Abstract
Diabetes and cancer are two heterogenous diseases which are rapidly increasing in prevalence globally. A link between these two non-communicable diseases was first identified over 100 years ago; however, recent epidemiological studies and advances in genomic research have provided greater insight into the association between diabetes and cancer. Epidemiological studies have suggested that individuals with diabetes have an increased risk of several types of cancer (including liver, pancreas, colorectal, breast, and endometrial) and an increased risk of cancer mortality. However, this increased risk is not observed in all cancers, for example, there is a reduced risk of prostate cancer in individuals with diabetes. It has also been observed that cancer patients have an increased risk of developing diabetes, highlighting that the relationship between these diseases is not straightforward. Evidence of a shared genetic aetiology along with numerous lifestyle and clinical factors have made it challenging to establish if the relationship between the two diseases is causal or a result of confounding factors. This review takes a pan-cancer approach to highlight the complexities of the interactions between type 2 diabetes and cancer development, indicating where advances in genomic research have enabled a greater insight into these two diseases.
Keywords: carcinoma, diabetes, molecular genetics
Introduction
Diabetes is a complex metabolic disease characterised by hyperglycaemia as a result of abnormal insulin function (Cho et al. 2018, Cole & Florez 2020). Circulating glucose levels are regulated by glucagon and insulin which work together in a negative feedback loop to increase and decrease glucose levels, respectively. In people without diabetes, glucose levels are maintained at 4–7 mmol/L prior to eating and rise to no more than 9 mmol/L 2 hours after consuming a meal (https://www.nhs.uk/conditions/high-blood-sugar-hyperglycaemia/), whilst those with diabetes commonly present glucose levels above the expected range of 7 mmol/L (hyperglycaemia) and are often unable to regulate glucose without clinical intervention. If left untreated, prolonged hyperglycaemia can increase the risk of chronic diabetic complications, including retinopathy, neuropathy, nephropathy, cardiovascular disease, and cancer (Nguyen et al. 2012, Chilelli et al. 2013, Baena-Díez et al. 2016). An association between diabetes and cancer was first described over 100 years ago with an observed positive correlation in male death rates (Claremont 1916). Although unable to determine causality at this time, in 1893, King and Newsholme determined that the increase in cancer death rates coincided with more accurate diagnosis and certification of death (King & Newsholme 1893), whilst Claremont (1916) observed the correlation over 20 years later and found the uniformity of the increase unlikely to be a result of improved diagnosis alone. Further studies in the early 1900s went on to question whether individuals with diabetes were more at risk of developing cancer or whether individuals with cancer developed symptoms that could be characterised as diabetes (Wilson & Maher 1932).
More recent observational epidemiological studies have demonstrated that individuals with type 2 diabetes (T2D) have a higher lifetime risk of several types of cancer, including liver, pancreas, colorectal, breast, and endometrial (Giovannucci et al. 2010, Duan et al. 2014, Ryu et al. 2014, Vincent & Yaghootkar 2020, Hu et al. 2021, Pearson-Stuttard et al. 2021). Individuals with prediabetes/metabolic syndrome are also associated with an increased risk of several common cancers, suggesting that the development of diabetes is not conditional to the development of cancer (Esposito et al. 2012). In addition, it has been repeatedly shown that cancer patients with pre-existing diabetes have increased mortality rates than cancer patients without diabetes (Carstensen et al. 2014). However, it is difficult to dissociate whether this observed increase in mortality is due to the reduced life expectancy of both diseases or whether the interactions between cancer and diabetes contribute to the increased risk of mortality. Focussing on the shared genetic aetiology between T2D and cancer, this review aims to provide an overview of the complex genetic relationship shared by these heterogenous diseases (Fig. 1). Diabetes encompasses several different forms, and the most common types are type 1 diabetes, T2D and gestational diabetes (Solis-Herrera et al. 2018). However, due to the differing aetiology of diabetes, this review will solely focus on the genetic overlap between T2D and cancer.
Figure 1.
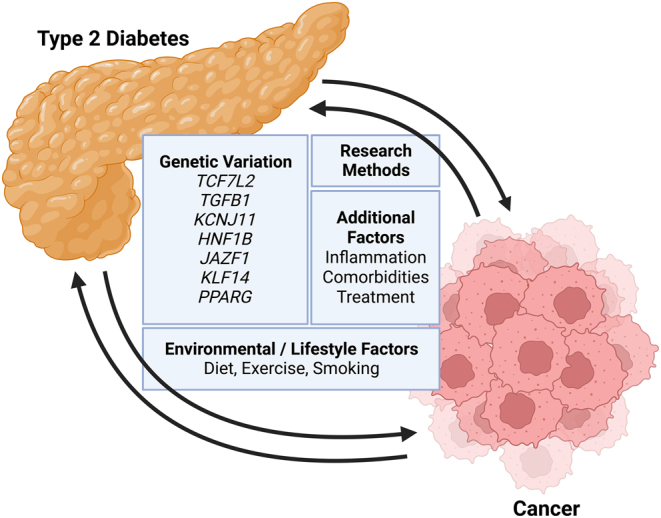
Review overview. This diagram depicts the multiple factors impacting the complex relationship between type 2 diabetes (T2D) and cancer. Focusing on the genetic overlap, this review covers seven genes which provide a plausible explanation for the interactions between T2D and cancer. It is important to note that this relationship is not limited to genetics and strongly influenced by environmental/lifestyle factors, treatments, comorbidities, and metabolic alterations such as inflammation. The review also discusses a range of research methods from observational epidemiological studies to Mendelian randomisation. Advancing genomic technologies and in silico methods are enabling a greater insight into the interactions between T2D and cancer (data fromLeedy et al. 2022).
Genetic variation
The biological drivers of many complex diseases remained largely unclear until the development of genome-wide association studies (GWAS) in 2005. GWAS have demonstrated that late-onset diseases such as T2D and cancer are more commonly comprised of multiple low-impact variants rather than single-gene causation (Barroso & McCarthy 2019). Multiple genetic loci have been identified which increase the risk of both diabetes and cancer; however, the degree to which these explain the overlap in the occurrence of these diseases is currently poorly understood. Advances in genomic analysis technologies and the generation of increasingly well-powered and curated datasets have provided an opportunity to look more closely at the association between diabetes and cancer (Sud et al. 2017, Barroso & McCarthy 2019). Genetic variation within the protein coding gene TCF7L2 is a well-evidenced example in which genetic variants and risk alleles independently predispose an individual to both T2D and cancer. The TCF7L2 gene encodes a transcription factor which acts as a nuclear receptor for β-catenin and subsequently mediates canonical Wnt signalling (Smith 2007, Adams & Vella 2018). Wnt signalling is an essential pathway for regulating cellular function, embryonic development and stem cell renewal (Flanagan et al. 2018). However, the role of Wnt signalling in carcinogenesis has also been well characterised most prominently in colorectal cancer (Zhan et al. 2017). Wnt signalling is essential for regulating homeostasis within intestinal tissue and alterations to the Wnt pathway resulting from genetic alterations have been shown to disrupt intestinal–crypt villus architecture and therefore cellular homeostasis (Korinek et al. 1998, Flanagan et al. 2018). Mutations impacting the signalling of canonical pathways have also been associated with cancer treatment resistance. In order to combat treatment resistance, studies of transcription factor TCF4 (encoded by TCF7L2) have demonstrated TCF4 as a potential metabolic target to sensitise treatment-resistant cells (Kendziorra et al. 2011, Osher & Macaulay 2019). Mutations in TCF7L2 have also been shown in breast, hepatocellular, and aggressive prostate cancer, supporting the role of Wnt signalling in tumourigenesis.
Variation on the TCF7L2 locus has also been associated with T2D, leading to a predisposition to the disease, most notably in the T allele at rs7903146. The diabetes-associated T allele has been observed to impair glucose tolerance through glucagon and insulin secretion (Shah et al. 2016). In addition, Shim et al. (2016) andSainz et al. (2012) replicated an association between T2D risk alleles within the TCF7L2 gene and a higher risk of colorectal cancer, supporting an association between the two diseases. Despite this supporting evidence, other studies have been unable to replicate strong associations and highlight inconsistencies when investigating other cancers such as breast cancer (Hou et al. 2012, Zhao et al. 2016). This suggests that despite the TCF7L2 gene demonstrating an association with an increased susceptibility to T2D and cancer development, shared causality is more difficult to determine.
Transforming growth factor-beta (TGFB) signalling also has shown an important role in the association between diabetes and cancer. The gene TGFB1 has a regulatory role in TGFB signalling through activation of the SMAD pathway (Polfus et al. 2021). Inhibition of TGFB signalling has been shown to promote pancreatic β cell replication in an adaptive response to insulin demand (Polfus et al. 2021).Dhawan et al. (2016) demonstrated that TGFB signalling induces INK4a expression. INK4a proteins are important for cellular senescence and apoptosis; therefore, the increased INK4a expression results in the decline of β cells and has thus been associated with T2D. The therapeutic inhibition of TGFB signalling could reduce the production of INK4a proteins specifically p16INK4a and subsequently increase β cell replication as shown in murine models (Dhawan et al. 2016). p16INK4a is a protein used in the regulation of the cell cycle and demonstrates altered expression in cancer (Romagosa et al. 2011), providing a potential mechanism linking both diabetes and cancer. A greater understanding of the interaction of these genetic and epigenetic pathways could inform the identification of novel therapeutic targets for both diseases (Dhawan et al. 2016).
Moving away from signalling pathways, another gene commonly associated with diabetes and cancer is KCNJ11. Pancreatic β cells mediate insulin secretion through ATP-sensitive potassium channels. These channels comprise of four potassium ion channel tetramers (Kir6.2), which work together to form the pore of the potassium channel (Haghvirdizadeh et al. 2015). The Kir6.2 tetramers are encoded by the KCNJ11 gene, which has been linked to an increased susceptibility of T2D through the disruption of insulin secretion pathways. Although the exact mechanism remains unclear, it is suggested that single-nucleotide polymorphisms (SNPs) within the KCNJ11 gene influence the structure and function of Kir6.2. Therefore, SNPs within this gene would disrupt insulin secretion through the ATP potassium channel and subsequently disrupt the glucose metabolism, β cell homeostasis and give rise to hyperinsulinaemia over time (McTaggart et al. 2010). It has also been shown that the SNPs within KCNJ11 which increase susceptibility to diabetes also increase colorectal cancer risk, which could suggest that hyperinsulinaemia plays a role in colorectal carcinogenesis (Giovannucci et al. 2010, Cheng et al. 2011).
Whilst there is evidence of a shared genetic aetiology between diabetes and many forms of cancer, there are exceptions to this in which T2D appears to ameliorate the risk of certain types of cancer and reduce cancer mortality. Epidemiological studies have observed that individuals with diabetes demonstrated a reduced risk of prostate cancer when compared to those without diabetes (Kasper et al. 2009, Tsilidis et al. 2015). Whilst this relationship is multifactorial, the HNF1B and JAZF1 genes have been associated with an increased risk of diabetes and decreased risk of prostate cancer. One study utilised luciferase reporter assays to demonstrate that SNPs altered the binding of microRNAs and subsequently suggested that the binding of these microRNAs regulates HNF1B gene expression and therefore T2D susceptibility (Goda et al. 2015). Examining the relationship through two large cohorts (the Cancer Prevention Study II Nutrition Cohort and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial), one study indicated that HNF1B variants are directly associated with both diabetes and prostate cancer. However, it was clear that the variants investigated do not mediate the relationship between the two diseases (Stevens et al. 2010). Evidence surrounding this gene in the risk of different types of cancer is conflicting, as the overexpression of HNF1B has also been associated with clear cell epithelial ovarian cancer. However, the effect varies dependent on the cancer subtype, highlighting that this relationship is not straightforward (Shen et al. 2013). Similar conflicting evidence also occurs in prostate cancer; a GWAS of European ancestry demonstrated that one SNP within intron 2 of the JAZF1 gene provided an association with prostate cancer (Prokunina-Olsson et al. 2010). Whilst a genetic relationship has been noted between T2D and prostate cancer, other studies have associated the reduction in cancer risk with alterations in insulin, insulin-like growth factor 1, and testosterone levels in individuals with diabetes. However, reduced detection of prostate cancer in individuals with diabetes must not be overlooked. Obese males with diabetes generally demonstrate lower prostate-specific antigen levels than individuals without diabetes. This is likely to reduce the detection and diagnosis of prostate cancer, which may also contribute to the reduced incidence of prostate cancer demonstrated in people with diabetes (Wu et al. 2011, Preston et al. 2014).
In contrast to HNF1B, JAZF1 also mediates metabolic stress and p53 stress pathways, including the metabolic process of T2D via interactions with nuclear receptors and protein kinases similar to TCF7L2 (Kobiita et al. 2020). SNPs within JAZF1 have been strongly associated with T2D through multiple mechanisms, including obesity, which epidemiological studies have shown to be the most important risk factor for T2D impacting insulin resistance and disease progression (Wu et al. 2014). Studies have shown that overexpression of JAZF1 reduces the expression of acetyl–coenzyme A carboxylase, fatty acid synthetase, and sterol regulatory element–binding protein 1 messenger RNA, all of which are essential for maintaining cellular homeostasis (Li et al. 2011). Li et al. (2011) also demonstrated that the overexpression of Jazf1 in a murine cell line reduced lipogenesis and increased lipolysis, which subsequently impacts the glucose metabolism and could play a role in the association with T2D (Li et al. 2011). Similarly linked with obesity through a build-up in adipose tissue, genetic variants in close proximity to the KLF14 gene have been associated with metabolic disease and increased cancer risk, providing another example of genetic variation impacting both diabetes and cancer (Yang & Civelek 2020).
Further investigation of the relationship with adipose tissue and obesity identified that PPARG is associated with adipogenesis and the development of insulin resistance commonly found in T2D (Schwenk et al. 2013). PPARG encodes the peroxisome proliferator-activated receptor subfamily of nuclear receptors. The overexpression of PPARG has been demonstrated in multiple cancers, including colon, breast, and pancreas; however, the biological role of PPARG is deemed controversial. PPARG has demonstrated anticancer effects through the promotion of cancer cell differentiation, cell-cycle arrest, and apoptosis, suggesting a tumour-suppressive role (Tang et al. 2011). PPARG has also demonstrated tumour-promoting properties through the stimulation of angiogenesis (Eibl 2008). This unclear evidence suggests that the role of PPARG and many other genetic variants is context dependent (Vincent & Yaghootkar 2020). This conflicting association provides a final example of the vast variation of genes shared by T2D and cancer. Genetic variants not only provide a plausible explanation for the complex associations between diabetes and cancer but also provide an opportunity for novel therapeutics. PPARG for example has also been utilised for its therapeutic properties as the binding of fatty acids, fatty acid derivatives, and ligands such as thiazolidinediones activates PPARGdemonstrating a role in insulin sensitivity and provides a therapeutic target. A novel thiazolidinedione (LPSF/SF-13) has shown a good affinity for PPARG, further supporting its potential therapeutic role (De Melo Rêgo et al. 2014). In order to utilise the potential therapeutic role of genetic variants, there is a requirement for a greater understanding of causality.
Mendelian randomisation
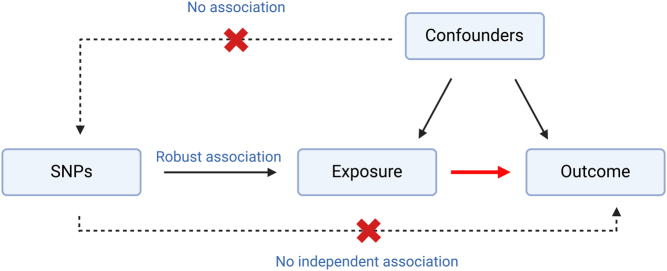
In recent studies, Mendelian randomisation (MR) has provided a technique to investigate causality and confounding (Fig. 2). Large-scale studies have begun to investigate the role that specific glycaemic traits (including fasting glucose, 2-hour glucose, and HbA1c) may have in the development of cancer, using MR approaches (Murphy et al. 2022). The role these traits have in the development of cancer have the potential to give insight into how T2D influences cancer due to the disruption of traits primarily involved in T2D development. MR is an analysis tool that uses genetic variants to establish causal relationships from observational data (Davies et al. 2018). The analysis is based on Mendel’s law of inheritance and instrumental variable estimation methods (Sanderson et al. 2022). Recent approaches using two-sample MR found no significant genetic evidence supporting the increased risk of cancer as a result of diabetes (Goto et al. 2020, Yuan et al. 2020). Although overall cancer risk differed from retrospective epidemiological studies, Yuan et al. (2022) demonstrated 6 of 22 site-specific cancers risks were influenced by diabetes, four sites of which demonstrated an increased cancer risk whilst two sites demonstrated a decreased cancer risk (Yuan et al. 2020). The study demonstrated little evidence supporting a causal association between fasting glucose and cancer. However, genetically predicted fasting insulin levels were positively associated with some site-specific cancers included in the study, suggesting that insulin resistance in early diabetes may contribute to cancer risk (Yuan et al. 2020).
Figure 2.
Summary schematic of Mendelian randomisation (MR). Single-nucleotide polymorphisms (SNPs) are selected as an instrumental variable to look at the causal relationship between an exposure and an outcome indicated by the red arrow. The method of MR relies on three assumptions indicated by the blue text. Assumption 1: There must be a robust association between the chosen SNPs and exposure variable. Assumption 2: The chosen SNPs must be independent of any confounding factors. Assumption 3: There must be no independent association between the SNPs and outcome variable. Any violation of these assumptions invalidates the study and can limit the method of MR (data from Gala & Tomlinson 2020).
Murphy et al. (2022) also identified an association between fasting insulin and carcinogenesis in the largest MR analysis of glycaemic traits and colorectal cancer to date. The study determined the causal effect of glycaemic traits on colorectal cancer risk including 48,214 cases and 64,159 controls and provided evidence that higher fasting insulin levels increased colorectal cancer risk (Murphy et al. 2022). Murphy et al. (2022) demonstrated no evidence in support for other glycaemic traits including fasting glucose or 2-hour glucose, which suggests that increased insulin levels are likely to be the primary driver of positive associations between T2D and colorectal cancer in this study. These results support a causal effect of higher fasting insulin levels, which creates an avenue for novel therapeutics and supports further investigation into the use of insulin as a clinical marker. These findings also suggest that interventions to decrease insulin levels may inhibit tumourigenesis (Murphy et al. 2022). Overall, MR studies demonstrate the necessity for large, highly powered datasets which are required to investigate the complex relationship between glucose metabolism and tumourigenesis. However, there are limitations to MR (Fig. 2), and it is important to note the many other factors influencing the relationship between diabetes and cancer which cannot be explored using this method.
Additional influences
There are multiple factors to consider in addition to genetics, including metabolic alterations, inflammation, and treatment of T2D. It is beyond the scope of this review to discuss the numerous factors which contribute to the complex relationship between diabetes and cancer in detail. However, it is important to highlight that these factors may also be contributing or confounding to this relationship. For example, obesity is considered to be one of the most significant confounders of T2D. Increased volume of adipose tissue can increase the secretions of adipokines such as leptin and inflammatory molecules such as macrophages which accumulate in the adipose tissue and can stimulate a downstream inflammatory response such as the release of proinflammatory cytokines, tumour necrosis factor alpha, and interleukin-6 (Weisberg et al. 2003, Olli et al. 2013, Spielman et al. 2014). Whilst inflammation is often used as a protective response, overstimulation can contribute to disease initiation and progression of cancer and diabetes. Comorbidities such as obesity and cardiovascular complications can also have significant implications in treatment choice.Lega et al. (2018) highlighted that individuals with diabetes often receive less aggressive cancer treatment, which is likely due to their diabetic comorbidities. This could lead to a poorer cancer prognosis and increased cancer progression in individuals with diabetes.
There is also significant evidence of a treatment overlap between diabetes and cancer through metformin. Metformin is currently the first-line treatment for patients with T2D (Foretz et al. 2019). The impressive safety profile of metformin and pharmacological benefits demonstrated in individuals with diabetes has led to investigations into the use of metformin as a cancer therapeutic (Liu et al. 2016). In vitro and in vivo studies have demonstrated decreased malignant cell proliferation and increased apoptosis in lung cancer and prostate cancer when treated with metformin (Mallik & Chowdhury 2018), whilst improved patient outcomes have also been observed in many other cancers including endometrial, hepatocellular, and ovarian (Mallik & Chowdhury 2018). However, controversy shrouds the preclinical success as studies have utilised doses 10–1000-fold greater than peak human in vivo plasma levels, making the success of metformin as a cancer therapeutic uncertain (Dowling et al. 2012, Lord et al. 2018). Advancing systematic bioinformatic approaches are also providing an opportunity for drug discovery and drug repurposing using genomic data. When investigating potential drug targets for T2D, Imamura et al. (2016) discussed T2D-susceptibility genes such as KIF11 to be involved in cancer treatment as inhibitors of the KIF11 gene product have been developed as chemotherapeutic agents, further supporting a novel opportunity for therapeutics using genetic information.
Whilst discussing the multitude of factors impacting both diabetes and cancer, it is evident that overall improvements in lifestyle such as diet and exercise can aid in the prevention of T2D and the subsequent disease complications. Further clinical trials into metformin and other treatments indicate the potential adaptability of existing therapeutics and support overlapping biological mechanisms between these two diseases. Whilst discussing the shared genetic factors, a concerted effort into whole-genome sequencing has the potential to provide a novel insight into the genetic overlap between diabetes and cancer. A greater understanding into the associations between T2D and cancer through a combination of approaches is essential to improve treatments and patient outcomes.
Conclusion
Despite significant efforts across the fields of diabetes and cancer research, the biological drivers shared between these two diseases are complex, and much remains to be understood. The heterogeneity shared by both diseases highlights the growing need for novel and innovative research to understand the underlying mechanisms to improve current therapeutics. Existing studies have taken a pan-cancer approach to extensively investigated genetic variants, impacting both diabetes and cancer. A shared genetic aetiology between T2D and cancer provides a plausible explanation for an increased cancer risk within individuals with T2D, most notably through TCF7L2. However, while many T2D-susceptibility genes are also involved in cancer development, this does not necessarily imply there is a shared causality. Increasingly highly powered datasets and advancing in silico methods are providing novel insights into the genetic drivers. Methods such as MR provide insight into causality which has previously been difficult to determine. This review sets out to highlight the relationship between diabetes and cancer, not only focussing on shared genetic variants but also emphasising the importance of lifestyle and clinical factors which influence an individuals’ risk of diabetes and cancer. Moving forward in this field, third-generation sequencing is providing a novel opportunity to investigate this complex relationship. Long-read whole-genome sequencing is beginning to uncover large structural variants which have previously been left undiscovered when using short-read sequencing techniques. Precision medicine approaches such as nanopore technologies are enabling research into both the genetic and epigenetic landscape of cancer and subsequently the impact of diabetes on cancer biology. In addition, prospective population-based studies are also providing novel discoveries into this relationship, with new robust MR methods and larger datasets beginning to investigate site-specific, sex-specific, and ancestral differences. These advances are working to differentiate the possible mechanisms involved in the interactions of diabetes and cancer, which will allow improvements to clinical care through precision medicine.
Declaration of interest
No conflict of interest.
Funding
This work was supported by the University of Exeter Medical School and Mireille Gillings Professorial Fellowship (Professor Chrissie Thirlwell).
Author contribution statement
M.E. planned and wrote the paper; A.P.W. and C.T. contributed to the planning and outline of the paper in addition to reviewing the manuscript.
Acknowledgements
This work was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- Adams JD & Vella A. 2018What can diabetes-associated genetic variation in TCF7L2 teach us about the pathogenesis of type 2 diabetes? Metabolic Syndrome and Related Disorders 16383–389. ( 10.1089/met.2018.0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Díez JM, Peñafiel J, Subirana I, Ramos R, Elosua R, Marín-Ibañez A, Guembe MJ, Rigo F, Tormo-Díaz MJ, Moreno-Iribas C, et al. 2016Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care 391987–1995. ( 10.2337/dc16-0614) [DOI] [PubMed] [Google Scholar]
- Barroso I & McCarthy MI. 2019The genetic basis of metabolic disease. Cell 177146–161. ( 10.1016/j.cell.2019.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen B Jørgensen ME & Friis S. 2014The epidemiology of diabetes and cancer. Current Diabetes Reports 14535. ( 10.1007/s11892-014-0535-8) [DOI] [PubMed] [Google Scholar]
- Cheng I, Caberto CP, Lum-Jones A, Seifried A, Wilkens LR, Schumacher FR, Monroe KR, Lim U, Tiirikainen M, Kolonel LN, et al. 2011Type 2 diabetes risk variants and colorectal cancer risk: the Multiethnic Cohort and PAGE studies. Gut 601703–1711. ( 10.1136/gut.2011.237727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilelli NC Burlina S & Lapolla A. 2013AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a “glycoxidation-centric” point of view. Nutrition, Metabolism, and Cardiovascular Diseases 23913–919. ( 10.1016/j.numecd.2013.04.004) [DOI] [PubMed] [Google Scholar]
- Cho NH Shaw JE Karuranga S Huang YD da Rocha Fernandes JD Ohlrogge AW & Malanda B. 2018IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice 138271–281. ( 10.1016/j.diabres.2018.02.023) [DOI] [PubMed] [Google Scholar]
- Claremont CA.1916On the correlation between the “corrected” cancer and diabetes deathrates. Biometrika 11191–200. ( 10.1093/biomet/11.3.191) [DOI] [Google Scholar]
- Cole JB & Florez JC. 2020Genetics of diabetes mellitus and diabetes complications. Nature Reviews. Nephrology 16377–390. ( 10.1038/s41581-020-0278-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NM Holmes MV & Smith GD. 2018Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362k601. ( 10.1136/bmj.k601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Melo Rêgo MJB Galdino-Pitta MR Pereira DTM da Silva JC Rabello MM de Lima MDCA Hernandes MZ da Rocha Pitta I Galdino SL & da Rocha Pitta MG. 2014Synthesis, in vitro anticancer activity and in silico study of new disubstituted thiazolidinedione derivatives. Medicinal Chemistry Research 233220–3226. ( 10.1007/s00044-013-0902-z) [DOI] [Google Scholar]
- Dhawan S Dirice E Kulkarni RN & Bhushan A. 2016Inhibition of TGF-β signaling promotes human pancreatic β-cell replication. Diabetes 651208–1218. ( 10.2337/db15-1331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ Niraula S Stambolic V & Goodwin PJ. 2012Metformin in cancer: translational challenges. Journal of Molecular Endocrinology 48R31–R43. ( 10.1530/JME-12-0007) [DOI] [PubMed] [Google Scholar]
- Duan W Shen X Lei J Xu Q Yu Y Li R Wu E & Ma Q. 2014Hyperglycemia, a neglected factor during cancer progression. BioMed Research International 2014461917. ( 10.1155/2014/461917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl G.2008The role of PPAR-γ and its interaction with COX-2 in pancreatic cancer. PPAR Research 2008326915. ( 10.1155/2008/326915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K Chiodini P Colao A Lenzi A & Giugliano D. 2012Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 352402–2411. ( 10.2337/dc12-0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan DJ Austin CR Vincan E & Phesse TJ. 2018Wnt signalling in gastrointestinal epithelial stem cells. Genes 9 178. ( 10.3390/genes9040178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M Guigas B & Viollet B. 2019Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nature Reviews. Endocrinology 15569–589. ( 10.1038/s41574-019-0242-2) [DOI] [PubMed] [Google Scholar]
- Gala H & Tomlinson I. 2020The use of Mendelian randomisation to identify causal cancer risk factors: promise and limitations. Journal of Pathology 250541–554. ( 10.1002/path.5421) [DOI] [PubMed] [Google Scholar]
- Giovannucci E Harlan DM Archer MC Bergenstal RM Gapstur SM Habel LA Pollak M Regensteiner JG & Yee D. 2010Diabetes and cancer: a consensus report. Diabetes Care 331674–1685. ( 10.2337/dc10-0666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda N Murase H Kasezawa N Goda T & Yamakawa-Kobayashi K. 2015Polymorphism in microRNA-binding site in HNF1B influences the susceptibility of type 2 diabetes mellitus: a population based case–control study. BMC Medical Genetics 1675. ( 10.1186/s12881-015-0219-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A, Yamaji T, Sawada N, Momozawa Y, Kamatani Y, Kubo M, Shimazu T, Inoue M, Noda M, Tsugane S, et al. 2020Diabetes and cancer risk: a Mendelian randomization study. International Journal of Cancer 146712–719. ( 10.1002/ijc.32310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghvirdizadeh P Mohamed Z Abdullah NA Haghvirdizadeh P Haerian MS & Haerian BS. 2015KCNJ11: genetic polymorphisms and risk of diabetes mellitus. Journal of Diabetes Research 2015908152. ( 10.1155/2015/908152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou N, Zheng Y, Gamazon ER, Ogundiran TO, Adebamowo C, Nathanson KL, Domchek SM, Rebbeck TR, Simon MS, John EM, et al. 2012Genetic susceptibility to Type 2 diabetes and breast cancer risk in women of European and African AncestryType. Cancer Epidemiology, Biomarkers and Prevention 21552–556. ( 10.1158/1055-9965.EPI-11-0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhang X, Ma Y, Yuan C, Wang M, Wu K, Tabung FK, Tobias D, Hu FB, Giovannucci E, et al. 2021Incident type 2 diabetes duration and cancer risk: a prospective study in two US cohorts. Journal of the National Cancer Institute 113381–389. ( 10.1093/jnci/djaa141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, Takahashi A, Yamauchi T, Hara K, Yasuda K, Grarup N, Zhao W, Wang X, Huerta-Chagoya A, Hu C, et al. 2016Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nature Communications 7 10531. ( 10.1038/ncomms10531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper JS Liu Y & Giovannucci E. 2009Diabetes mellitus and risk of prostate cancer in the health professionals follow‐up study. International Journal of Cancer 1241398–1403. ( 10.1002/ijc.24044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendziorra E, Ahlborn K, Spitzner M, Rave-Fränk M, Emons G, Gaedcke J, Kramer F, Wolff HA, Becker H, Beissbarth T, et al. 2011Silencing of the Wnt transcription factor TCF4 sensitizes colorectal cancer cells to (chemo-) radiotherapy. Carcinogenesis 321824–1831. ( 10.1093/carcin/bgr222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G & Newsholme A. 1893On the alleged increase of cancer. The Hospital 14124. ( 10.1098/rspl.1893.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiita A Godbersen S Araldi E Ghoshdastider U Schmid MW Spinas G Moch H & Stoffel M. 2020The diabetes gene JAZF1 is essential for the homeostatic control of ribosome biogenesis and function in metabolic stress. Cell Reports 32 107846. ( 10.1016/j.celrep.2020.107846) [DOI] [PubMed] [Google Scholar]
- Korinek V Barker N Moerer P van Donselaar E Huls G Peters PJ & Clevers H. 1998Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genetics 19379–383. ( 10.1038/1270) [DOI] [PubMed] [Google Scholar]
- Leedy D Tiwana JK Mamas M Hira R & Cheng R. 2022Coronary revascularisation outcomes in patients with cancer. Heart 108507–516. ( 10.1136/heartjnl-2020-318531) [DOI] [PubMed] [Google Scholar]
- Lega IC Austin PC Fischer HD Fung K Krzyzanowska MK Amir E & Lipscombe LL. 2018The impact of diabetes on breast cancer treatments and outcomes: a population-based study. Diabetes Care 41755–761. ( 10.2337/dc17-2012) [DOI] [PubMed] [Google Scholar]
- Li L Yang Y Yang G Lu C Yang M Liu H & Zong H. 2011The role of JAZF1 on lipid metabolism and related genes in vitro. Metabolism: Clinical and Experimental 60523–530. ( 10.1016/j.metabol.2010.04.021) [DOI] [PubMed] [Google Scholar]
- Liu X Romero IL Litchfield LM Lengyel E & Locasale JW. 2016Metformin targets central carbon metabolism and reveals mitochondrial requirements in human cancers. Cell Metabolism 24728–739. ( 10.1016/j.cmet.2016.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Cheng WC, Liu D, Gaude E, Haider S, Metcalf T, Patel N, Teoh EJ, Gleeson F, Bradley K, et al. 2018Integrated pharmacodynamic analysis identifies two metabolic adaption pathways to metformin in breast cancer. Cell Metabolism 28679–688.e4. ( 10.1016/j.cmet.2018.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R & Chowdhury TA. 2018Metformin in cancer. Diabetes Research and Clinical Practice 143409–419. ( 10.1016/j.diabres.2018.05.023) [DOI] [PubMed] [Google Scholar]
- McTaggart JS Clark RH & Ashcroft FM. 2010The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. Journal of Physiology 5883201–3209. ( 10.1113/jphysiol.2010.191767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N, Song M, Papadimitriou N, Carreras-Torres R, Langenberg C, Martin RM, Tsilidis KK, Barroso I, Chen J, Frayling TM, et al. 2022Associations between glycemic traits and colorectal cancer: a Mendelian randomization analysis. Journal of the National Cancer Institute 114740–752. ( 10.1093/jnci/djac011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DV Shaw LC & Grant MB. 2012Inflammation in the pathogenesis of microvascular complications in diabetes. Frontiers in Endocrinology 3 170. ( 10.3389/fendo.2012.00170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olli K Lahtinen S Rautonen N & Tiihonen K. 2013Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. British Journal of Nutrition 10943–49. ( 10.1017/S0007114512000888) [DOI] [PubMed] [Google Scholar]
- Osher E & Macaulay VM. 2019Therapeutic targeting of the IGF axis. Cells 8 895. ( 10.3390/cells8080895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, Rizos EC, Monori G, Ward HA, Kyrgiou M, et al. 2021Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiology and Prevention Biomarkers 301218–1228. ( 10.1158/1055-9965.EPI-20-1245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polfus LM, Darst BF, Highland H, Sheng X, Ng MCY, Below JE, Petty L, Bien S, Sim X, Wang W, et al. 2021Genetic discovery and risk characterization in type 2 diabetes across diverse populations. HGG Advances 2 100029. ( 10.1016/j.xhgg.2021.100029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MA Riis AH Ehrenstein V Breau RH Batista JL Olumi AF Mucci LA Adami HO & Sørensen HT. 2014Metformin use and prostate cancer risk. European Urology 661012–1020. ( 10.1016/j.eururo.2014.04.027) [DOI] [PubMed] [Google Scholar]
- Prokunina-Olsson L, Fu YP, Tang W, Jacobs KB, Hayes RB, Kraft P, Berndt SI, Wacholder S, Yu K, Hutchinson A, et al. 2010Refining the prostate cancer genetic association within the JAZF1 gene on Chromosome 7p15. 2JAZF1 and prostate cancer. Cancer Epidemiology, Biomarkers and Prevention 191349–1355. ( 10.1158/1055-9965.EPI-09-1181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagosa C Simonetti S Lopez-Vicente L Mazo A Lleonart ME Castellvi J & Ramon y Cajal S. 2011p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 302087–2097. ( 10.1038/onc.2010.614) [DOI] [PubMed] [Google Scholar]
- Ryu TY Park J & Scherer PE. 2014Hyperglycemia as a risk factor for cancer progression. Diabetes and Metabolism Journal 38330–336. ( 10.4093/dmj.2014.38.5.330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz J Rudolph A Hoffmeister M Frank B Brenner H Chang-Claude J Hemminki K & Försti A. 2012Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. Journal of Clinical Endocrinology and Metabolism 97E845–E851. ( 10.1210/jc.2011-2565) [DOI] [PubMed] [Google Scholar]
- Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, Palmer T, Schooling CM, Wallace C, Zhao Q, et al. 2022Mendelian randomization. Nature Reviews Methods Primers 21–21. ( 10.1038/s43586-021-00092-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk RW Vogel H & Schürmann A. 2013Genetic and epigenetic control of metabolic health. Molecular Metabolism 2337–347. ( 10.1016/j.molmet.2013.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M Varghese RT Miles JM Piccinini F Dalla Man C Cobelli C Bailey KR Rizza RA & Vella A. 2016TCF7L2 genotype and α-cell function in humans without diabetes. Diabetes 65371–380. ( 10.2337/db15-1233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, Cicek MS, Tyrer J, Stram D, Larson MC, et al. 2013Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nature Communications 41–10. ( 10.1038/ncomms2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim HJ Lee R Shin MH Kim HN & Kweon SS. 2016Association between the TCF7L2 polymorphism and colorectal cancer does not differ by diabetes and obesity statuses. Cancer Epidemiology 45108–111. ( 10.1016/j.canep.2016.10.012) [DOI] [PubMed] [Google Scholar]
- Smith U.2007TCF7L2 and type 2 diabetes—we WNT to know. Diabetologia 505–7. ( 10.1007/s00125-006-0521-z) [DOI] [PubMed] [Google Scholar]
- Solis-Herrera C Triplitt C Reasner C DeFronzo RA & Cersosimo E. 2018Classification of diabetes mellitus. Endotext. (https://pubmed.ncbi.nlm.nih.gov/25905343/) [Google Scholar]
- Spielman LJ Little JP & Klegeris A. 2014Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. Journal of Neuroimmunology 2738–21. ( 10.1016/j.jneuroim.2014.06.004) [DOI] [PubMed] [Google Scholar]
- Stevens VL Ahn J Sun J Jacobs EJ Moore SC Patel AV Berndt SI Albanes D & Hayes RB. 2010HNF1B and JAZF1 genes, diabetes, and prostate cancer risk. Prostate 70601–607. ( 10.1002/pros.21094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud A Kinnersley B & Houlston RS. 2017Genome-wide association studies of cancer: current insights and future perspectives. Nature Reviews. Cancer 17692–704. ( 10.1038/nrc.2017.82) [DOI] [PubMed] [Google Scholar]
- Tang H Dong X Hassan M Abbruzzese JL & Li D. 2011Body mass index and obesity-and diabetes-associated genotypes and risk for pancreatic cancer. Cancer Epidemiology, Biomarkers and Prevention 20779–792. ( 10.1158/1055-9965.EPI-10-0845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilidis KK, Allen NE, Appleby PN, Rohrmann S, Nöthlings U, Arriola L, Gunter MJ, Chajes V, Rinaldi S, Romieu I, et al. 2015Diabetes mellitus and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. International Journal of Cancer 136372–381. ( 10.1002/ijc.28989) [DOI] [PubMed] [Google Scholar]
- Vincent EE & Yaghootkar H. 2020Using genetics to decipher the link between type 2 diabetes and cancer: shared aetiology or downstream consequence? Diabetologia 631706–1717. ( 10.1007/s00125-020-05228-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP McCann D Desai M Rosenbaum M Leibel RL & Ferrante AW. 2003Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation 1121796–1808. ( 10.1172/JCI19246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB & Maher HC. 1932Cancer and tuberculosis with some comments on cancer and other diseases. American Journal of Cancer 16227–250. ( 10.1158/ajc.1932.227) [DOI] [Google Scholar]
- Wu C Moreira DM Gerber L Rittmaster RS Andriole GL & Freedland SJ. 2011Diabetes and prostate cancer risk in the REDUCE trial. Prostate Cancer and Prostatic Diseases 14326–331. ( 10.1038/pcan.2011.28) [DOI] [PubMed] [Google Scholar]
- Wu Y Ding Y Tanaka Y & Zhang W. 2014Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. International Journal of Medical Sciences 11 1185–1200. ( 10.7150/ijms.10001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q & Civelek M. 2020Transcription factor KLF14 and metabolic syndrome. Frontiers in Cardiovascular Medicine 7 91. ( 10.3389/fcvm.2020.00091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S Kar S Carter P Vithayathil M Mason AM Burgess S & Larsson SC. 2020Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample Mendelian randomization study. Diabetes 691588–1596. ( 10.2337/db20-0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T Rindtorff N & Boutros M. 2017Wnt signaling in cancer. Oncogene 361461–1473. ( 10.1038/onc.2016.304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Wen W, Michailidou K, Bolla MK, Wang Q, Zhang B, Long J, Shu XO, Schmidt MK, Milne RL, et al. 2016Association of genetic susceptibility variants for type 2 diabetes with breast cancer risk in women of European ancestry. Cancer Causes and Control 27679–693. ( 10.1007/s10552-016-0741-6) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a