Abstract
The herpes simplex virus type 1 (HSV-1) LAT gene is the only viral gene abundantly transcribed during latency. LAT null mutants created with strains McKrae and 17syn+ are impaired for both in vivo spontaneous and in vivo-induced reactivation. Thus, LAT is essential for efficient in vivo-induced and spontaneous reactivation. Different investigators have studied two LAT mutants containing a StyI-StyI region deletion corresponding to LAT nucleotides 76 to 447. One mutant, dLAT371 (parent strain, McKrae), had parental high frequencies of spontaneous reactivation. In vivo-induced reactivation was not examined. The other mutant, 17ΔSty (parent strain, 17syn+), had parental frequencies of in vitro reactivation following cocultivation of explanted ganglia but reduced frequencies of in vivo-induced reactivation. Spontaneous reactivation frequency was not reported for 17ΔSty. These combined results suggested the possibility that in vivo spontaneous reactivation and in vivo-induced reactivation may map to different regions within the LAT domain. We now report that dLAT371 has in vivo-induced reactivation frequencies of the parent and that 17ΔSty has reduced frequencies of in vivo spontaneous reactivation. Thus, dLAT371 demonstrated the parental phenotype for both in vivo spontaneous and -induced reactivation while the apparently identical 17ΔSty was impaired for both in vivo spontaneous and -induced reactivation. These results suggest that one or more differences between the genetic backgrounds of McKrae and 17syn+ result in different in vivo reactivation phenotypes of otherwise identical deletion mutations and that McKrae may have compensating sequences sufficient to overcome the loss of the StyI-StyI region of the LAT transcript.
Herpes simplex virus type 1 (HSV-1) can infect the eye, after which it travels by retrograde axonal transport to the trigeminal ganglia and establishes a latent infection that lasts throughout the life of the infected individual. The virus can reactivate sporadically, return to the eye, and cause recurrent disease. This in turn can produce corneal scarring leading to loss of vision. In the industrialized nations, recurrent ocular HSV-1 is the leading cause of infectious corneal blindness (16).
The only viral gene abundantly transcribed during latency is the latency-associated transcript (LAT) (21, 24). LAT is located in the long repeats of the HSV-1 genome and is therefore present in two copies per genome (Fig. 1). LAT is transcribed as an 8.3-kb RNA that gives rise to a family of LAT RNAs, including a very stable 2.0-kb LAT intron (5, 23, 26–28). LAT null mutants incapable of LAT transcription reactivate poorly by explant culture or induced reactivation in the mouse (12, 13, 22), by induced reactivation in the rabbit(2, 10, 25), and by spontaneous reactivation in the rabbit (1, 9, 17, 18).
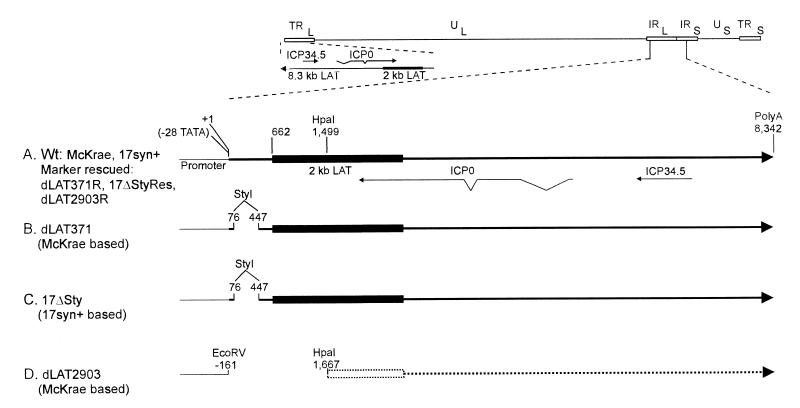
FIG. 1.
Structures of dLAT371 and 17ΔSty. The top of the figure shows a schematic representation of the prototypic orientation of HSV-1 (strains McKrae and 17syn+). HSV-1 contains a unique long region and a unique short region, each of which is flanked by inverted repeats. The unique regions are shown as solid lines. The repeats are shown as open rectangles. UL, unique long; US, unique short; TRL, terminal repeat, long; IRL, internal repeat, long; TRS, terminal repeat, short; IRS, internal repeat, short. The long-repeat regions are expanded to show details of the LAT domain and are indicated by the dashed lines. The locations of the genes for ICP0 and ICP34.5 are shown for reference. (A) Detailed enlargement of the internal-repeat region of parental and marker-rescued viruses. This region contains the 8.3-kb primary LAT transcript. The direction of transcription is indicated by the arrowhead. TATA indicates the location (in the genomic DNA) of the LAT promoter TATA box. The start of LAT transcription is indicated as +1, corresponding to nt 118801 of the genome (15, 20). The filled rectangle within the primary 8.3-kb LAT transcript indicates the location of the stable 2-kb LAT intron starting at LAT nt 662. The locations and directions of the ICP34.5 and ICP0 transcripts are shown for reference. In all viruses shown here, the copy of LAT in the terminal long repeat is identical to the internal long repeat (enlargement shown). (B) dLAT371, a McKrae mutant. The 371-nt deletion is indicated by the break in the line and is bounded by the StyI restriction sites at LAT nt 76 and 447. This deletion is present in both copies of LAT (one in each long repeat). (C) 17ΔSty, a 17syn+ mutant. The StyI-StyI deletion is identical to that shown in panel B. (D) dLAT2903, a McKrae LAT null mutant. The deletion from nt −161 to +1667 encompasses part of the LAT promoter containing the TATA box. The region of LAT downstream of the HpaI site is drawn with a dashed line to indicate that dLAT2903 makes no LAT RNA.
In a previous study, the HSV-1 McKrae-based mutant dLAT371 (Fig. 1B) with a deletion of a 371-nucleotide (nt) StyI-StyI region corresponding to LAT nt 76 to 447 did not affect in vivo spontaneous reactivation in the rabbit ocular model (19). This finding suggests that this region is not essential for efficient spontaneous reactivation. In vivo-induced reactivation was not examined in that study. 17ΔSty (Fig. 1C), a mutant constructed from HSV-1 17syn+ that had the same StyI-StyI deletion as dLAT371 (14), reactivated poorly in the rabbit ocular model following induced in vivo reactivation by iontophoresis of epinephrine (7). 17ΔSty-Res (Fig. 1A), the rescued 17ΔSty mutant, had the high-reactivation phenotype of the parent, 17syn+. Spontaneous reactivation of 17ΔSty was not reported at that time. Thus, in vivo spontaneous reactivation of dLAT371 is at the parental frequency while in vivo-induced reactivation of 17ΔSty is reduced.
The present study was directed at determining if dLAT371, like 17ΔSty, is impaired for in vivo-induced reactivation and if 17ΔSty, like dLAT371, exhibits the parental phenotype for in vivo spontaneous reactivation. If so, these findings may indicate that in vivo-induced reactivation and in vivo spontaneous reactivation are due to different LAT gene functions. If, however, dLAT371 has the McKrae phenotype for in vivo-induced reactivation and 17ΔSty is impaired for spontaneous reactivation, there may be a difference between HSV-1 strains McKrae and 17syn+, such that McKrae is able to compensate for a deletion of 371 nt within the LAT gene.
A genetic map of the viruses used in this report is shown schematically in Fig. 1. The top of Fig. 1 illustrates the HSV-1 genome of both McKrae and 17syn+. In each virus, both copies of LAT (one in each long repeat) are identical. For simplicity, in Fig. 1A to D, only an enlargement of the internal long repeat region is shown. Panel A shows the LAT region of both wild-type parental viruses and the marker-rescued viruses, dLAT371R (19), dLAT2903R (17), and 17ΔSty-Res (14), that have been restored back to the parental phenotype. Panels B and C show the LAT regions of the McKrae-based dLAT371 and the 17syn+-based 17ΔSty, respectively. Both viruses contain a StyI-StyI deletion in both copies of their LAT genes and are otherwise structurally unaltered from their parents (14, 19). The LAT promoters in dLAT371 and 17ΔSty are intact, and both mutants appear to have normal LAT expression and RNA processing despite the fact that the primary LAT transcript is 371 nt shorter. Panel D shows dLAT2903, a McKrae-based LAT null mutant that contains a deletion of LAT nt −161 to +1667 relative to the transcription start sites in both copies of the LAT gene. This mutant does not transcribe any detectable LAT RNA and has previously been shown to be impaired for both in vivo spontaneous and -induced reactivation in latently infected rabbits (17).
To ensure that any differences seen between dLAT371 and 17ΔSty were not due to subtle differences in the rabbit model employed by the two testing laboratories, dLAT371 (constructed and analyzed for spontaneous reactivation by Perng et al. [19]) was sent to the Hill laboratory (the original analysis of 17ΔSty was by Hill et al. [7]). To examine in vivo spontaneous and -induced reactivation, rabbits were infected with either dLAT371 or 17ΔSty, their parent viruses McKrae or 17syn+ (positive controls), dLAT2903 (negative control), or the rescuant of 17ΔSty (17ΔSty-Res). Inoculations with the McKrae viruses were done in unscarified eyes, since McKrae infects unscarified eyes with very high efficiency. Inoculations with the 17syn+ viruses were done in mildly scarified eyes (2 by 2 crosshatch), to increase the efficiency of the acute infection. Kaufman et al. (11) and Garza and Hill (6) used scarification to infect with McKrae to allow for the synchronization of the initial times the lesions appeared. They did not use scarification when inoculating with 17syn+ and saw similar levels of severity of the lesions with only an alteration in the time interval to the visualization of the lesions. Scarification prior to acute infection does not alter reactivation. The rabbits received 2 × 105 PFU of virus in a 25-μl suspension of tissue culture medium. The acute infection was monitored by slit-lamp microscopy until all eyes demonstrated dendritic lesions (postinoculation [p.i.] days 4 through 7 [7]). Rabbits were considered latently infected when the epithelium recovered from the corneal lesion (p.i. day 21). Latently infected rabbits were allowed to reactivate spontaneously or were induced to reactivation by epinephrine iontophoresis. In either case, a tear film was collected daily from each eye and incubated in tubes containing indicator (primary rabbit kidney) cells for detection of cytopathic effect (7, 8).
To examine in vivo spontaneous reactivation, rabbits were inoculated as described above. Cohorts of latently infected rabbits were established as described above, and spontaneous reactivation was analyzed as previously described (1). The numbers of rabbits, eyes, and swabs tested are shown in Table 1 with respect to the number of positive results over the total number of specimens tested for each parameter. The data shown are totals from two experiments, which produced similar results. Spontaneous reactivation of 17ΔSty was significantly reduced compared to that of 17syn+ with respect to rabbits (P = 0.03), eyes (P = 0.0005), and swabs (P = 0.0001). The parental phenotype was restored in the marker-rescued strain 17ΔSty-Res (rabbits and eyes, P = 1.0; swabs, P = 0.53). Thus, the StyI-StyI deletion in 17ΔSty causes impaired in vivo spontaneous reactivation compared to that of 17syn+.
TABLE 1.
In vivo spontaneous reactivationa
| Virus or P value comparison | No. of virus-positive rabbits/total no. of rabbits (%) or P value | No. of virus-positive eyes/total no. of eyes (%) or P value | No. of virus-positive eye swabs/total no. of eye swabsb (%) or P value |
|---|---|---|---|
| Viruses | |||
| 17syn+ | 6/8 (75) | 11/15 (73) | 35/297 (11.8) |
| 17ΔSty | 3/13 (23) | 4/25 (16) | 11/492 (2.2) |
| 17ΔSty-Res | 7/10 (70) | 13/19 (64) | 38/377 (10.1) |
| P value comparisonc | |||
| 17ΔSty vs 17syn+ | 0.03 | 0.0005 | <0.0001 |
| 17ΔSty vs 17ΔSty-Res | 0.04 | 0.0006 | <0.0001 |
| 17syn+ vs 17ΔSty-Res | 1.0 | 1.0 | 0.53 |
| Virusesd | |||
| McKrae | 25/30 (83) | 44/60 (73) | 183/1,570 (11.7) |
| dLAT371 | 5/6 (83) | 9/12 (75) | 38/290 (13.1) |
| dLAT2903 | 13/28 (46) | 15/56 (27) | 37/1,516 (2.4) |
| P value comparisonc | |||
| 17syn+ vs McKrae | 0.62 | 1.0 | 0.42 |
| 17ΔSty vs dLAT371 | 0.04 | 0.0008 | <0.0001 |
| 17ΔSty vs dLAT2903 | 0.19 | 0.40 | 0.93 |
Rabbits were infected with 2 × 105 PFU of the indicated virus per eye. Tears (eye swabs) were collected daily from latently infected rabbits from day 20 to 39 p.i. Spontaneous reactivation was monitored by culturing tears for the presence of infectious HSV-1 as described previously (7).
The total numbers of cultures are slightly less than the numbers of eyes times 20 days, because occasional cultures were lost due to contamination.
Two-sided Fisher exact test or chi-square test if the numbers were too large for the Fisher exact test. Analyses were done with the personal computer program Instat. A P of <0.05 was considered significant.
Data reproduced from a previous publication (19).
The reduced spontaneous reactivation frequencies for 17ΔSty shown in Table 1 differ from data previously reported for dLAT371, which had the parental phenotype for spontaneous reactivation. For comparison purposes, previous results for McKrae, dLAT371, and dLAT2903 (19) are also reported in Table 1. McKrae and 17syn+ had similar frequencies of spontaneous reactivation (rabbits, P = 0.62; eyes, P = 1.0; swabs, P = 0.42). In contrast, spontaneous reactivation of dLAT371 and 17ΔSty were significantly different (rabbits, P = 0.04; eyes, P = 0.0008; swabs, P < 0.0001). Furthermore, the reduced spontaneous reactivation of 17ΔSty appeared similar to the reduced spontaneous reactivation of dLAT2903 (Table 1). These findings confirm that dLAT371 and 17ΔSty have different spontaneous reactivation phenotypes and indicate that spontaneous reactivation of the 17ΔSty mutant is similar to that of dLAT2903, a LAT null mutant.
The numbers of rabbits, eyes, and swabs tested for in vivo-induced reactivation are shown in Table 2 with respect to the number of positive results over the total number of specimens tested. Within all three parameters (rabbits, eyes, and swabs), the in vivo-induced reactivation of dLAT371 was not statistically different than that of McKrae but was significantly greater than that of dLAT2903 (Table 2). Thus, in vivo-induced reactivation of dLAT371 did not appear to be reduced. As previously shown by Perng et al. (17), the LAT null mutant dLAT2903 had significantly reduced in vivo-induced reactivation compared to that of the parent McKrae (Table 2) when results for eyes and swabs were compared. The data from rabbits latently infected with dLAT2903 were not significantly different from data for rabbits latently infected with McKrae, and this is probably due to the smaller than usual number of latently infected McKrae rabbits that were induced to reactivate.
TABLE 2.
In vivo epinephrine-induced ocular reactivation in latently infected rabbitsa
| Virus or P value comparison | No. of virus-positive rabbitsb/total no. of rabbits (%) or P value | No. of virus-positive eyes/total no. of eyes (%) or P value | No. of virus-positive eye swabs/total no. of eye swabs (%) or P value |
|---|---|---|---|
| Viruses | |||
| McKrae | 4/7 (57) | 7/14 (50) | 23/98 (24) |
| dLAT371 | 7/7 (100) | 9/14 (64) | 20/98 (20) |
| dLAT2903 | 4/16 (25) | 4/32 (13) | 8/192 (4) |
| P value comparisonsc | |||
| dLAT371 vs McKrae | 0.19 | 0.70 | 0.73 |
| dLAT371 vs dLAT2903 | 0.001 | 0.0008 | <0.0001 |
| dLAT2903 vs McKrae | 0.18 | 0.01 | <0.0001 |
| Virusesc | |||
| 17syn+ | 7/11 (64) | 12/22 (55) | 48/154 (31) |
| 17ΔSty | 2/9 (22) | 2/18 (11) | 4/126 (3) |
| 17ΔSty-Res | 5/8 (63) | 10/16 (63) | 35/112 (31) |
| P value comparisons | |||
| McKrae vs 17syn+ | 1.0 | 1.0 | 0.2 |
| dLAT371 vs 17ΔSty | 0.003 | 0.003 | <0.0001 |
| dLAT2903 vs 17ΔSty | 1.0 | 1.0 | 0.77 |
| 17ΔSty-Res vs 17syn+ | 1.0 | 0.74 | 1.0 |
Rabbits were infected with 2 × 105 PFU of the indicated virus per eye and subjected to epinephrine iontophoresis approximately 30 days p.i., and in vivo-induced reactivation was monitored by culturing tears daily for 7 consecutive days, as described previously (7). Data presentation and statistical analyses are as described for Table 1.
Number of rabbits in each group that had at least one virus-positive tear culture within 7 days of induction.
Data reproduced from a previous publication (7).
The results shown in Table 2 indicate that dLAT371 was induced by epinephrine iontophoresis to reactivate. 17ΔSty had significantly reduced reactivation by epinephrine iontophoresis compared to that of its parent virus, 17syn+ (7). For comparison purposes, the previous 17ΔSty results are also shown in Table 2. While McKrae and 17syn+ had similar induced reactivation frequencies as evidenced by the swab data (P = 0.2) and rabbit and eye data (P = 1.0), dLAT371 and 17ΔSty were significantly different with respect to the swab data (P < 0.0001) and rabbit and eye data (P = 0.003). Furthermore, the induced reactivation frequency of 17ΔSty was similar to that of dLAT2903 (P > 0.7), both of which were reduced. These findings indicate that dLAT371 and 17ΔSty have different induced reactivation phenotypes and that in vivo-induced reactivation of 17ΔSty is similar to that of the LAT null mutant dLAT2903.
The McKrae-based mutant dLAT371 and the 17syn+-based mutant 17ΔSty have identical deletions of the StyI-StyI region within the 5′ end of the primary LAT transcript. The structures of both mutants were confirmed by Southern blot analysis (7, 14, 19). Both mutants appear to be unimpaired for LAT transcription, with the exception of a smaller transcript, due to the deletion of LAT nt 76 to 447. The sequences for the first 1.5 kb of the LAT genes in McKrae and 17syn+ are very similar, with approximately 98% identity (4). Their StyI-StyI subregions (LAT nt 76 to 447) have a similar level of homology. Within this region, compared to McKrae, 17syn+ has nine mismatched nucleotides, one inserted nucleotide, and one deleted nucleotide, for an overall nucleotide homology of 97%. Without atypical splicing, there are no well-conserved potential open reading frames in this region of LAT (4). Furthermore, both mutants appear to have their respective parental wild-type phenotypes for in vitro and in vivo replication, acute eye disease, neurovirulence, and rate at which latency is established (7). In addition, the results reported here indicate that the apparent in vivo reactivation differences between results for the dLAT371 and 17ΔSty mutants from the two laboratories were not due to analysis of in vivo spontaneous reactivation by one laboratory and analysis of in vivo-induced reactivation by the second laboratory. The results were also not due to differences in the techniques or methodologies used by the laboratories analyzing the mutants. It therefore appears that the difference between the in vivo reactivation phenotypes of these otherwise identical mutants was due to genotypic differences in the parental strains.
The finding that the StyI-StyI region of LAT in strain 17syn+ is essential for in vivo-induced reactivation but that this region of LAT in strain McKrae is not essential for this function was unexpected. Since the StyI-StyI region of LAT in 17syn+ plays a role in in vivo reactivation, it is likely that this region is also important in McKrae; however, we found that StyI-StyI is not necessary for induced reactivation in strain dLAT371. This is not the first reported paradox found in HSV reactivation experiments. Bloom et al. (3) reported that a 348-bp deletion in 17syn+ has reduced in vivo-induced reactivation frequencies. In attempting to define the region responsible for the phenotype, three shorter, nonoverlapping deletion mutants were generated (17Δ110, 17Δ91, and 17Δ116) from the 348-bp deletion. These viral constructs had the high in vivo reactivation frequencies of the parent strain, 17syn+ (3). To date, this paradox is not fully understood and new constructs are being generated and additional experiments are being planned. Our findings here suggest that the McKrae background can compensate for the loss of this region in the LAT gene. The compensatory genomic region may lie either within LAT or elsewhere in the genome. However, since LAT has been shown to be essential for parental frequencies of spontaneous reactivation in McKrae, it is likely that the compensatory region lies within LAT. One possibility is that, at least in McKrae, the LAT domain has multiple functional regions. These regions need not all be present to achieve parental in vivo reactivation frequencies. Also, based on the data from 17Δ348, 17ΔSty, and dLAT371, it is evident that small manipulations of the HSV-1 genome are enough to disturb the in vivo reactivation phenotypes of these viruses. This hypothesis would help explain why natural selection has resulted in conservation of the entire 8.3-kb primary LAT transcript in all HSV-1 strains examined, despite the finding that just the first 1.5 kb of this transcript is sufficient for high frequencies of spontaneous reactivation in rabbits (18).
Acknowledgments
This work was supported by Public Health Service grants EY07566 (S.L.W.), EY10243 (S.L.W.), EY06311 (J.M.H.), and EY02377 (Eye Core Grant); Research to Prevent Blindness; the Discovery Fund for Eye Research; and the Skirball Program in Molecular Ophthalmology.
We thank Maxine Simpson-Evans for expert technical support.
REFERENCES
- 1.Berman E J, Hill J M. Spontaneous ocular shedding of HSV-1 in latently infected rabbits. Investig Ophthalmol Vis Sci. 1985;26:587–590. [PubMed] [Google Scholar]
- 2.Bloom D C, Devi-Rao G B, Hill J M, Stevens J G, Wagner E K. Molecular analysis of herpes simplex virus type 1 during epinephrine-induced reactivation of latently infected rabbits in vivo. J Virol. 1994;68:1283–1292. doi: 10.1128/jvi.68.3.1283-1292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom D C, Hill J M, Devi-Rao G, Wagner E K, Feldman L T, Stevens J G. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J Virol. 1996;70:2449–2459. doi: 10.1128/jvi.70.4.2449-2459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drolet B S, Perng G C, Cohen J, Slanina S M, Yukht A, Nesburn A B, Wechsler S L. The region of the herpes simplex virus type 1 LAT gene involved in spontaneous reactivation does not encode a functional protein. Virology. 1998;242:221–232. doi: 10.1006/viro.1997.9020. [DOI] [PubMed] [Google Scholar]
- 5.Farrell M J, Dobson A T, Feldman L T. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA. 1991;88:790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garza H H, Jr, Hill J M. Effect of a beta-adrenergic antagonist, propranolol, on induced HSV-1 ocular recurrence in latently infected rabbits. Curr Eye Res. 1997;16:453–458. doi: 10.1076/ceyr.16.5.453.7051. [DOI] [PubMed] [Google Scholar]
- 7.Hill J M, Maggioncalda J B, Garza H H, Jr, Su Y-H, Fraser N W, Block T M. In vivo epinephrine reactivation of ocular herpes simplex virus type 1 in the rabbit is correlated to a 370-base-pair region located between the promoter and the 5′ end of the 2.0-kilobase latency-associated transcript. J Virol. 1996;70:7270–7274. doi: 10.1128/jvi.70.10.7270-7274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill J M, O’Callaghan R J, Hobden J A. Ocular iontophoresis. In: Mitra A K, editor. Ophthalmic drug delivery systems. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 331–354. [Google Scholar]
- 9.Hill J M, Sederati F, Javier R T, Wagner E K, Stevens J G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990;174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 10.Hill J M, Wen R, Halford W P. Pathogenesis and molecular biology of HSV latency and ocular reactivation in the rabbit. In: Brown M S, Maclean A R, editors. Herpes simplex virus protocols. Totowa, N.J: Humana Press, Inc.; 1998. pp. 291–315. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman H E, Varnell E D, Gebhardt B M, Thompson H W, Hill J M. Propanolol suppression of ocular HSV-1 recurrence and associated corneal lesions following spontaneous reactivation in the rabbit. Curr Eye Res. 1996;15:680–684. doi: 10.3109/02713689609008909. [DOI] [PubMed] [Google Scholar]
- 12.Leib D A, Bogard C L, Kosz-Vnenchak M, Hicks K A, Coen D M, Knipe D M, Schaffer P A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leib D A, Nadeau K C, Rundle S A, Schaffer P A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci USA. 1991;88:48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggioncalda J, Mehta A, Fraser N W, Block T M. Analysis of a herpes simplex virus type 1 LAT mutant with a deletion between the putative promoter and the 5′ end of the 2.0-kilobase transcript. J Virol. 1994;68:7816–7824. doi: 10.1128/jvi.68.12.7816-7824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 16.Nesburn A B, editor. Report of the corneal disease panel: vision research—a national plan, 1983–1987. II, part III. St. Louis, Mo: C. V. Mosby; 1983. [Google Scholar]
- 17.Perng G-C, Dunkel E C, Geary P A, Slanina S M, Ghiasl H, Kaiwar R, Nesburn A B, Wechsler S L. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perng G-C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J Virol. 1996;70:976–984. doi: 10.1128/jvi.70.2.976-984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perng G-C, Slanina S M, Ghiasi H, Nesburn A B, Wechsler S L. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J Virol. 1996;70:2014–2018. doi: 10.1128/jvi.70.3.2014-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry L J, McGeoch D J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- 21.Rock D L, Nesburn A B, Ghiasl H, Ong J, Lewis T L, Lokensgard J R, Wechsler S L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987;61:3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spivack J G, Fraser N W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988;62:1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesvirus a gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 25.Trousdale M D, Steiner I, Spivack J G, Deshmane S L, Brown S M, MacLean A R, Subak-Sharpe J H, Fraser N W. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991;65:6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler S L, Nesburn A B, Watson R, Slanina S M, Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988;62:4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wechsler S L, Nesburn A B, Watson R, Slanina S M, Ghiasi H. Fine mapping of the major latency-related RNA of herpes simplex virus type 1 in humans. J Gen Virol. 1988;69:3101–3106. doi: 10.1099/0022-1317-69-12-3101. [DOI] [PubMed] [Google Scholar]
- 28.Zwaagstra J C, Ghiasi H, Slanina S M, Nesburn A B, Wheatley S C, Lillycrop K, Wood J, Latchman D S, Patel K, Wechsler S L. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64:5019–5028. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]