Summary
Background
Identifying potential risk factors related to severe COVID-19 outcomes is important. Repeated intermittent antibiotic use is known be associated with adverse outcomes. This study aims to examine whether prior frequent antibiotic exposure is associated with severe COVID-19 outcomes.
Methods
With the approval of NHS England, we used the OpenSAFELY platform, which integrated primary and secondary care, COVID-19 test, and death registration data. This matched case–control study included 0.67 million patients (aged 18–110 years) from an eligible 2.47 million patients with incident COVID-19 by matching with replacement. Inclusion criteria included registration within one general practice for at least 3 years and infection with incident COVID-19. Cases were identified according to different severity of COVID-19 outcomes. Cases and eligible controls were 1:6 matched on age, sex, region of GP practice, and index year and month of COVID-19 infection. Five quintile groups, based on the number of previous 3-year antibiotic prescriptions, were created to indicate the frequency of prior antibiotic exposure. Conditional logistic regression used to compare the differences between case and control groups, adjusting for ethnicity, body mass index, comorbidities, vaccination history, deprivation, and care home status. Sensitivity analyses were done to explore potential confounding and the effects of missing data.
Findings
Based on our inclusion criteria, between February 1, 2020 and December 31, 2021, 98,420 patients were admitted to hospitals and 22,660 died. 55 unique antibiotics were prescribed. A dose–response relationship between number of antibiotic prescriptions and risk of severe COVID-19 outcome was observed. Patients in the highest quintile with history of prior antibiotic exposure had 1.80 times greater odds of hospitalisation compared to patients without antibiotic exposure (adjusted odds ratio [OR] 1.80, 95% Confidence Interval [CI] 1.75–1.84). Similarly, the adjusted OR for hospitalised patients with death outcomes was 1.34 (95% CI 1.28–1.41). Larger number of prior antibiotic type was also associated with more severe COVID-19 related hospital admission. The adjusted OR of quintile 5 exposure (the most frequent) with more than 3 antibiotic types was around 2 times larger than quintile 1 (only 1 type; OR 1.80, 95% CI 1.75–1.84 vs. OR 1.03, 95% CI 1.01–1.05).
Interpretation
Our observational study has provided evidence that antibiotic exposure frequency and diversity may be associated with COVID-19 severity, potentially suggesting adverse effects of repeated intermittent antibiotic use. Future work could work to elucidate causal links and potential mechanisms. Antibiotic stewardship should put more emphasis on long-term antibiotic exposure and its adverse outcome to increase the awareness of appropriate antibiotics use.
Funding
Health Data Research UK and National Institute for Health Research.
Keywords: Antibiotics, Primary care, COVID-19, Severe outcome
Research in context.
Evidence before this study
Accumulating research indicates that repeated intermittent antibiotic use is associated with adverse outcomes, yet few research examined its effect on COVID-19. Patients with long-term exposure to more antibiotics might be at higher risk of infection-related complications or autoimmune disease compared to those with less antibiotics. Possible explanations might include antibiotic resistance or antibiotic perturbation of gut microbiota, which has been reported in relation to immune and metabolism regulation. Current Research indicated that SARS-CoV-2 virus infected patients through both respiratory and gastrointestinal tracts and then possibly induced later serious immune response depends on individual conditions or characteristics. Some clinical data also found that the composition of gut microbiome was altered in COVID-19 patients, especially for those exposed to antibiotic treatment. It is, therefore, hypothesised that patients who use antibiotics frequently may be more susceptible to COVID-19 infection.
Added value of this study
In our case–control study, patients with more frequent antibiotic exposure in the past 3 years were at higher odds to experience severe COVID-19 outcome, including hospital admission and 30-day mortality. In addition, the diversity of antibiotic type use was associated with COVID-19 hospital admission. It seems advisable to discourage the regular practice of indiscriminately prescribing antibiotics, repeatedly and intermittently, given their uncertain benefit and likely risks.
Implications of all the available evidence
Our observational findings have provided evidence to suggest that antibiotic exposure frequency and diversity may be associated with COVID-19 severity. Although no causal links can be determined, these data suggest potential adverse effects of repeated intermittent antibiotic use. Future work could work to elucidate causal links and potential mechanisms. Antibiotic stewardship efforts should put more emphasis on long-term antibiotic exposure and its adverse outcome to increase the awareness of appropriate antibiotics use.
Introduction
The pandemic caused by the SARS-CoV-2 virus overwhelmed the world since March 2020. To date, the World Health Organization reports that there have been 533 million confirmed cases and 6 million deaths globally.1 In the UK, 22 million cases led to 196,000 deaths.2 The Coronavirus disease 2019 (COVID-19) can have different outcomes depending on patient characteristics. For most healthy people, COVID-19-related symptoms are mild, but older age, non-white ethnicity, and presence of comorbidities are associated with developing more severe symptoms and increased risk of COVID-19 related death.3, 4, 5, 6 It is important to understand what factors are associated with more severe COVID-19, including prior medication use. Observational studies have found that long-term history of oral corticosteroid use was associated with increased risk of death, while non-steroidal anti-inflammatory drug use (NSAIDs) was not.4,7 Research evidence is essential for supporting clinical treatment decisions in pandemics.
Repeated intermittent antibiotic use is known be associated with adverse outcomes. A dose–response relationship has been reported in previous studies, where a higher frequency of prior antibiotic exposure was associated with increased risk of infection-related complications and autoimmune disease.8,9 One potential explanation may be that frequent antibiotic use increases the likelihood of patients becoming colonised and infected with antibiotic-resistant pathogens, leading to antibiotic treatment failure and increased susceptibility to adverse consequences of infection. Other studies also pointed out that antibiotic treatment might alter gut microbiota, which can impact metabolic and immune function.10 While in most situations, gut microbiota will recover to baseline within a few weeks or even months after stopping an antibiotic course, frequent antibiotic perturbation may affect the resilience of gut microbiomes.11,12
In the UK, 71.4% of all antibiotics are prescribed in primary care settings.13 However, most antibiotic research in the COVID-19 pandemic focused on bacterial co-infections for COVID-19 patients admitted to hospital. No studies have explored how the severity of COVID-19 disease is affected by prior antibiotic use. Therefore, this population-based case–control study aimed to determine the relationship between prior antibiotic use and clinical outcomes for COVID-19 patients.
Methods
Data sources
On behalf of NHS England, we used primary care records form OpenSAFELY-TPP, which comprises nearly 22 million patients' electronic health records (EHRs) covering 40% of the population in England. They contain information about demographics, diagnosis and medications. These EHRs were linked, at patient-level, to the following databases: (1) SARS-CoV-2 PCR testing results from the Second Generation Surveillance System (SGSS), (2) Hospital admission data from the NHS Digital Secondary Use Service (SUS): part of Hospital Episode Statistics (HES), providing information about admissions, diagnosis, and treatments for discharged patients, (3) COVID-19 inpatient death data from the COVID-19 Patient Notification System (CPNS), and (4) death registration data from the Office for National Statistics (ONS). Data linkage was provided via the OpenSAFELY integrated platform, which is governed by National Health Service (NHS) England.
Ethics
This study was approved by the Health Research Authority and NHS Research Ethics Committee [REC reference 21/SC/0287] (Supplementary Protocol).
Study design
This population-based matched case–control study was divided into two parts. Study 1 evaluated hospital admission among COVID-19 patients identified from the general population. To evaluate the severity among cases from study 1 with COVID-19 related hospitalisation, study 2 evaluated severe outcome by measuring death.
Case-control study 1
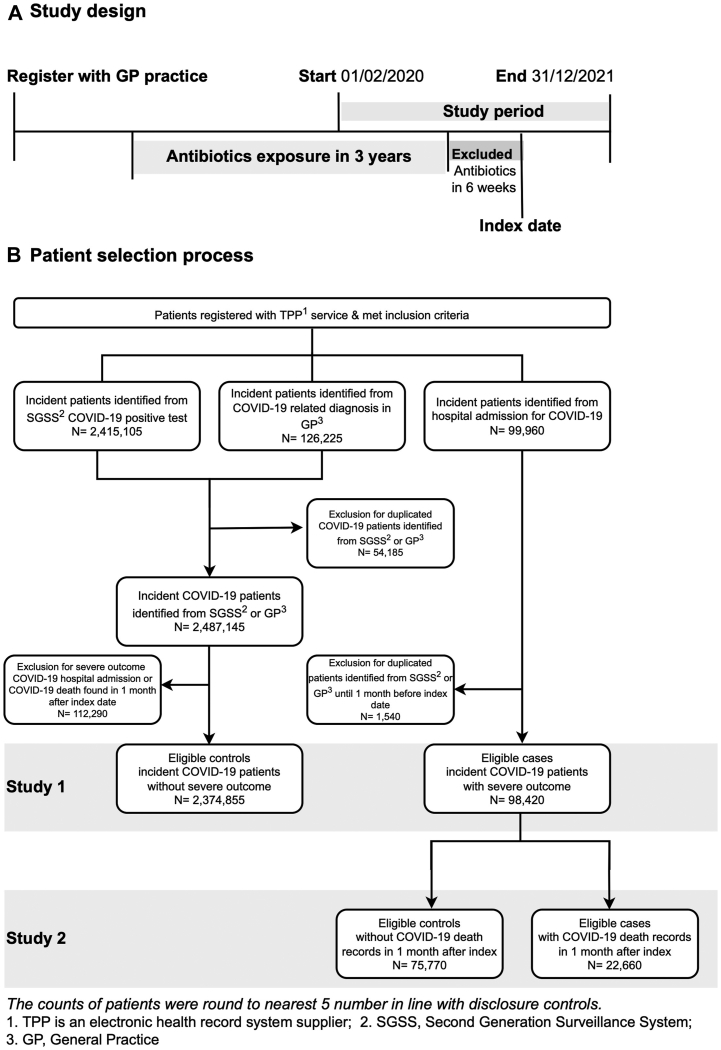
Eligible patients were selected from the beginning of the pandemic (01-02-2020), until the end of the year 2021 (31-12-2021) (Fig. 1A). Inclusion criteria consisted of: (1) complete age, sex, and region information; (2) aged 18–110 years; (3) registered with one practice for at least 3 years before the index date for a more complete prior history of antibiotics exposure; (4) incident COVID-19 infection identified from SGSS or coded in general practices (GP) in primary care and SUS in secondary care. Repeat COVID-19 records within 1 month were regarded as the same infection episode and the delineation of study design was in Supplementary Figure S1. For the small number of patients with more than one COVID-19 positive result (> one month apart), the first episode was used in this analysis and subsequent episodes excluded as the main focus was to investigate the effect of prior antibiotic exposure in patients with first exposure to COVID-19.
Fig. 1.
Study design (A) andpatient selection (B).
Cases were defined as patients admitted to hospital for COVID-19 with a primary diagnosis of International Classification of Diseases, 10th revision (ICD-10) codes U07.1 and U07.2. Controls were patients who had either COVID-19 positive test results or COVID-19 related diagnosis. The index date of cases was the incident date of COVID-19 hospital admission from SUS, while controls were identified from first COVID-19 record in GP records or confirmed positive test from SGSS. Prior COVID-19 history was searched from all databases and excluded for analysis. The flowchart of patient selection is illustrated in Fig. 1B. Since controls were limited to patients with minor COVID-19 clinical outcomes, patients with hospital admission or death records identified from SUS, CPNS or ONS within 1 month of index date were excluded.
Case-control study 2
All patients hospitalised for COVID-19 in study 1 were selected for study 2 (Fig. 1B). Cases were patient with death certificate record (from ONS and CPNS) of COVID-19 related death within 1 month following admission. All other admitted patients were considered controls.
Matching
For each study, cases were matched to up to 6 controls on age (within a maximum of 5 years), sex, region of general practices (NHS England regions divided by 44 Sustainability and Transformation Plan areas) to account for variation in infection prevalence, along with calendar year and month of the index date. The study inclusion period was divided into 3 waves according to index date, which were Wave 1 (early pandemic and first national lockdown, limited COVID-19 test availability for healthcare workers and severe symptoms patients): February–August 2020; Wave 2 (second national lockdown, COVID-19 availability for wider population): September 2020–April 2021; Wave 3 (end of national lockdown): May–December 2022.14,15 The distribution was shown in Table 1. To achieve comparable groups, matching with replacement was performed due to limited potential control pool for patients with COVID-19 (via R package MatchIT v4.2.0).16
Table 1.
Characteristics of study cohorts before and after matching.
| Study 1: admitted to hospitals |
||||||||
|---|---|---|---|---|---|---|---|---|
| Before matching |
Matched |
|||||||
| Case |
Control |
Case |
Control |
|||||
| na | % | na | % | na | % | na | % | |
| Number of patients | 98,420 | 4.0 | 2,374,855 | 96.0 | 97,880 | 14.6 | 576,640 | 85.5 |
| Inclusion timeb | ||||||||
| Wave 1 | 20,295 | 20.6 | 65,680 | 2.8 | 19,880 | 20.3 | 111,605 | 19.4 |
| Wave 2 | 50,375 | 51.2 | 781,415 | 32.9 | 50,295 | 51.4 | 299,675 | 52.0 |
| Wave 3 | 27,750 | 28.2 | 1,527,760 | 64.3 | 27,705 | 28.3 | 165,360 | 28.7 |
| Sex | ||||||||
| Male | 54,600 | 55.5 | 1,098,125 | 46.2 | 54,270 | 55.4 | 318,360 | 55.2 |
| Female | 43,825 | 44.5 | 1,276,730 | 53.8 | 43,610 | 44.6 | 258,280 | 44.8 |
| Mean age (SD) | 65.7 (17.7) | 43.3 (15.8) | 65.6 (17.6) | 65.2 (17.5) | ||||
| Age group | ||||||||
| 18–29 | 2880 | 2.9 | 541,030 | 22.8 | 2880 | 2.9 | 17,270 | 3.0 |
| 30–39 | 6290 | 6.4 | 486,925 | 20.5 | 6285 | 6.4 | 37,625 | 6.5 |
| 40–49 | 9885 | 10.0 | 524,480 | 22.1 | 9885 | 10.1 | 59,160 | 10.3 |
| 50–59 | 16,055 | 16.3 | 453,170 | 19.1 | 16,045 | 16.4 | 96,185 | 16.7 |
| 60–69 | 17,000 | 17.3 | 224,400 | 9.4 | 16,975 | 17.3 | 101,470 | 17.6 |
| 70–79 | 20,625 | 21.0 | 100,740 | 4.2 | 20,545 | 21.0 | 121,855 | 21.1 |
| 80+ | 25,680 | 26.1 | 44,115 | 1.9 | 25,265 | 25.8 | 143,075 | 24.8 |
| Practice region | ||||||||
| East | 20,270 | 20.6 | 528,820 | 22.3 | 20,130 | 20.6 | 118,310 | 20.5 |
| East Midlands | 19,140 | 19.4 | 435,115 | 18.3 | 19,070 | 19.5 | 111,940 | 19.4 |
| London | 6910 | 7.0 | 125,925 | 5.3 | 6855 | 7.0 | 40,425 | 7.0 |
| North East | 6350 | 6.5 | 137,785 | 5.8 | 6345 | 6.5 | 37,805 | 6.6 |
| North West | 10,960 | 11.1 | 254,725 | 10.7 | 10,910 | 11.1 | 64,455 | 11.2 |
| South East | 5380 | 5.5 | 137,805 | 5.8 | 5315 | 5.4 | 31,050 | 5.4 |
| South West | 7175 | 7.3 | 266,175 | 11.2 | 7105 | 7.3 | 41,305 | 7.2 |
| West Midlands | 5915 | 6.0 | 106,190 | 4.5 | 5835 | 6.0 | 33,735 | 5.9 |
| Yorkshire and Humber | 16,330 | 16.6 | 382,310 | 16.1 | 16,315 | 16.7 | 97,615 | 16.9 |
| Study 2: death |
||||||||
|---|---|---|---|---|---|---|---|---|
| Before matching |
Matched |
|||||||
| Case |
Control |
Case |
Control |
|||||
| na | % | na | % | na | % | na | % | |
| Number of patients | 22,660 | 23.0 | 75,770 | 77.0 | 22,330 | 14.9 | 127,220 | 85.1 |
| Inclusion timeb | ||||||||
| Wave 1 | 6240 | 27.5 | 14,055 | 18.5 | 6160 | 27.6 | 34,895 | 27.4 |
| Wave 2 | 12,350 | 54.5 | 38,030 | 50.2 | 12,195 | 54.6 | 70,505 | 55.4 |
| Wave 3 | 4070 | 18.0 | 23,685 | 31.3 | 3975 | 17.8 | 21,820 | 17.2 |
| Sex | ||||||||
| Male | 13,680 | 60.4 | 40,920 | 54.0 | 13,500 | 60.5 | 77,270 | 60.7 |
| Female | 8980 | 39.6 | 34,850 | 46.0 | 8830 | 39.5 | 49,945 | 39.3 |
| Mean age (SD) | 77.2 (12.2) | 62.3 (17.6) | 77.10 (12.2) | 76.5 (11.9) | ||||
| Age group | ||||||||
| 18–29 | 40 | 0.2 | 2845 | 3.8 | 35 | 0.2 | 160 | 0.1 |
| 30–39 | 155 | 0.7 | 6135 | 8.1 | 150 | 0.7 | 875 | 0.7 |
| 40–49 | 450 | 2.0 | 9440 | 12.5 | 445 | 2.0 | 2565 | 2.0 |
| 50–59 | 1440 | 6.4 | 14,615 | 19.3 | 1435 | 6.4 | 8585 | 6.7 |
| 60–69 | 3145 | 13.9 | 13,855 | 18.3 | 3120 | 14.0 | 18,290 | 14.4 |
| 70–79 | 6285 | 27.7 | 14,345 | 18.9 | 6250 | 28.0 | 37,200 | 29.2 |
| 80+ | 11,150 | 49.2 | 14,530 | 19.2 | 10,890 | 48.8 | 59,545 | 46.8 |
| Practice region | ||||||||
| East | 5275 | 23.3 | 14,995 | 19.8 | 5215 | 23.4 | 29,950 | 23.5 |
| East Midlands | 4440 | 19.6 | 14,695 | 19.4 | 4400 | 19.7 | 25,095 | 19.7 |
| London | 1340 | 5.9 | 5570 | 7.4 | 1325 | 5.9 | 7580 | 6.0 |
| North East | 1390 | 6.1 | 4960 | 6.5 | 1375 | 6.2 | 8050 | 6.3 |
| North West | 2530 | 11.2 | 8430 | 11.1 | 2495 | 11.2 | 14,190 | 11.2 |
| South East | 1115 | 4.9 | 4260 | 5.6 | 1080 | 4.8 | 5985 | 4.7 |
| South West | 1495 | 6.6 | 5675 | 7.5 | 1435 | 6.4 | 7500 | 5.9 |
| West Midlands | 1350 | 6.0 | 4565 | 6.0 | 1300 | 5.8 | 7005 | 5.5 |
| Yorkshire and Humber | 3715 | 16.4 | 12,615 | 16.7 | 3705 | 16.6 | 21,865 | 17.2 |
The counts of patients were round to nearest 5 number in line with disclosure controls.
Wave 1 (early pandemic and first national lockdown, limited COVID-19 test availability for healthcare workers and severe symptoms patients): February–August, 2020; Wave 2 (second national lockdown, COVID-19 availability for wider population): September 2020–April 2021; Wave 3 (end of national lockdown): May–December, 2022.
Exposures
To measure the long-term impact of repeated antibiotic use on COVID-19 infection outcomes, the maximum exposure time frame was set at 3 years. Since acute effects of antibiotics were not of interest in this study, prescriptions issued in the 6 weeks before the index date were excluded (Fig. 1A). Antibiotics in this study were systemic antibiotics for common infections listed in the British National Formulary (BNF) chapter 5.1 (Antibacterial Drugs), except for BNF5.1.9 (Antituberculosis drugs) and BNF5.1.10 (Antileprotic drugs). A total of 55 unique antibiotic (by molecular structure) was prescribed to this study population. These different antibiotic names were referred to as antibiotic types hereafter to indicate the variety of antibiotics use. Quintile groups based on the number of prior antibiotic prescriptions were created to indicate the frequency of prior antibiotic exposure, where the 1st quintile represents low-frequency users and the 5th quintile high-frequency users. Each quintile was further divided into 1–3 groups based on the number of different antibiotic types a patient was prescribed (i.e., group 1, all antibiotics were the same type; group 3, the patient received a total of three different antibiotic types). Patients without antibiotic use in the prior 3 years were classified as an individual group for both variables.
Confounding
Demographic variables including patient-level index of multiple deprivation (IMD) quintile (1–5), ethnicity (White, mixed, south Asian, Black, other), smoking status (current, former, never), and care home residence (yes, no) were extracted from the most recent records. Variables of health status were measured in the most recent 5 years, including body mass index (BMI) categorised into underweight, healthy weight, overweight, obese, and a weighted Charlson Comorbidity Index (CCI) group (no comorbidities, low, medium, high, very high).17 COVID-19 vaccine or influenza vaccine were recorded within 1 year before index date. Detailed classification criteria are listed in Table 2.
Table 2.
Baseline characteristics for cases and controls stratified by outcome.
| Study 1: admitted to hospitals |
Study 2: death |
|||||||
|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Case |
Control |
|||||
| na = 97,880 |
na = 576,640 |
na = 22,330 |
na = 127,220 |
|||||
| na | % | na | % | na | % | na | % | |
| Ethnicity | ||||||||
| White | 76,040 | 77.7 | 404,850 | 70.2 | 18,570 | 83.2 | 107,545 | 84.5 |
| South Asian | 9895 | 10.1 | 44,275 | 7.7 | 1820 | 8.2 | 8720 | 6.9 |
| Black | 3090 | 3.2 | 7535 | 1.3 | 510 | 2.3 | 2650 | 2.1 |
| Mixed | 1045 | 1.1 | 3910 | 0.7 | 145 | 0.6 | 940 | 0.7 |
| Other | 2670 | 2.7 | 8445 | 1.5 | 345 | 1.5 | 2385 | 1.9 |
| Unknown | 5140 | 5.3 | 107,620 | 18.7 | 940 | 4.2 | 4975 | 3.9 |
| BMI categoryb | ||||||||
| Healthy weight (<18.5 kg/m2) | 15,590 | 15.9 | 117,195 | 20.3 | 4720 | 21.1 | 25,690 | 20.2 |
| Underweight (18.5–24.9 kg/m2) | 1490 | 1.5 | 8115 | 1.4 | 560 | 2.5 | 2255 | 1.8 |
| Overweight (25–29.9 kg/m2) | 24,030 | 24.6 | 160,760 | 27.9 | 5940 | 26.6 | 36,675 | 28.8 |
| Obese (≥30 kg/m2) | 35,655 | 36.4 | 144,830 | 25.1 | 7230 | 32.4 | 41,340 | 32.5 |
| Unknown | 21,115 | 21.6 | 145,740 | 25.3 | 3875 | 17.4 | 21,265 | 16.7 |
| CCI groupc | ||||||||
| No comorbidities (0) | 41,975 | 42.9 | 336,100 | 58.3 | 6060 | 27.1 | 42,930 | 33.7 |
| Low (1–2) | 45,315 | 46.3 | 208,655 | 36.2 | 12,210 | 54.7 | 66,440 | 52.2 |
| Medium (3–4) | 9515 | 9.7 | 29,340 | 5.1 | 3595 | 16.1 | 15,990 | 12.6 |
| High (5–6) | 1030 | 1.1 | 2350 | 0.4 | 435 | 1.9 | 1800 | 1.4 |
| Very high (≥7) | 45 | <0.1 | 190 | <0.1 | 25 | 0.1 | 65 | 0.1 |
| Smoking statusd | ||||||||
| Never | 38,095 | 38.9 | 246,155 | 42.7 | 6635 | 29.7 | 42,105 | 33.1 |
| Current | 7395 | 7.6 | 51,305 | 8.9 | 1510 | 6.8 | 7805 | 6.1 |
| Former | 51,695 | 52.8 | 275,020 | 47.7 | 14,130 | 63.3 | 76,975 | 60.5 |
| Unknown | 690 | 0.7 | 4155 | 0.7 | 55 | 0.2 | 335 | 0.3 |
| IMDe | ||||||||
| 1 (least deprived) | 13,125 | 13.4 | 96,390 | 16.7 | 3135 | 14.0 | 19,180 | 15.1 |
| 2 | 16,005 | 16.4 | 108,345 | 18.8 | 3790 | 17.0 | 22,700 | 17.8 |
| 3 | 19,015 | 19.4 | 118,705 | 20.6 | 4360 | 19.5 | 26,210 | 20.6 |
| 4 | 21,270 | 21.7 | 117,735 | 20.4 | 4855 | 21.8 | 26,215 | 20.6 |
| 5 (most deprived) | 26,655 | 27.2 | 121,510 | 21.1 | 5765 | 25.8 | 30,580 | 24.0 |
| Unknown | 1805 | 1.8 | 13,950 | 2.4 | 415 | 1.9 | 2330 | 1.8 |
| Care home residents | 3100 | 3.2 | 37,315 | 6.5 | 1565 | 7.0 | 4630 | 3.6 |
| COVID-19 vaccinef | 22,270 | 22.8 | 193,430 | 33.5 | 4325 | 19.4 | 26,120 | 20.5 |
| Flu vaccineg | 54,925 | 56.1 | 329,720 | 57.2 | 15,845 | 71.0 | 89,650 | 70.5 |
The counts of patients were round to nearest 5 number in line with disclosure controls.
BMI, Body Mass Index as recorded within previous 5 years.
CCI, Charlson Comorbidities Index, measured from 17 weighted conditions, including Myocardial infarction, Congestive heart failure, Peripheral vascular disease, Cerebrovascular disease, Dementia, Chronic pulmonary disease, Connective tissue disease, Ulcer disease, Mild liver disease, Diabetes, Hemiplegia, Moderate or severe renal disease, Diabetes with complications, Any malignancy (including leukaemia and lymphoma), Moderate or severe liver disease, Metastatic solid tumour, AIDS.
Smoking status and care home residents identified from the most recent clinical records.
IMD (Index of Multiple Deprivation) quintile measured from patient-level address.
COVID-19 vaccine identified since vaccination programme started.
Influenza vaccine identified in previous 1 years.
Statistical analysis
Descriptive statistics were used to summarise the characteristics of matching variables for the study population, as well as the distribution of confounders for cases and controls at baseline. Conditional logistic regression was conducted to compare the frequency of antibiotic exposure between cases and controls (using R package survival v3.2-3).18 Odds ratios (ORs) with 95% confidence intervals (95% CI) were estimated based on unadjusted (crude) and fully adjusted models. Models were adjusted for all confounders listed above. Models included a missingness indicator for ethnicity, BMI, IMD, and smoking status; these are less likely to be less recorded for healthy people so did not meet the “missing at random” assumption for imputation.19
Sensitivity analysis
To check for survival bias, selection criteria for COVID-19 hospital admission and death were tested by using a looser definition that either (1) included COVID-19 as a secondary diagnosis of hospital admission and secondary cause of death or (2) extended the assessment time of COVID-19 outcome from 1 month to 3 months. To investigate potential effect of the definition of severe COVID-19 outcomes, further analyses were done using broader criteria for identifying patients: (1) including both COVID-19 hospital admissions and deaths for study 1 and (2) including both Intensive Care Units (ICU) admissions and COVID-19 deaths for study 2. Furthermore, complete case analysis was used to diagnose the consequence of missing data for ethnicity, BMI, smoking, and IMD. More sensitivity analyses were done to investigate potential confounding effects, including analyses stratifying by age group and sex, removing outliers of total antibiotics consumed (90th, 99th and 99.9th percentiles), adjusting for timing of latest antibiotic prescriptions (in days), as well as 6-week exclusion period of antibiotics. Further conditional logistic regression analysis stratified by age and sex, adjusted for individual diseases, and descriptive statistics for comparing matched and non-matched cases were conducted (See Supplementary Tables S2–S9 and Supplementary Figures S2–S8).
Software and reproducibility
Data management was performed using Python 3.8.2, with analysis carried out using R 4.0.2. Code for data management and analysis, as well as code lists (Supplementary Table S10), are archived online (https://github.com/opensafely/amr-uom-brit).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Study participants
Overall, 2.47 million incident COVID-19 patients were identified before matching between February 1, 2020, and December 31, 2021. Of those, 98,420 (4%) were hospitalised for COVID-19. Among all COVID-19 hospitalised patients, 22,660 (23%) died in 30 days after hospital admission (for study 2). A total of 0.67 million patients was included after matching with replacement. Table 1 shows patient characteristics after matching. Compared with controls, cases were less healthy including higher BMI, CCI, and smoking percentage, more likely to be deprived and less likely to be care home residents (as shown in Table 2). To compare the difference between case and control within matched strata, we randomly picked one control case from multiple matches and found similar results (Supplementary Table S1).
Antibiotic counts and COVID-19 outcome
Cases had more frequent antibiotic exposure in the prior 3 years compared to controls in both studies (Table 3). The case group had higher odds of receiving antibiotics than controls and the risk rose along with increased exposure quintiles in both crude and adjusted models. For the highest antibiotic exposure quintile, the adjusted OR was 1.80 (95% CI 1.75–1.84) for hospital admissions, and 1.34 (95% CI 1.28–1.41) for death, compared with patients without antibiotic exposure (reference category). Sensitivity analysis stratified by sex and age groups found no distinct change but adjusted OR were slightly higher in 40–59 age group (OR 2.59, 95% CI 2.44–2.75 for study 1; OR 2.26, 95% CI 1.87–2.74 for study 2) (Supplementary Table S2). To investigate the effect of recent antibiotic exposure time, models were adjusted by the timing of the latest antibiotic prescription, though little difference was found (Supplementary Table S3 & Supplementary Figure S2).
Table 3.
Conditional logistic regression analysis of prior antibiotic exposure levels and COVID-19 outcomes.
| Antibiotic quintile | Study 1: admitted to hospitals |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Crude model |
Adjusted modelc |
|||||||
| Med (25th, 75th)a | nb | % | Med (25th, 75th)a | nb | % | OR | 95% CI | OR | 95% CI | |
| No antibiotics | 0 (0, 0) | 31,830 | 32.5 | 0 (0, 0) | 237,815 | 41.2 | ref | ref | ||
| 1 | 1 (1, 1) | 16,095 | 16.4 | 1 (1, 1) | 105,505 | 18.3 | 1.17 | 1.14–1.19 | 1.03 | 1.01–1.05 |
| 2 | 2 (2, 2) | 10,875 | 11.1 | 2 (2, 2) | 64,515 | 11.2 | 1.32 | 1.29–1.35 | 1.10 | 1.07–1.13 |
| 3 | 3 (3, 3) | 7590 | 7.8 | 3 (3, 3) | 40,695 | 7.1 | 1.48 | 1.44–1.52 | 1.19 | 1.15–1.22 |
| 4 | 5 (4, 6) | 15,450 | 15.8 | 5 (4, 6) | 73,125 | 12.7 | 1.70 | 1.66–1.74 | 1.32 | 1.29–1.35 |
| 5 (most frequent) | 14 (10, 23) | 16,035 | 16.4 | 12 (9, 19) | 54,985 | 9.5 | 2.41 | 2.36–2.47 | 1.80 | 1.75–1.84 |
| Antibiotic quintile | Study 2: death |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Crude model |
Adjusted modelc |
|||||||
| Med (25th, 75th)a | nb | % | Med (25th, 75th)a | nb | % | OR | 95% CI | OR | 95% CI | |
| No antibiotics | 0 (0, 0) | 10,845 | 48.6 | 0 (0, 0) | 67,925 | 53.4 | ref | ref | ||
| 1 | 1 (1, 1) | 3695 | 16.5 | 1 (1, 1) | 22,060 | 17.3 | 1.05 | 1.01–1.10 | 1.01 | 0.97–1.06 |
| 2 | 2 (2, 2) | 1980 | 8.9 | 2 (2, 2) | 10,875 | 8.5 | 1.15 | 1.09–1.21 | 1.07 | 1.02–1.13 |
| 3 | 3 (3, 3) | 1305 | 5.8 | 3 (3, 3) | 6315 | 5.0 | 1.30 | 1.22–1.39 | 1.21 | 1.13–1.29 |
| 4 | 5 (4, 5) | 1830 | 8.2 | 5 (4, 5) | 8625 | 6.8 | 1.35 | 1.28–1.42 | 1.25 | 1.18–1.32 |
| 5 (most frequent) | 13 (9, 28) | 2680 | 12.0 | 13 (9, 24) | 11,415 | 9.0 | 1.48 | 1.41–1.55 | 1.34 | 1.28–1.41 |
Median (25th precentile, 75th percentile) of number of antibiotic prescriptions in each quintile group.
The counts of patients were round to nearest 5 number in line with disclosure controls.
Adjusted for ethnicity, BMI category, CCI group, smoking status, IMD, care home residents, COVID-19 and influenza vaccine.
Antibiotic variety and COVID-19 outcome
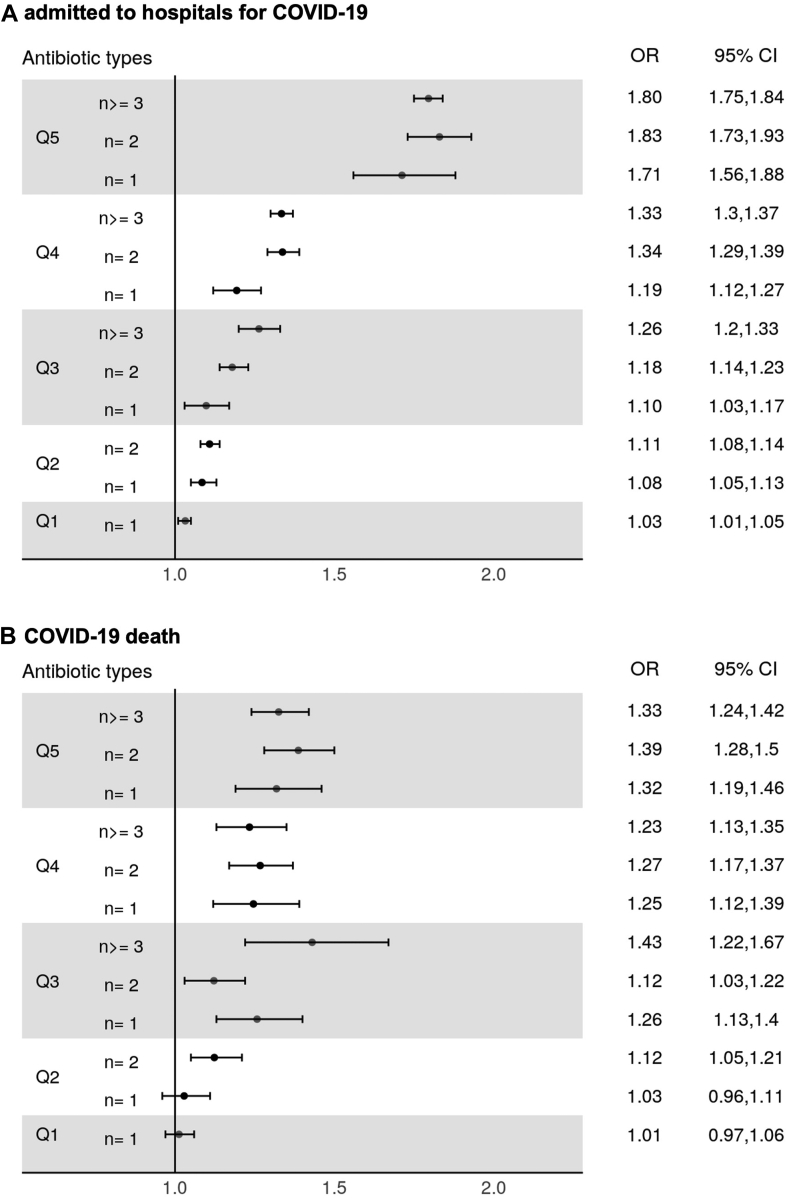
To understand the impact of antibiotics variety on COVID-19 outcome, the breakdown of antibiotic type for each frequency quintile was divided by 1, 2, and 3+ types. Fig. 2 shows that cases were at a higher probability to be exposed to diverse antibiotics and this association increased with the frequency of antibiotic exposure. In study 1, the adjusted OR of quintile 5 exposure (the most frequent) with more than 3 antibiotic types was around 2 times larger than quintile 1 exposure with only 1 type (OR 1.80, 95% CI 1.75–1.84 vs. OR 1.03, 95% CI 1.01–1.05). In study 2, a similar exposure-response association was observed although the magnitude of ORs was smaller. The highest OR was for quintile 3 (middle quintile antibiotic use) with 3 or more types (OR 1.43, 95% CI 1.22–1.67) where no significant difference was found in quintile 1 and 2 with only 1 type. Sensitivity analysis was used to detect the possible outliers of antibiotics prescriptions. After removing patients with the 99.99th, 99th, and 90th percentile of total prescriptions, the overall trend of OR still increased with quintiles and number of types (Supplementary Figure S3), except for quintile 5 with number of types greater than 3 in study 1, where excluding 90th percentile outliers reduced the odds to 1.48 (95% CI 1.42–1.53).
Fig. 2.
Adjusted ORs for COVID-19 outcomes (A. admitted to hospitals; B. death) stratified by number of antibiotic types in the 3 years by quintile (Q1–Q5) of total number of prior antibiotic prescription.
Additional analysis for exclusion period
The main analysis excluded antibiotics in the previous 6 weeks to investigate the long-term effects of varying antibiotic exposure frequency. However, considering that there might be potential confounding from recent antibiotic use, an additional analysis was conducted to adjust for history of antibiotic use during the exclusion period. Three different approaches were applied to measure the confounders including antibiotic exposure (yes/no), the number of antibiotic prescriptions and the number of antibiotic types from 6 weeks before the index date. No obvious change was observed for severe outcome in study 2 but overall adjusted OR decreased in study 1. For example, after adjusting counts of antibiotics in recent 6 weeks and all confounders, OR of quintile 5 decreased to 1.33 (95% CI 1.30–1.37) (Supplementary Table S4) and OR of more than 3 types in quintile 5 decreased to 1.33 (95% 1.29–1.37) (Supplementary Figure S4).
Sensitivity analysis for study definition
The results did not change by relaxing the definition of COVID-19 hospital admissions and COVID-19 deaths as secondary diagnosis inclusive or extending the time frame for same COVID-19 infection episode (Supplementary Table S5 & Supplementary Figure S5). Results were similar when applying broader definition of severe outcome (adding COVID-19 deaths for study 1). However, including ICU admission as severe outcome for study 2 reduced the OR (Supplementary Table S6 & Supplementary Figure S6).
Sensitivity analysis for comorbidities
The prevalence difference of 17 different comorbidities between case and control groups ranged from <1% to 8.2% (Supplementary Table S7A), which showed no major difference in distribution. To understand whether adjustment for a weighted CCI affected the results, an additional model adjusted for 17 individual diseases. This found similar results to the main analysis (Supplementary Table S7B & Supplementary Figure S7).
Sensitivity analysis for exclusion and missing data
Though implementing matching with replacement improved the matching performance, less than 1% cases still failed to find matched controls due to very old ages (Supplementary Table S8 & Supplementary Figure S8). Around 20% of BMI were missing in our analysis. A complete case sensitivity analysis was performed. The adjusted OR of antibiotic exposure frequency only changed minimally compared the original models which included missing data in both studies (Supplementary Table S9 & Supplementary Figure S9).
Discussion
We found an association between prior antibiotic exposure frequency and severe COVID-19 outcomes with a dose–response. This finding was consistent with a previous Spanish study. However, that study used a simpler measure for antibiotic exposure and only covered the first 4 months of the pandemic.20 To our knowledge, the present study is the first to explore the relationship between repeated prior 3-year antibiotics exposure and subsequent severity of COVID-19 infections covering most of the pandemic. Additionally, an association was observed between antibiotic diversity (number of different prior antibiotic types) and COVID-19 related hospital admission.
There may be several explanations for our findings, ranging from a direct effect of antibiotics to potential confounding. A direct effect of antibiotics could involve disruption of the gut microbiome. According to a recent review, the structure of the gut microbiome is relatively stable in adulthood. However, antibiotics are thought to cause acute gut dysbiosis and affect resilience to perturbation due to reduced diversity or richness of bacteria. The gut microbiota play an important role in regulating human immune and metabolic systems, thus frequent antibiotic perturbation might result in adverse effects.21 For example, Sultan (2019) discovered in a population-based database an association between frequency of antibiotic exposure and risk of rheumatoid arthritis (an autoimmune disease).9 Alternatively, antibiotics might also impact the gut resistome which comprises of antibiotic resistance genes (ARG) in gut flora and thus lead to antibiotic resistance, increasing COVID-19 patients' susceptibility to secondary bacterial infection and difficulty in treatment. A previous observational study found that patients with frequent intermittent antibiotic exposure were more likely to suffer from infection-related complications.8 A meta-analysis also reported that 24% of COVID-19 hospital patients presented bacterial resistant co-infection.22 Furthermore, a number of studies have analysed faecal samples and found that the composition of gut microbiota in particular, bacterial species for potential immunomodulation were depleted in COVID-19 patients.23,24 The study by Yeoh et al. (2021) evaluated stool samples and found that gut microbiome composition was significantly altered in patients with COVID-19 compared with non-COVID-19 individuals, which was also correlated with the increased levels of cytokines and inflammatory markers in patients with COVID-19.24 In addition, a recent study found that COVID-19 patients with empiric antibiotic treatment presented with an abundance of ARGs compared to healthy controls.25 Since the samples were collected during COVID-19 hospital admission, a temporal relationship between antibiotics and human health cannot be determined, but these findings support the hypothesis that antibiotics could adversely impact human health through disruption of gut microbiota.
The association observed in this study might be subject to confounding as patients using antibiotics repeatedly may have been more immunocompromised, increasing susceptibility to infections and adverse clinical outcomes. A South Korean study estimated that around 13.5% of COVID-19 patients had history of malignancy, HIV/AIDS, organ transplantation or prescriptions for immunosuppressants.26 However, our sensitivity analysis adjusting for 17 individual diseases including HIV/AIDS and malignancy showed that the ORs did not change substantially. A future study may attempt to elaborate immunosuppressing conditions among patients exposed to antibiotics. However, the high prevalence of repeated antibiotic exposure in patients in primary care may indicate that confounding by being immunocompromised may not fully explain the current findings. Importantly, there is little evidence to suggest that repeated intermittent antibiotic exposure is actually effective in reducing infection-related complications.8 A review by Costelloe et al. (2010) reported that individuals prescribed an antibiotic in primary care for a respiratory or urinary infection are more likely to develop bacterial resistance to that antibiotic.27
The strength of this study was the ability to look at longer-term antibiotic prescribing using OpenSAFELY that linked multiple data sources. It covered a population of more than 20 million patients in England with comprehensive health care data provided. This study has several limitations. First, there might be potential misclassification of cases and controls in this study for several reasons. A COVID-19 episode was defined as lasting one month in line with previous research suggesting the natural trajectory of a COVID-19 infection.28,29 Besides, a sensitivity analysis considering COVID-19 infections lasting for 3 months found no difference. Further analyses by relaxing the criteria of selecting cases with severe outcome also presented similar results, suggesting that prior antibiotic exposure had effect on the severe COVID-19 outcomes regardless of using strict or loose case definitions. Secondly, as we could not fully match the oldest cases (<1%), the results from this study cannot be inferred to this population. Finally, despite that current data had data missing BMI, ethnicity, smoking, and IMD, a complete case analysis found consistent results.
As a trial randomising patients to different antibiotic histories cannot be conducted, the interpretation of this observational study findings may need to consider wider evidence of benefits as well as adverse effects of repeated intermittent antibiotic exposure. There is little evidence to suggest that repeated intermittent antibiotic exposure is effective in reducing infection-related complications. On the other hand, there is accumulating evidence that this antibiotic use may be ineffective or even unsafe, particularly when other research has found that gut microbiome composition was significantly altered in patients with COVID-19, this may explain the accumulative effect of prior antibiotic exposure on covid-19 infection severity observed in this study. A recent commentary around repeated antibiotic prescribing highlighted the need to promote behavioural change. Antibiotic stewardship strategies to reduce inappropriate repeat antibiotic prescribing could include review of these patients with specialised toolkits and habits of collegial reviews.30 Additional strategies could include to clearly outline in antibiotic prescribing guidelines what type of antibiotic is unlikely to be of value.31 Currently, the common infection guidelines in England (as developed by the National Institute for Health and Care Excellence) do not provide guidance around commonly encountered challenges such as the high levels of antibiotic treatment failure, repeated antibiotic use and a patient's risk of developing resistance to the antibiotic.32,33 Furthermore, shared decision-making between patients and clinicians could also include a balanced discussion of the risks for a patient of repeated antibiotic use and future resistance.31
To conclude, we found that antibiotic exposure frequency and diversity were associated with COVID-19 clinical outcome severity. Given the known effects of antibiotics on the gut microbiome, it seems advisable to discourage the regular practice of indiscriminately prescribing antibiotics repeatedly and intermittently given their uncertain benefits and likely risks.
Contributors
All authors contributed to and approved the final manuscript. Design of study, TVS, VP, DW, YY. Analysis: YY. Interpretation of results and first draft of manuscript: YY, VP, DW, TvS. YT, XZ, AF, VP, and JM accessed and verified the underlying data. TvS is the guarantor for the article, and accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
All data were linked, stored and analysed securely within the OpenSAFELY platform https://opensafely.org/. Data include pseudonymised data such as coded diagnoses, medications and physiological parameters. No free text data are included. All code is shared openly for review and re-use under MIT open license (https://github.com/opensafely/amr-uom-brit/). Detailed pseudonymised patient data is potentially re-identifiable and therefore not shared. We rapidly delivered the OpenSAFELY data analysis platform without prior funding to deliver timely analyses on urgent research questions in the context of the global COVID-19 health emergency: now that the platform is established we are developing a formal process for external users to request access in collaboration with NHS England; details of this process will be published shortly on OpenSAFELY.org.
Access to the underlying identifiable and potentially re-identifiable pseudonymised electronic health record data is tightly governed by various legislative and regulatory frameworks, and restricted by best practice. The data in OpenSAFELY is drawn from General Practice data across England where TPP is the Data Processor. TPP developers (CB, JP, FH, SH, JC) initiate an automated process to create pseudonymised records in the core OpenSAFELY database, which are copies of key structured data tables in the identifiable records. These are linked onto key external data resources that have also been pseudonymised via SHA-512 one-way hashing of NHS numbers using a shared salt. DataLab developers and PIs holding contracts with NHS England have access to the OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools. These tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis without direct access to the underlying raw pseudonymised patient data, and to review the outputs of this code. All code for the full data management pipeline—from raw data to completed results for this analysis—and for the OpenSAFELY platform as a whole is available for review at github.com/OpenSAFELY. The data management and analysis code for this paper was led by YY and contributed to by JM, VP, XZ and AF.
Declaration of interests
All authors declare the following: BG and OpenSAFELY has received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. AM has received consultancy fees (from https://inductionhealthcare.com) and is member of RCGP health informatics group and the NHS Digital GP data Professional Advisory Group that advises on access to GP Data for Pandemic Planning and Research (GDPPR). For the latter, he received payment for the GDPPR role.
Acknowledgements
We are very grateful for all the support received from the TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England and the NHS England Transformation Directorate. This work was supported by Health Data Research UK (Better prescribing in frail elderly people with polypharmacy: learning from practice and nudging prescribers into better practice -BetterRx) and by National Institute for Health research (NIHR130581 – Cluster randomised trial to improve antibiotic prescribing in primary care: individualised knowledge support during consultation for general practitioners and patients – BRIT2). This research used data assets made available as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant ref MC_PC_20058). In addition, the OpenSAFELY Platform is supported by grants from the Wellcome Trust (222097/Z/20/Z); MRC (MR/V015757/1, MC_PC-20059, MR/W016729/1); NIHR (NIHR135559, COV-LT2-0073), and Health Data Research UK (HDRUK2021.000, 2021.0157).
NHS England is the data controller; TPP is the data processor and the key researchers on OpenSAFELY are acting with the approval of NHS England. This implementation of OpenSAFELY is hosted within the TPP environment which is accredited to the ISO 27001 information security standard and is NHS IG Toolkit compliant34; patient data has been pseudonymised for analysis and linkage using industry standard cryptographic hashing techniques; all pseudonymised datasets transmitted for linkage onto OpenSAFELY are encrypted; access to the platform is via a virtual private network (VPN) connection, restricted to a small group of researchers; the researchers hold contracts with NHS England and only access the platform to initiate database queries and statistical models; all database activity is logged; only aggregate statistical outputs leave the platform environment following best practice for anonymisation of results such as statistical disclosure control for low cell counts.35 The OpenSAFELY research platform adheres to the obligations of the UK General Data Protection Regulation (GDPR) and the Data Protection Act 2018. In March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 (COPI) to require organisations to process confidential patient information for the purposes of protecting public health, providing healthcare services to the public and monitoring and managing the COVID-19 outbreak and incidents of exposure; this sets aside the requirement for patient consent.36 This was extended in November 2022 for the NHS England OpenSAFELY COVID-19 research platform.37 In some cases of data sharing, the common law duty of confidence is met using, for example, patient consent or support from the Health Research Authority Confidentiality Advisory Group.38 Taken together, these provide the legal bases to link patient datasets on the OpenSAFELY platform. GP practices, from which the primary care data are obtained, are required to share relevant health information to support the public health response to the pandemic, and have been informed of the OpenSAFELY analytics platform.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102064.
Appendix A. Supplementary data
References
- 1.World Health Organization WHO Coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Coronavirus (COVID-19) in the UK. GOV.UK; 2022. https://coronavirus.data.gov.uk/ [Google Scholar]
- 3.Williamson E.J., McDonald H.I., Bhaskaran K., et al. Risks of covid-19 hospital admission and death for people with learning disability: population based cohort study using the OpenSAFELY platform. BMJ. 2021;374 doi: 10.1136/bmj.n1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott J., Bodinier B., Whitaker M., et al. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors. Eur J Epidemiol. 2021;36(3):299–309. doi: 10.1007/s10654-021-00722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultze A., Walker A.J., MacKenna B., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong A.Y.S., MacKenna B., Morton C.E., et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80(7):943–951. doi: 10.1136/ANNRHEUMDIS-2020-219517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Staa T.P., Palin V., Li Y., et al. The effectiveness of frequent antibiotic use in reducing the risk of infection-related hospital admissions: results from two large population-based cohorts. BMC Med. 2020;18(1) doi: 10.1186/s12916-020-1504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultan A.A., Mallen C., Muller S., et al. Antibiotic use and the risk of rheumatoid arthritis: a population-based case-control study. BMC Med. 2019;17(1) doi: 10.1186/s12916-019-1394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange K., Buerger M., Stallmach A., Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 11.Elvers K.T., Wilson V.J., Hammond A., et al. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10(9) doi: 10.1136/BMJOPEN-2019-035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz D.J., Langdon A.E., Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12(1) doi: 10.1186/s13073-020-00782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health England. Ashiru-Oredope D., Hopkins S., ESPAUR Oversight Group Writing Committee . 2020. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2019 to 2020.www.gov.uk/phe%0Awww.facebook.com/PublicHealthEngland [Google Scholar]
- 14.Institute for Government analysis . 2021. Timeline of UK government coronavirus lockdowns and restrictions.https://www.instituteforgovernment.org.uk/data-visualisation/timeline-coronavirus-lockdowns [Google Scholar]
- 15.Mathur R., Rentsch C.T., Morton C.E., et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397(10286):1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho D.E., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 17.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Therneau T.M. 2020. A package for survival analysis in R.https://cran.r-project.org/package=survival [Google Scholar]
- 19.Bhaskaran K., Smeeth L. What is the difference between missing completely at random and missing at random? Int J Epidemiol. 2014;43(4):1336–1339. doi: 10.1093/IJE/DYU080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llor C., Ouchi D., Giner-Soriano M., García-Sangenís A., Bjerrum L., Morros R. Correlation between previous antibiotic exposure and covid-19 severity. A population-based cohort study. Antibiotics. 2021;10(11) doi: 10.3390/antibiotics10111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez J., Guarner F., Bustos Fernandez L., Maruy A., Sdepanian V.L., Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:731. doi: 10.3389/fcimb.2020.572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kariyawasam R.M., Julien D.A., Jelinski D.C., et al. Antimicrobial resistance (AMR) in COVID-19 patients: a systematic review and meta-analysis (November 2019-June 2021) Antimicrob Resist Infect Control. 2022;11(1) doi: 10.1186/S13756-022-01085-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albrich W.C., Ghosh T.S., Ahearn-Ford S., et al. A high-risk gut microbiota configuration associates with fatal hyperinflammatory immune and metabolic responses to SARS-CoV-2. Gut Microbes. 2022;14(1) doi: 10.1080/19490976.2022.2073131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeoh Y.K., Zuo T., Lui G.C.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Y., Chen S., Chen Y., et al. Alterations of fecal antibiotic resistome in COVID-19 patients after empirical antibiotic exposure. Int J Hyg Environ Health. 2022;240 doi: 10.1016/j.ijheh.2021.113882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek M.S., Lee M.T., Kim W.Y., Choi J.C., Jung S.Y. COVID-19-related outcomes in immunocompromised patients: a nationwide study in Korea. PLoS One. 2021;16(10) doi: 10.1371/journal.pone.0257641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costelloe C., Metcalfe C., Lovering A., Mant D., Hay A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 28.Marschner I.C. Estimating age-specific COVID-19 fatality risk and time to death by comparing population diagnosis and death patterns: Australian data. BMC Med Res Methodol. 2021;21(1):126. doi: 10.1186/s12874-021-01314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Poel E., Vanden Bussche P., Klemenc-Ketis Z., Willems S. How did general practices organize care during the COVID-19 pandemic: the protocol of the cross-sectional PRICOV-19 study in 38 countries. BMC Prim Care. 2022;23(1) doi: 10.1186/s12875-021-01587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krockow E.M., Harvey E.J., Ashiru-Oredope D. Addressing long-term and repeat antibiotic prescriptions in primary care: considerations for a behavioural approach. BMJ Qual Saf. 2022;31(11):782–786. doi: 10.1136/bmjqs-2022-014821. [DOI] [PubMed] [Google Scholar]
- 31.Tarrant C., Krockow E.M. Antibiotic overuse: managing uncertainty and mitigating against overtreatment. BMJ Qual Saf. 2021;31(3):163–167. doi: 10.1136/bmjqs-2021-013615. [DOI] [PubMed] [Google Scholar]
- 32.Vincent E. Managing common infections: guidance for primary care. Prim Health Care. 2016;26(7):14. doi: 10.7748/phc.26.7.14.s15. [DOI] [Google Scholar]
- 33.Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. JAC Antimicrob Resist. 2019;1(2) doi: 10.1093/jacamr/dlz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NHS Digital Data security and protection toolkit - NHS Digital. https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit
- 35.NHS Digital ISB1523: anonymisation standard for publishing health and social care data - NHS digital. https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb1523-anonymisation-standard-for-publishing-health-and-social-car
- 36.Secretary of State for Health and Social Care - UK Government . 2020. Coronavirus (COVID-19): notification to organisations to share information.https://web.archive.org/web/20200421171727/https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information [Google Scholar]
- 37.Secretary of State for Health and Social Care - UK Government . 2022. Coronavirus (COVID-19): notice under regulation 3(4) of the health service (control of patient information) Regulations 2002 Apr 2020.https://www.gov.uk/government/publications/coronavirus-covid-19-notification-toorganisations-to-share-information/coronavirus-covid-19-notice-under-regulation-34-of-thehealth-service-control-of-patient-information-regulations-2002 [Google Scholar]
- 38.Confidentiality Advisory Group - Health Research Authority. https://www.hra.nhs.uk/about-us/committees-and-services/confidentiality-advisory-group/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.