Abstract
Alazami syndrome is a rare autosomal recessive neurodevelopmental disorder due to loss-of-function variants in the La ribonucleoprotein 7 (LARP7) gene. Children with Alazami syndrome are most often affected by a combination of primordial dwarfism, intellectual disability, and distinctive facial features. Previous cases have been primarily found in consanguineous families from the Middle East, Asia, and North Africa. We present a 21-month-old Caucasian male from the Midwest United States with nonconsanguineous parents who presented with frequently reported findings of unusual facial features, poor growth, cardiac and genitourinary findings, and developmental delay; less-frequently reported findings, including transient erythroblastopenia of childhood (TEC) and immune deficiency; and never-before reported findings of periventricular nodular heterotopia and stroke. He developed stroke during a hospitalization for Hemophilus influenzae meningitis. The possible contributions of LARP7 to TEC, immune deficiency, brain malformation, and stroke are discussed. Guidelines for the care of Alazami patients are proposed.
Keywords: LARP7, Alazami syndrome, developmental disabilities, primordial dwarfism, intellectual disability, transient erythroblastemia of childhood, immune deficiency, periventricular nodular heterotopia, stroke
Introduction
Alazami syndrome (OMIM: 615071) is a rare autosomal recessive neurodevelopmental disorder caused by pathogenic variants in LARP7 (La ribonucleoprotein 7). 1 It was first identified as a cause of intellectual disability in 2011 2 then described in detail in 2012 in a consanguineous Saudi Arabian family with multiple individuals with intellectual disability, growth retardation, dysmorphic facial features, and microcephaly. 3 At the time of this review, 53 cases were accessible in the literature. Of these cases, 26 cases derive from 13 consanguineous families.
LARP7 encodes for La- related protein 7, a chaperone protein that stabilizes the 7SK non-coding RNA. This RNA chaperone complex then inhibits the p-TEFb transcription factor, preventing RNA polymerase II phosphorylation and transcriptional elongation. The downstream effect is a change in transcription and expression of multiple other genetic and regulatory factors. LARP7 is expressed in all tissues but is most highly expressed in the brain. 4 A LARP7 knockdown leads to growth failure in embryonic cells due to as downregulation of Lin28, which promotes organismal growth. Embryonic stem cells differentiate instead of undergoing cell division, resulting in fewer somatic cells and thus smaller size, resulting in the primordial dwarfism seen in patients with loss of function of LARP7. 5
Affected individuals show a wide range of physical features and developmental outcomes. Facial features reported included deep-set eyes, broad noses, malar hypoplasia and short philtrum. Musculoskeletal and skin findings described include mild epiphyseal changes in proximal phalanges, scoliosis, and thickened skin over hands and feet. 3 Behavioral difficulties such as hyperactivity, aggression, and anxiety have been described, as well as hand wringing and involuntary movements.6–8 We present a new case of Alazami Syndrome in a child with rarely reported findings of transient erythroblastopenia of childhood (TEC) and immune deficiency, and never-before reported findings of periventricular nodular heterotopia and stroke.
Clinical Report
This child initially presented to the Developmental Pediatric Clinic at 20 months of age with concerns for poor growth, global developmental delay and feeding difficulties. He was from a non-consanguineous Caucasian family of European background with no family history of developmental delay. After his premature birth at 34 weeks, he spent 6 weeks in the neonatal intensive care unit where he received continuous positive airway pressure, and nasogastric tube feeds due to aspiration on his initial swallow study. Echocardiogram showed a moderate-sized secundum atrial septal defect (ASD) with left to right shunt. He had pyelectasis; renal and bladder ultrasound revealed bilateral hydronephrosis. Voiding cystourethrogram was normal. His newborn metabolic and hearing screens were normal.
He developed obstructive sleep apnea which was managed with adenoidectomy and supraglottoplasty at 11 months. He had frequent ear infections (>10) requiring myringotomy, and multiple upper respiratory infections. He had 6 weeks history of diarrhea that self-resolved. Gastroenterology workup revealed an elevated erythrocyte sedimentation rate, twice, two months apart, with normal C-reactive protein, thyroid stimulating hormone, IGF1, TTG IgA and total IgA, lactoferrin, calprotectin and pancreatic elastase. Computed tomography of the abdomen and pelvis, revealed bilateral testicles in the inguinal canals.
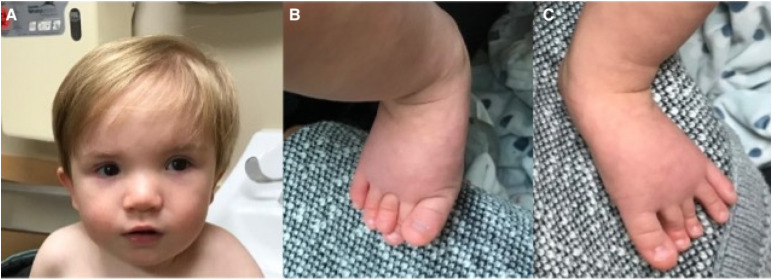
At 20 months old, his developmental age was about 12 months. He could sit unsupported, pull to stand, and cruise. He would wave bye-bye. He made sounds, but no words; speech delay was initially attributed to frequent ear infections. He was unable to complete any task on the 18-month Ages and Stages Questionnaire, third edition. 9 His head circumference was 46 cm (SD -1.37), weight 8.2 kg (SD -3.03), and height 71 cm (SD – 4.91). He was diagnosed with severe malnutrition. He had hypertelorism, telecanthus, bilateral syndactyly and overlapping of toes 2 and 3 bilaterally (Figure 1). He had oropharyngeal dysphasia and delayed swallow trigger on repeat swallow study and received gastric tube placement.
Figure 1.
Physical features of an 18-month-old with Alazami syndrome. (A) Telecanthus (inner canthus outside the insertion of the nasal ala) and hypertelorism (pupils are aligned later to the outer corners of the mouth). (B) Overlapping toes on the right foot. (C) third digit brachydactyly on the left foot.
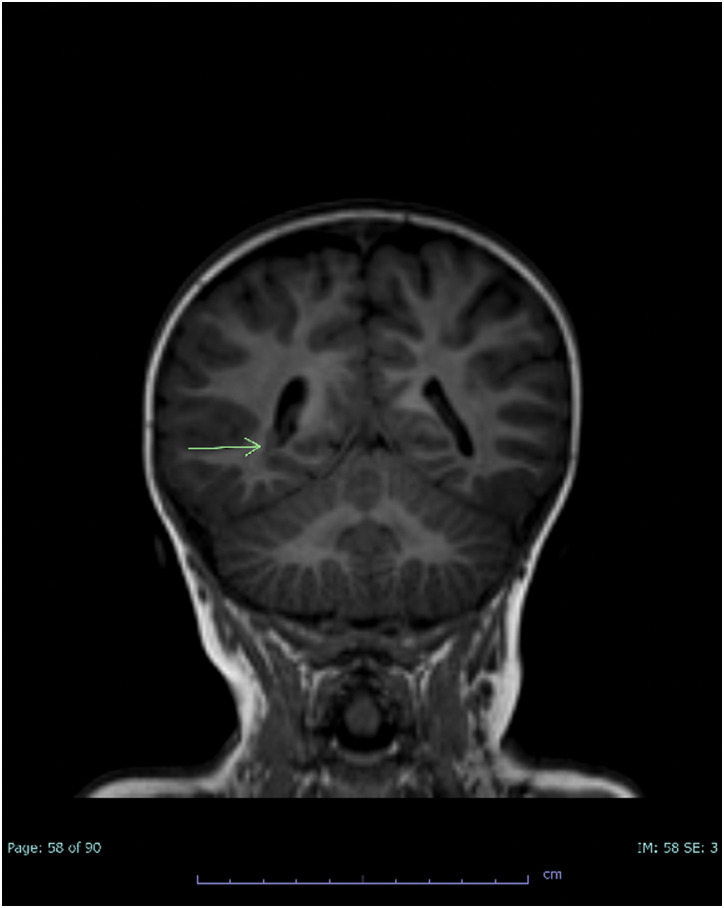
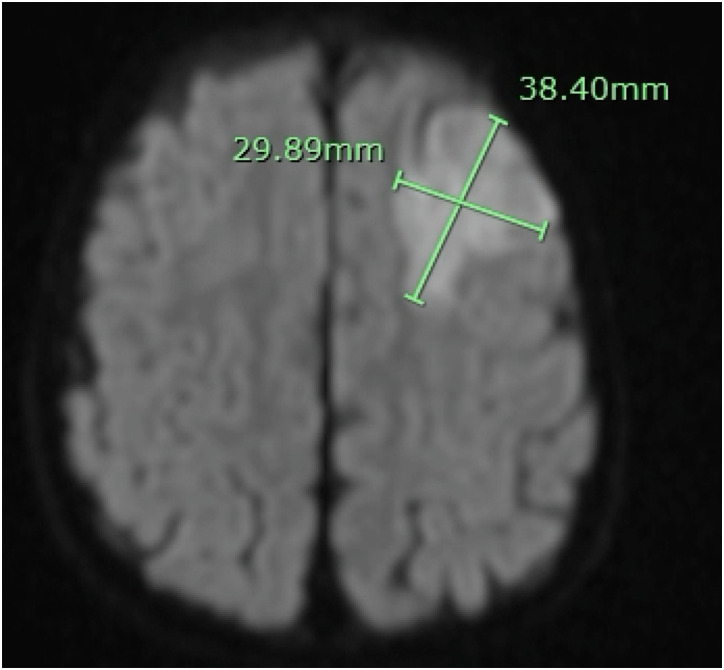
At 21 months old he was admitted with H. influenzae meningitis, serotyping was not performed. He was not up-to-date on all vaccinations. Magnetic resonance imaging (MRI) revealed mild prominence of the frontal horns of the lateral ventricles related to periventricular white matter loss, periventricular nodular heterotopia (Figure 2), leptomeningeal enhancement related to meningitis, a tiny empyema, and infarction in the left anterior frontal lobe (Figure 3). Computed tomography angiography of head and neck were obtained. No large vessel occlusion was found. A retroesophageal right subclavian artery and persistent left trigeminal artery was observed. He was anemic with hemoglobin of 6.8 GM/dl and red blood cells at 2.31 million/cumm and was diagnosed with transient erythroblastopenia of childhood. Prothrombotic workup revealed heterozygosity for Factor V Leiden and elevated lipoprotein a (156 mg/dL, repeat 101 mg/dL when fasting). Repeat echocardiogram showed no hemodynamically significant ASD, and trivial mitral regurgitation. Audiology exam found sensorineural hearing loss.
Figure 2.
Coronal T1 brain MRI demonstrating right periventricular nodular heterotopia (arrow).
Figure 3.
Diffusion-weighted brain MRI demonstrating 3.8 × 3 cm area of restricted diffusion in the left anterior frontal lobe involving the left anterior MCA territory.
Initial immunological evaluation revealed normal T cell, B cell, and natural killer cell enumeration, tetanus response, IgG, IgA, IgM, IgE, and normal CH50. Computed tomography of the abdomen showed normal-sized spleen. Some vaccinations were off-schedule, including H influenzae and pneumococcal conjugate. Vaccine records indicated he received Hib Conjugate (PRP-t) and pneumococcal conjugate (PCV13) at 8 weeks and 8 months, receiving 2 doses out of each 4-dose series. His initial pneumococcal titers were consistent with weak response. He was seen in immunology clinic at 24 months of age, pneumovax vaccination was given. However, repeat pneumococcal titers 4 weeks after vaccination did not show proper response. Subcutaneous immunoglobulin treatment was started due to concern for specific antibody deficiency in the setting of invasive infection history. Unfortunately, further immune phenotyping was not done because the family moved out of state.
Genetic Testing
Consent was obtained from parents and buccal samples were obtained from this patient and both parents. Chromosomal microarray showed a duplication: (arr[GRCh37} 8p23.1 (10391498_10579840) x3 with a duplication of at least 188 kb within 8p23.1. This duplication was initially classified as a variant of unknown significance, then downgraded to likely benign after being confirmed in the patient's mother. Trio whole exome sequencing identified a pathogenic biallelic homozygous variant in LARP7 (c.728_729dup p.S244Qfs*9). The patient was also heterozygous for a pathological patient in G6PC gene (c.79del p.(Q27Rfa*9) p and was considered a carrier for GSD1A.
Discussion
The majority of previous Alazami cases have been found in consanguineous families from the Middle East, Asia, and North Africa. Our patient is a Caucasian male from the Midwest United States with nonconsanguineous parents. He has both common and rare features of Alazami syndrome, as well as several that have not been previously reported.
Common Features in Alazami Syndrome
Unusual facial features, short stature, cardiac defects, and genitourinary defects have been frequently reported in Alazami Syndrome.6,10
Facial features and height: Our patient has a triangular-shaped face, deep-set eyes, narrow palpebral fissures, malar hypoplasia, a prominent forehead, and short stature, all of which have been reported on other Alazami patients. 1 The mechanisms behind the development of typical facial features in Alazami Syndrome are not clear.
Cardiac findings: This patient had a resolved moderate- sized ASD, ASD has been described in other Alazami patients. Other reported cardiac defects include pulmonary stenosis, and patent foramen ovale. 6 Loss of LARP7 contributes to increased endothelial-mesenchymal transition at the endocardial cushion during embryogenesis, leading to abnormalities in heart valve and septum formation.11,12
Genitourinary findings: Our patient had hydronephrosis, undescended testes and a buried penis; hydronephrosis and undescended testes have been found in other Alazami patients. Genitourinary abnormalities described in Alazami patients include hypospadias, inguinal hernias, small kidneys and vesical diverticulum.13–16 Defective spermatogenesis occurred in an Alazami rodent model. 12 The mechanisms behind these are not clear.
Less Common Features of Alazami Syndrome
Facial and other physical features: Our patient had less-frequently reported features including hypertelorism, syndactyly and overlapping toes, strabismus and thickened skin have also been reported in Alazami our patient did not have these.1,6,13,15–19
Hematological and immunological findings: Our patient had TEC, a self-limiting red cell aplasia which may be triggered by infection, but appears to have a genetic component. 20 One case of TEC and several cases of borderline anemia have been reported in Alazami Syndrome. 18 Loss of function in one or both copies of LARP7 leads to decreased telomerase enzymatic activity and stability; telomeropathies are a known cause of anemia and bone marrow disorders.18,21
Our patient also had documented poor response to vaccination that was worse than would be expected due to poor nutrition alone. 22 While immunological workups have not been reported in other children with Alazami, there have been a few other reports of children with frequent ear infections and respiratory infections, which could suggest an associated inborn error of immunity.18,23 LARP7 knockdown suppresses intracellular proliferation of intracellular Legionella bacterial infections, the effect on other bacteria more commonly involved in children's illnesses has not been well studied. 24 Further studies are required to link Alazami syndrome with possible humoral immune deficiency.
Brain malformations: Our patient had periventricular nodular heterotopia, which has not been reported previously in Alazami patients. Brain malformations have been described in a few children with Alazami, but several papers in the Alazami literature do not report brain MRIs. Of the documented MRIs, findings included normal exam, unilateral mild insular and anterior frontal gyrus cortical thickening,3,17 increased signal in periventricular white matter, 4 corpus callosum agenesis and absence of septum pellucidum, 15 thin corpus callosum and disorganized cerebellar hemispheres, 1 Arnold Chiari malformation type 1, venous angioma, 25 and mild myelination delay. 26 LARP7 is most highly expressed in the brain. In rat studies, knockdown of LARP7 reduces perikaryal ribosome content and protein synthesis in rat hippocampal neurons. 27 Further studies may provide additional information on LARP7 role in neurodevelopment.
Clinical Issues in Alazami
Feeding issues: Our patient had ongoing feeding issues; similar problems occurred in 6 other patients.1,14,17,18
Hearing loss: Our patient had hearing loss possibly due to ear infections, issues with tympanostomy tubes and meningitis, possibly related to his immunological issues, Another Alazami patient with mild conductive hearing loss has been described. 16
Sleep disorders: Our patient had sleep apnea; this has been reported in another Alazami patient. 7
Epilepsy: Our patient has not had any signs of epilepsy, but epilepsy has been diagnosed in many other cases of Alazami syndrome.4,7,17,18,23,25
Stroke in This Alazami Patient, and Potential Risk in Others
Our patient appears to be the first reported Alazami patient with stroke. Pediatric stroke is often multifactorial. Meningitis is a known risk factor, 28 and immunological dysfunction in other children with Alazami could predispose to meningitis and stroke. Cardiac defects are another risk factor; our patient's ASD had resolved, but congenital cardiac defects in other Alazami patients could increase stroke risk. Our patient had two prothrombotic risk factors, Factor V Leiden and elevated lipoprotein a that were likely contributory. Prothrombotic evaluation should be part of the pediatric stroke workup in Alazami patients, as it is for the general population of pediatric stroke patients.
Suggested Guidelines for the Care of Alazami patients
The authors suggest that initial exam include a search for common and less-common facial and extremity findings and genitourinary abnormalities; height and weight; and auscultation for heart murmur (Table 1). Heart murmur and genitourinary abnormalities should prompt additional imaging. Brain MRI should be considered in all Alazami patients. EEGs should be obtained if there is a clinical concern for seizures. We suggest immune evaluation, particularly in patients with recurrent, invasive, or unusual infections, or autoimmunity; and at least one complete blood cell count with differential to screen for blood disorders. Other possible health issues to watch for include feeding dysfunction, hearing loss possibly due to multiple ear infections or a factor related to the syndrome, higher rates of infection and sleep apnea.
Table 1.
Propose Guidelines for the Management of Patients with Alazami Syndrome.
| Genetics | Obtain whole exome sequencing to confirm the diagnosis. Offer genetic testing to first-degree relatives for family counseling. |
| Neurology | Obtain a brain MRI in all patients to assess for numerous brain anomalies found including periventricular nodular heterotopia, unilateral mild insular and anterior frontal gyrus cortical thickening corpus callosum agenesis, absence of septum pellucidum, Arnold Chiari malformation type 1, venous angioma and mild myelination delay. Counsel parents on clinical signs of seizures. Obtain an EEG if there are clinical concerns. Counsel parents on signs concerning for stroke. |
| Musculoskeletal system | Monitor for scoliosis and consider spine x-ray if there are clinical concerns. Examine for prominent forehead, triangular shaped faces short stature, syndactyly, overlapping toes, thickened skin, deep set eyes, hypertelorism, narrow palpebral fissures and malar hypoplasia. |
| Cardiology | Assess for heart murmur on clinical exam. Obtain an echocardiogram in all patients. |
| Hematology and Immunology | Obtain a baseline CBC with differential to rule out anemia. Obtain baseline lymphocyte subsets, T and B cell phenotypes, IgG, Ig A, IgM and IgE. Obtain titers to assess vaccine responses to tetanus and pneumococcal vaccines. Repeat a CBC with differential if there are clinical concerns for anemia to rule out transient erythroblastopenia of childhood. Monitor for repeated infections or poor response to vaccinations. |
| Pulmonology | Obtain a polysomnogram if there are concerns for snoring, restless sleep or apneic events |
| Gastrointestinal | Monitor for poor feeding. Obtain a swallow study for patients with poor weight gain or concern for aspiration. Examine for signs of malnourishment, Consult dietician if there are clinical concerns. |
| Genitourinary | Obtain a renal bladder ultrasound to assess for anomalies such as hydronephrosis, small kidneys, or vesical diverticulum. Monitor blood pressure due to reports of premature hypertension. Refer to urology if there are clinical exam findings of buried penis or hypospadias. |
| Ophthalmology | Examine for strabismus and refer to ophthalmology if indicated. |
| Audiology | Obtain a baseline hearing assessment. Repeat hearing test if there are clinical concerns, a history of multiple ear infection or meningitis. |
| Development | Assess for developmental delay. Refer to speech therapy, occupational therapy and physical therapy where indicated. |
Conclusion
As whole exome sequencing becomes more commonly used, the authors expect more Alazami patients to be identified around the world. We welcome additional insights into the evaluation and care of these children.
Acknowledgments
The authors would like to thank the family of this patient for their assistance and cooperation in the preparation of this management. The family hopes that other cases will be reported so long-term care guidelines can be developed.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iDs: Kristin D. Fauntleroy-Love https://orcid.org/0000-0002-8579-3200
Meredith R. Golomb https://orcid.org/0000-0002-9707-6735
References
- 1.Wojcik MH, Linnea K, Stoler JM, Rappaport L. Updating the neurodevelopmental profile of Alazami syndrome: illustrating the role of developmental assessment in rare genetic disorders. Am J Med Genet A. 2019;179(8):1565-1569. doi: 10.1002/ajmg.a.61189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478(7367):57-63. doi: 10.1038/nature10423 [DOI] [PubMed] [Google Scholar]
- 3.Alazami AM, Al-Owain M, Alzahrani F, et al. Loss of function mutation in LARP7, chaperone of 7SK ncRNA, causes a syndrome of facial dysmorphism, intellectual disability, and primordial dwarfism. Hum Mutat. 2012;33(10):1429-1434. doi: 10.1002/humu.22175 [DOI] [PubMed] [Google Scholar]
- 4.Kazemi G, Peymani F, Mohseni M, et al. Novel mutation in LARP7 in two Iranian consanguineous families with syndromic intellectual disability and facial dysmorphism. Arch Iran Med. 2020;23(12):842-847. doi: 10.34172/aim.2020.112 [DOI] [PubMed] [Google Scholar]
- 5.Dai Q, Luan G, Deng Let al. et al. Primordial dwarfism gene maintains Lin28 expression to safeguard embryonic stem cells from premature differentiation. Cell Rep. 2014;7(3):735-746. doi: 10.1016/j.celrep.2014.03.053 [DOI] [PubMed] [Google Scholar]
- 6.Ivanovski I, Caraffi SG, Magnani E, et al. Alazami syndrome: the first case of papillary thyroid carcinoma. J Hum Genet. 2020;65(2):133-141. doi: 10.1038/s10038-019-0682-5 [DOI] [PubMed] [Google Scholar]
- 7.Imbert-Bouteille M, Mau Them FT, Thevenon J, et al. LARP7 Variants and further delineation of the Alazami syndrome phenotypic spectrum among primordial dwarfisms: 2 sisters. Eur J Med Genet. 2019;62(3):161-166. doi: 10.1016/j.ejmg.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Ling TT, Sorrentino S. Compound heterozygous variants in the LARP7 gene as a cause of Alazami syndrome in a Caucasian female with significant failure to thrive, short stature, and developmental disability. Am J Med Genet A. 2016;170(1):217-219. doi: 10.1002/ajmg.a.37396 [DOI] [PubMed] [Google Scholar]
- 9.Squires J, Bricker D. Ages & Stages Questionnaires®, Third Edition (ASQ®-3): A Parent-Completed Child Monitoring System. Paul H. Brookes Publishing Co., Inc.; 2009. [Google Scholar]
- 10.Patalan M, Lesniak A, Bernatowicz Ket al. et al. Patient with phenylketonuria and intellectual disability-problem not always caused exclusively by insufficient metabolic control (coexistence of PKU and Alazami syndrome). Int J Envron Res Public Health. 2022;19(5):2574. doi: 10.3390/ijerph19052574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anbara T, Sharifi M, Aboutaleb N. Endothelial to mesenchymal transition in the cardiogenesis and cardiovascular diseases. Curr Cardiol Rev. 2020;16(4):306-314. doi: 10.2174/1573403X15666190808100336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X, Wu S, Geng Z, et al. LARP7 Suppresses endothelial-to-mesenchymal transition by coupling with TRIM28. Circ Res. 2021;129(9):843-856. doi: 10.1161/CIRCRESAHA.121.319590 [DOI] [PubMed] [Google Scholar]
- 13.Dateki S, Kitajima T, Kihara T, Watanabe S, Yoshiura KI, Moriuchi H. Novel compound heterozygous variants in the LARP7 gene in a patient with Alazami syndrome. Hum Genome Var. 2018;5(1):18014. doi: 10.1038/hgv.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das S, Godbole K, Abraham SSC, Ganesan P, Kamdar P, Danda S. Alazami syndrome: report of three Indian patients with phenotypic spectrum from adolescence to adulthood. Am J Med Genet A. 2021;185(5):1606-1609. doi: 10.1002/ajmg.a.62118 [DOI] [PubMed] [Google Scholar]
- 15.Gana S, Plumari M, Rossi E, et al. Alazami syndrome: phenotypic expansion and clinical resemblance to Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2020;182(11):2722-2726. doi: 10.1002/ajmg.a.61832 [DOI] [PubMed] [Google Scholar]
- 16.Alzahrani MS. Alazami syndrome in an Afghani girl: a case report and review of literature. Global J Rare Dis. 2019;4(1):3-6. doi: 10.17352/2640-7876.000012 [DOI] [Google Scholar]
- 17.Al-Hinai A, Al-Hashmi S, Ganesh Aet al. Further phenotypic delineation of Alazami syndrome. Am J Med Genet. 2022;188(8):2485-2490. doi: 10.1002/ajmg.a.62778 [DOI] [PubMed] [Google Scholar]
- 18.Hollink IH, Alfadhel M, Al-Wakeel AS, et al. Broadening the phenotypic spectrum of pathogenic LARP7 variants: two cases with intellectual disability, variable growth retardation and distinct facial features. J Hum Genet. 2016;61(3):229-233. doi: 10.1038/jhg.2015.134 [DOI] [PubMed] [Google Scholar]
- 19.Holohan B, Kim W, Lai TP, et al. Impaired telomere maintenance in Alazami syndrome patients with LARP7 deficiency. BMC Genomics. 2016;17(Suppl 9):749. doi: 10.1186/s12864-016-3093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns RA, Woodward GA. Transient erythroblastopenia of childhood: a review for the pediatric emergency medicine physician. Pediatr Emerg Care. 2019;35(3):237-240. doi: 10.1097/PEC.0000000000001760 [DOI] [PubMed] [Google Scholar]
- 21.Collopy LC, Ware TL, Goncalves T, et al. LARP7 family proteins have conserved function in telomerase assembly. Nat Commun. 2018;9(1):557. doi: 10.1038/s41467-017-02296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671):20140141. doi: 10.1098/rstb.2014.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soengas-Gonda E, Perez de la Fuente R, Arteche-López A. Expanding the phenotypic spectrum of Alazami syndrome: two unrelated Spanish families. Neuropediatrics. 2022;54(01):031-036. doi: 10.1055/a-1947-8411 [DOI] [PubMed] [Google Scholar]
- 24.Von Dwingelo J, Chung I, Price CTet al. et al. Interaction of the Ankyrin H core effector of Legionella with the host LARP7 component of the 7SK snRNP complex. mBio. 2019;10(4):e01942-19. doi: 10.1128/mBio.01942-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmas M, Yıldız H, Erdoğan M, Gogus B, Avcı K, Solak M. Comparison of clinical parameters with whole exome sequencing analysis results of autosomal recessve patients; a center experience. Mol Biol Rep. 2019;46(1):287-299. doi: 10.1007/s11033-018-4470-7 [DOI] [PubMed] [Google Scholar]
- 26.Palumbo P, Palumbo O, Leone MP, et al. Compound phenotype due to recessive variants in LARP7 and OTOG genes disclosed by an integrated approach of SNP-array and whole exome sequencing. Genes (Basel). 2020;11(4):379. doi: 10.3390/genes11040379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slomnicki LP, Malinowska A, Kistowski M, et al. Nucleolar enrichment of brain proteins with critical roles in human neurodevelopment. Mol Cell Proteomics. 2016;15(6):2055-2075. doi: 10.1074/mcp.M115.051920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunbar M, Shah H, Shinde Set al. et al. Stroke in pediatric bacterial meningitis: population-based epidemiology. Pediatr Neurol. 2018;89:11-18. doi: 10.1016/j.pediatrneurol.2018.09.005 [DOI] [PubMed] [Google Scholar]