, a sign that the structures effectively promote stress attenuation. The presence of a constraint on the opposite side, on the other hand, generated a stress intensification in the hand and wrist area. This is more relevant in models para1 and manual; as expected, the densification of the area provided by the increased thickness of the para2 model resulted in a maximum stress and displacement decrease by 60% (Table 1, Table 2). On the other hand, the higher thickness negatively impacts both the printing time, which increases by 43%, and the final weight of the cast, which goes from 175 g to 241 g (+38%).
Table 2.
Maximum stress and displacement in the three models (2nd analysis).
| Model | para1 | para2 | manual |
|---|---|---|---|
| Maximum stress (MPa) | 9.41 | 3.09 | 7.77 |
| Maximum displacement (mm) | 2.12 | 0.87 | 2.20 |
4. Conclusions
This study shows that a semi-automatic, programmable tool allows to design anatomical customized orthopedic casts with optimized features for the treatment of forearm fractures.
Its main advantages are:
-
●
it does not require specific CAD skills to perform the design of the orthosis;
-
●
it does not take significant time for the generation of the model;
-
●
the designs can be subject to FEA analysis to foresee different load scenarios and validate the choice of geometry.
The parametric approach allows for automatic creation of a ventilation pattern that may provide multiple clinical benefits: it is lightweight and breathable, potentially reducing pain as well as the risk of cutaneous complications, moreover it allows for better inspection of the limb from the doctor. Thanks to an optimized design, the lightweight structure still possesses a high strength; it provides circumferential support for the fractured limb and can prevent injury from external impact, as demonstrated by the FEA analysis results. Parameters choice in the programmable tool allows to design for specific requests in terms of strength, support and pressure, according to the physician's requests. The anatomical customized design is expected to maintain the alignment of fractured bone parts through a moderate, custom-fit wearing pressure that could potentially prevent compartment syndrome and pressure sores. The proposed approach is not limited to forearm fractures, but it could be applied in all conditions requiring casting, thus in different anatomical areas (including lower limbs and spine).
FFF is a fast, economic technology enabling to produce the customized orthosis in less than 2 h. It is environmentally responsible as it greatly limits material waste and employs a recyclable plastic filament, PETg. Unlike plaster of Paris and fiberglass casts, the orthosis produced through FFF technology can be recycled after use.
The 3D printed casts could represent viable alternatives to traditional casts, both in hospitals and clinics settings, for the treatment of closed fractures; further future studies will be directed to clinical testing of the 3D printed casts in order to evaluate their effectiveness and advantages in the specific indication, compared to the conventional ones.
Funding
Playcast srl.
Declaration of competing interest
The authors are employes or consultants of Playcast srl.
References
- 1.Farner S., Malkani A., Lau E., Day J., Ochoa J., Ong K. Outcomes and cost of care for patients with distal radius fractures. Orthopedics. 2014 Oct;37(10):e866–e878. doi: 10.3928/01477447-20140924-52. PMID: 25275973. [DOI] [PubMed] [Google Scholar]
- 2.Søsborg-Würtz H., Corap Gellert S., Ladeby Erichsen J., Viberg B. Closed reduction of distal radius fractures: a systematic review and meta-analysis. EFORT Open Rev. 2018 Apr 26;3(4):114–120. doi: 10.1302/2058-5241.3.170063. PMID: 29780618; PMCID: PMC5941650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szostakowski B., Smitham P., Khan W.S. Plaster of paris-short history of casting and injured limb immobilzation. Open Orthop J. 2017;11:291–296. doi: 10.2174/1874325001711010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.rEkanayake C., Gamage J.C.P.H., Mendis P., Weerasinghe P. Revolution in orthopedic immobilization materials: a comprehensive review. Heliyon. 2023 Feb 16;9(3) doi: 10.1016/j.heliyon.2023.e13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake D.F., Ritzman T.F. Cast-related complications. Orthop Clin N Am. 2021 Jul;52(3):231–240. doi: 10.1016/j.ocl.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Tzavellas A.-N., Kenanidis E., Potoupnis M., Tsiridis E. In: 3D Printing: Applications in Medicine and Surgery. Tsoulfas G., Bangeas P.I., Suri J.S., editors. Elsevier Inc; 2020. 3D printing in orthopedic surgery; pp. 133–141. [Google Scholar]
- 7.Eltorai A.E., Nguyen E., Daniels A.H. Three-dimensional printing in orthopedic surgery. Orthopedics. 2015;38(11):684–687. doi: 10.3928/01477447-20151016-05. [DOI] [PubMed] [Google Scholar]
- 8.Munteanu A., Chitariu D., Cioata F. The FDM 3D printing application for orthopedic splints. Appl Mech Mater. 2015;809–810:375–380. [Google Scholar]
- 9.Li J., Tanaka H. Rapid customization system for 3D-printed splint using programmable modeling technique - a practical approach. 3D Print Med. 2018;4(1):5. doi: 10.1186/s41205-018-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonamici F., Furferi R., Governi L., et al. A practical methodology for computer-aided design of custom 3D printable casts for wrist fractures. Vis Comput. 2020;36(2):375–390. [Google Scholar]
- 11.Li J., Tanaka H. Feasibility study applying a parametric model as the design generator for 3D-printed orthosis for fracture immobilization. 3D Print Med. 2018;4(1):1. doi: 10.1186/s41205-017-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson A.M., Bibb R., Campbell R.I., Bingham G. Comparing additive manufacturing technologies for customised wrist splints. Rapid Prototyp J. 2015;21(3):230–243. [Google Scholar]
- 13.Górski F., Wichniarek R., Kuczko W., Żukowska M., Lulkiewicz M., Zawadzki P. Experimental studies on 3D printing of automatically designed customized wrist-hand orthoses. Materials. 2020;13(18):4091. doi: 10.3390/ma13184091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebrahim M.A.-B. 3D laser scanners' techniques overview. Int J Sci Res. 2015;4(10):323–331. [Google Scholar]