Abstract
Purpose:
Eganelisib (IPI-549) is a first-in-class, orally administered, highly selective phosphoinositide-3-kinase (PI3K)-γ inhibitor with anti-tumor activity alone and in combination with programmed cell death protein 1/ligand 1 (PD-1/PD-L1) inhibitors in preclinical studies. This phase 1/1b first-in-human, MAcrophage Reprogramming in Immuno-Oncology-1 (MARIO-1; NCT02637531) study evaluated the safety and tolerability of once-daily eganelisib as monotherapy and in combination with nivolumab in patients with solid tumors.
Patients and Methods:
Dose-escalation cohorts received eganelisib 10–60 mg as monotherapy (n=39) and 20–40 mg when combined with nivolumab (n=180). Primary endpoints included incidence of dose-limiting toxicities (DLTs) and adverse events (AEs).
Results:
The most common treatment-related grade ≥3 toxicities with monotherapy were increased alanine aminotransferase (ALT; 18%), aspartate aminotransferase (AST; 18%), and alkaline phosphatase (5%). No DLTs occurred in the first 28 days; however, toxicities meeting DLT criteria (mostly grade 3 reversible hepatic enzyme elevations) occurred with eganelisib 60 mg in later treatment cycles. In combination, the most common treatment-related grade ≥3 toxicities were increased AST (13%) and increased ALT and rash (10%). Treatment-related serious adverse events occurred in 5% of monotherapy patients (grade 4 bilirubin and hepatic enzyme increases in one patient each) and 13% in combination (pyrexia, rash, cytokine release syndrome, and infusion-related reaction in ≥2 patients each). Anti-tumor activity was observed in combination, including patients who had progressed on PD-1/PD-L1 inhibitors.
Conclusions:
Based on the observed safety profile, eganelisib doses of 30 mg and 40 mg once daily in combination with PD-1/PD-L1 inhibitors were chosen for phase 2 study.
Keywords: IPI-549, immune checkpoint inhibitors, phosphoinositide-3-kinase, nivolumab, phase I clinical trial
Introduction
Tumor-associated myeloid cells, including myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages, are an essential component of the tumor microenvironment. These cells have been implicated in tumor immune evasion, angiogenesis, and metastasis, as well as resistance to chemotherapies and immune checkpoint inhibitors (ICIs) (1, 2). The presence of tumor-associated myeloid cells in the tumor microenvironment is associated with shorter overall survival in several tumor types, including squamous cell cancer of the head and neck (SCCHN) and triple-negative breast cancer (TNBC) (3).
Phosphoinositide-3-kinase (PI3K)- is the predominant PI3K isoform in myeloid cells and has limited expression outside of the myeloid compartment (4, 5). Preclinical studies using PI3K- deletion and kinase-dead knock-in models revealed a key role for PI3K- as a “molecular switch” that controls immune suppression by myeloid cells in the tumor microenvironment. For example, syngeneic tumors inoculated into PI3K- null mice showed reduced growth rates, which was linked to reduced myeloid cell migration to tumors, and myeloid cell reprogramming from an immunosuppressive tumor-supporting phenotype to an immune-stimulatory, anti-tumor phenotype that promotes activated dendritic cell and CD8+ T-cell infiltration (4, 6, 7).
Eganelisib (IPI-549) is a potent, first-in-class PI3K- inhibitor (), with high selectivity (≥150-fold compared with class I PI3K isoforms and other kinases) (8). Preclinical pharmacokinetic (PK) and absorption, distribution, metabolism, and excretion studies showed that eganelisib has oral bioavailability of ≥31% across preclinical species and a PK profile that supports complete and sustained PI3K- inhibition with daily dosing (8). In vitro, eganelisib had no direct effect on cancer cell proliferation or, importantly, T-cell activation (4). Similar to the PI3K- knockout studies, in vivo studies of eganelisib in multiple syngeneic models also demonstrated immune activation within the myeloid compartment that led to T-cell infiltration and activation and resulted in tumor growth delay (4, 6). Moreover, the combination of eganelisib with ICIs targeting programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) overcame resistance to ICIs in myeloid-rich checkpoint-refractory tumor models (4, 9–11). Together, these data support that PI3K- inhibition by eganelisib can reprogram immunosuppressive tumor-associated myeloid cells to reshape the tumor microenvironment and promote anti-tumor activity in a nonredundant, complementary approach to ICIs, thereby providing the rationale to administer eganelisib in combination with ICIs for the treatment of solid tumors.
Here, we report the first clinical data on the administration of eganelisib in patients with cancer. This study was designed to evaluate the safety and tolerability of escalating doses of eganelisib as monotherapy and in combination with the PD-1 inhibitor nivolumab and assess the PK, pharmacodynamics (PD), and preliminary anti-tumor activity of eganelisib in patients with multiple advanced solid tumor types.
Patients and methods
Study design and participants
MAcrophage Reprogramming in Immuno-Oncology-1 (MARIO-1) was a phase 1/1b, open-label, first-in-human, dose-escalation and expansion study of eganelisib as monotherapy and in combination with nivolumab in patients with advanced solid tumors (ClinicalTrials.gov identifier: NCT02637531), which was conducted in the United States between 2016 and 2021. Eligible patients had histological or cytological evidence of solid cancer with at least one measurable disease lesion per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (12) for dose-escalation and dose-expansion cohorts. Patients with ongoing systemic bacterial, fungal, or viral infections or undergoing treatment with chronic immunosuppressants or systemic steroids were excluded. Additional cohort-specific inclusion and exclusion criteria are summarized below and in the Supplementary Materials and Methods. The study was conducted in accordance with the protocol, the Declaration of Helsinki, the International Council for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and all applicable local regulatory requirements. All patients provided written informed consent on forms approved by an Institutional Review Board and Internal Ethics Committee.
The study design included dose-escalation and dose-expansion cohorts to determine dosing of eganelisib as monotherapy and in combination with nivolumab (Figure 1). All patients were to receive study treatment in continuous 28-day treatment cycles until unacceptable toxicity or disease progression occurred. Nivolumab was administered as 240 mg every 2 weeks (Q2W) or 480 mg every 4 weeks (Q4W) per the Investigator’s discretion.
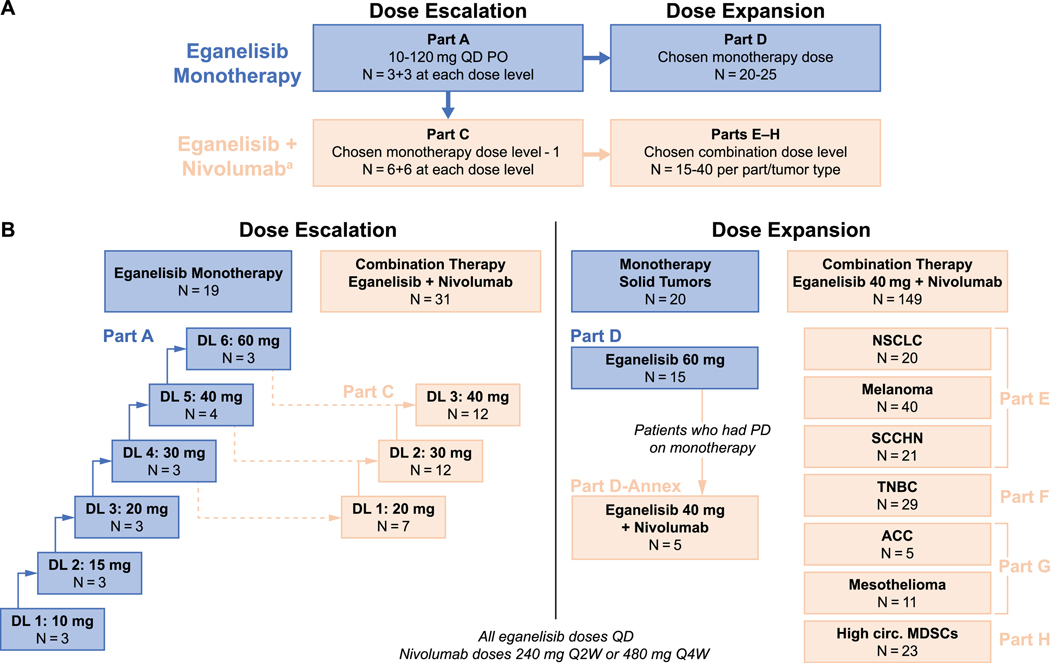
Figure 1. Study design and patient flow.
A) Study schema showing eganelisib dosing and numbers of patients planned for dose escalation and dose expansion cohorts. B) Numbers of patients enrolled at each dose level.
Abbreviations: ACC, adrenocortical carcinoma; DL, dose level; MDSCs, myeloid-derived suppressor cells; MDSCs, myeloid-derived suppressor cells; NSCLC, non-small cell lung cancer; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PO, oral route; Q2W, every 2 weeks; Q4W, every 4 weeks; QD, once daily; SCCHN, squamous cell carcinoma of the head and neck; TNBC, triple-negative breast cancer.
Part B (examining twice daily dosing) was planned if pharmacokinetic data indicated that QD dosing did not result in adequate eganelisib exposure. It was determined that Part B was not necessary, and it was not conducted. Part I, which was originally designed to evaluate patients with cisplatin-refractory, anti-PD-1/PD-L1 therapy-naïve advanced urothelial cancer receiving eganelisib in combination with nivolumab, was not conducted.
aNivolumab was administered at 240 mg Q2W or 480 mg Q4W per Investigator discretion.
Parts A-D included patients with advanced solid tumors who had failed to respond to standard therapy or for whom no appropriate therapies were available. Prior treatment with ICIs was permitted, including PD-1 or PD-L1 inhibitors, within 2–3 weeks prior to initiating study treatment. In the monotherapy dose-escalation phase (Part A), patients received eganelisib once daily (QD) in a standard 3+3 dose-escalation scheme starting at 10 mg and proceeding to the next dose if no dose-limiting toxicities (DLTs) occurred during the first treatment cycle at the current dose. The maximum tolerated dose (MTD) was defined as the dose immediately below the dose at which 2 or more patients experienced a DLT. Dose escalation could continue until the MTD was identified; PK futility (no substantive increase in exposure despite increasing dose) occurred; or the optimal biological dose (defined as no substantive change in PD markers despite increasing dose) was identified. Dose escalation in combination with nivolumab (Part C) was based on a 6+6 design in which patients proceeded to the next dose if no DLT occurred during the first treatment cycle in the first 6 patients, or fewer than 3 DLTs occurred in the first 12 patients, with the initial dose informed by the results of the monotherapy dose escalation (Part A). Dose escalation was continued until the recommended phase 2 dose was determined based on an aggregate assessment of safety and PK/PD data.
After the dose-escalation phase was complete, dose expansion for both monotherapy (Part D) and combination therapy (Parts E–H) was initiated. For Part D, eganelisib monotherapy dosing was informed by the results of the monotherapy dose expansion (Part A). If patients in Part D experienced disease progression, they could switch to Part D-annex in which they would receive the combination of eganelisib (dosing based on findings in Part C) with nivolumab. All patients in Parts E–H, who also received eganelisib dosing based on Part C in combination with nivolumab, were assigned by tumor type as follows: non-small cell lung cancer (NSCLC), melanoma, or SCCHN with disease progression on PD-1/PD-L1 inhibitors at study entry (Part E); anti-PD-1/PD-L1-naïve patients with advanced TNBC in the second line or later (Part F); advanced adrenocortical carcinoma (ACC) or mesothelioma in the second line or later (Part G; patients with mesothelioma were required to be anti-PD-1/PD-L1-naïve); or solid tumors with high-circulating MDSCs in any line, but must have received PD-1/PD-L1 inhibitors previously if indicated by specific tumor characteristics, such as high microsatellite instability (Part H).
Further details on treatment cohorts and the methodology and criteria for high-circulating MDSCs in Part H are provided in the Supplemental Material and Methods.
Study assessments and endpoints
The primary objective of the dose-escalation phase was to evaluate safety and tolerability, and determine the recommended dose of eganelisib for further clinical development as monotherapy in Part A, and in combination with nivolumab in Part C. Corresponding endpoints were the incidence of DLTs during the first 28-day cycle (primary endpoint), and incidence of adverse events (AEs), safety laboratory values, PK assessments for plasma eganelisib and metabolite (IPI-5421) concentrations, PD assessment of PI3K- inhibition by measuring phosphorylated (p)AKT levels, and QT interval corrected with Fridericia’s method (QTcF).
The primary objective of the dose-expansion phase was to evaluate the safety and tolerability of eganelisib as monotherapy (Part D) and in combination with nivolumab in specific tumor types (Parts E–H). Corresponding endpoints were the incidence of AEs and safety laboratory values (primary endpoint), PK/PD assessments as described above (secondary endpoint), and tumor response parameters including overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS) (secondary endpoints).
The PK of eganelisib and its major metabolite IPI-5421 were assessed using noncompartmental methods by a validated high-performance liquid chromatography-tandem mass spectrometry technique. Blood samples were collected at specified time points and assessed for maximum observed plasma concentration (), time of maximum observed plasma concentration (), area under the plasma concentration-time curve from time zero to the last quantifiable concentration (AUC), terminal elimination half-life (), and apparent clearance (CL/F). These findings were further used to determine dose proportionality, accumulation ratio (AR) for multiple-dose administration, and the relationship between eganelisib exposure and QTcF.
PD was assessed using whole-blood samples collected before, and 1, 2, 4, 6, and 24 hours after dose administration during cycles 1 and 2 and analyzed using an ex vivo whole-blood assay employing C-X-C motif chemokine ligand-12 (CXCL12) stimuli to measure levels of AKT phosphorylated at T308 in monocytes (PI3K- dependent) or at S473 in B cells (PI3K-δ dependent) using flow cytometry. Subtraction of baseline pAKT was performed using the corresponding unstimulated control samples collected at each time point. An inhibitory maximal effect () model was fitted to the PK/PD data from Parts A and D (eganelisib monotherapy); and Hill factor were fixed to 1 and the observed baseline pAKT was included as a covariate of the estimated baseline level. The 90% maximal effective concentration () was calculated from the half maximal effective concentration () value and the Hill factor of 1 using the formula:
Further details on the methodology for the PK and PD assays are provided in the Supplemental Material and Methods.
Safety was assessed continuously by monitoring treatment-emergent AEs, defined as events with onset or worsening after the first study treatment. AEs were coded per the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.03. In the dose-escalation phase (Parts A and C), DLTs were defined as drug-related, grade ≥4 neutropenia persisting for ≥7 days, or of any duration if complicated by infection or febrile neutropenia; grade ≥4 thrombocytopenia; or any drug-related, grade ≥3 non-hematological toxicity (except for grade 3 fatigue lasting <7 days or grade 3 nausea, emesis, or diarrhea that responded to supportive treatment within 5 days). In Part C, grade 3 uncomplicated rash (defined as demonstrating response to treatment within 7 days and not associated with fever, new-onset autoimmune symptoms, or bullous pemphigoid or lichen planus features) was added as an exception to grade ≥3 non-hematological toxicities and not counted as a DLT.
Tumor response was assessed based on computed tomography or magnetic resonance imaging performed at baseline (reference scan), then every 8 weeks between cycles 3–13, and every 3 months until treatment termination. Individual patients were assessed using the same imaging modality for the duration of the study. ORR was defined as the percentage of patients who achieved complete response (CR) or partial response (PR) as determined by the Investigator using RECIST 1.1. In addition to ORR, DOR, and PFS (secondary endpoints) and disease control rate (DCR) were determined.
Statistical considerations
PK/PD analyses were performed on patients who received at least one dose of eganelisib and had at least one measurable plasma concentration of eganelisib or metabolite. Safety analyses were performed on all patients who received any amount of study drug(s). Treatment response was assessed in all patients with measurable disease at baseline and received at least one dose of study drug(s) and had at least one on-study response assessment, or discontinued treatment for disease progression (including death) within 16 weeks of the first dose without a response assessment.
All results are summarized using descriptive statistics. PK and PD results are reported by dose at the actual sample collection times. Safety results are summarized by treatment group according to the number of patients who experienced each AE, and the frequency expressed as a percentage of the full treatment group. Tumor response endpoints are reported by the number and percent of patients with the best overall response in each response category in each treatment group. PFS is presented as median values with 2-sided 95% confidence intervals based on Kaplan-Meier estimates for all treated patients. Censoring rules are provided in the Supplemental Material and Methods.
Data availability statement
The data generated in this study are available upon request from the corresponding author.
Results
Patients
Of 219 patients enrolled and treated at 12 centers in the United States, 39 patients received eganelisib monotherapy while 180 patients received eganelisib in combination with nivolumab 240 mg Q2W or 480 mg Q4W. The number of patients enrolled in each study cohort is shown in Figure 1B.
The median age of patients across all cohorts was 62.0 years (range, 24–86), 50.7% of patients were male, 49.3% were female, and 98.6% had an Eastern Cooperative Oncology Group performance status of 0 or 1. The study population was heavily pretreated, with 65.3% of patients having received at least three lines of prior anticancer therapy, and 53.4% having received prior treatment with PD-1/PD-L1 inhibitors (in accordance with treatment group-specific eligibility criteria).
Pharmacokinetics and pharmacodynamics
The for eganelisib ranged from 1–6 hours in most patients, and the ranged from 10.6–39.8 hours. The geometric mean of the terminal value of 39.8 hours was based on the 10 mg dose cohort (n=3) on C1D1. The remaining cohorts had terminal values ranging from 10.6–23.6 hours. Sample collection was performed only during the first 24 hours after dosing, which may have affected estimates, although the findings are largely consistent with the AR estimate of approximately 1.2 to 2.1 after continuous QD dosing to steady state by day 1 of cycle 2. The effective values calculated from the AR estimate were 9.3−25.7 hours, consistent with excluding the 10 mg dose cohort value. Based on AUC values, exposure to the major metabolite IPI-5421 was <10% that of the parent compound. According to and AUC results, eganelisib exposure was approximately dose proportional from 10− 60 mg, although relatively large variability in exposure occurred (Supplemental Table S1). The geometric mean of CL/F was approximately 1 L/hr and the coefficient of variation ranged from 7% to 98% across different dose cohorts (Supplemental Table S1). No clinically relevant effects on QTcF were detected at any dose level (data not shown).
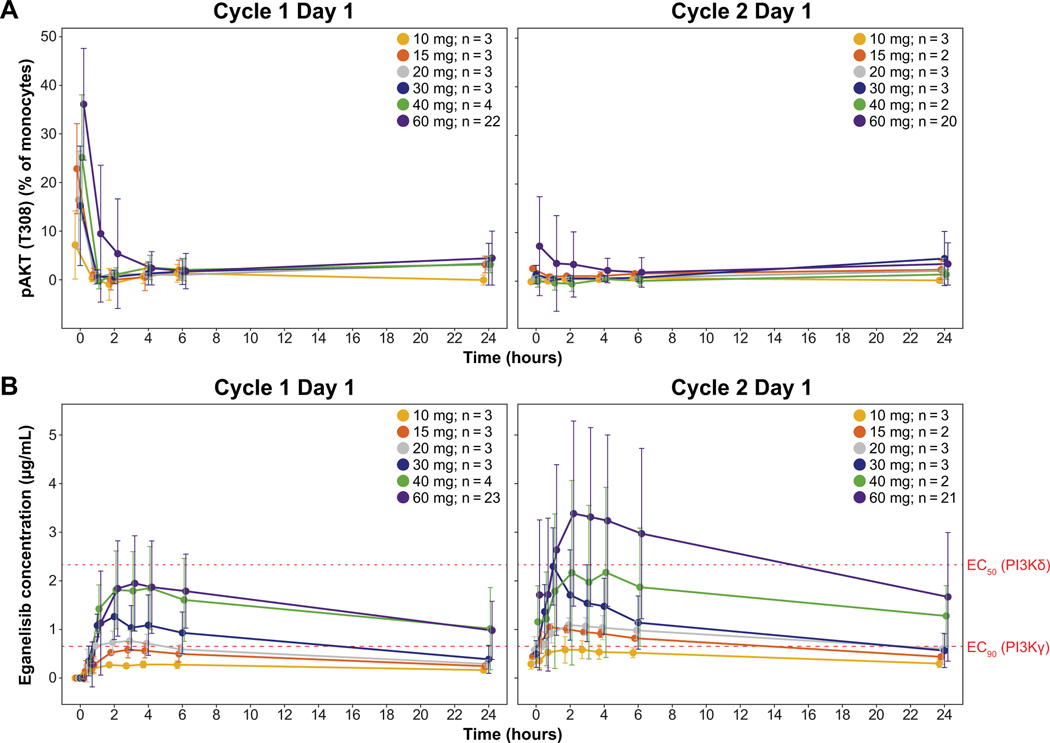
PD results revealed a rapid and large reduction of approximately 80% PI3K activity across all doses based on CXCL12 stimulated pAKT T308 levels in monocytes (Figure 2A). Peak inhibition was observed as early as 1–2 hours after dose administration and was maintained through day 1 of cycle 2. Based on an model of eganelisib plasma concentrations for PI3K inhibition, estimated and values for PI3K- inhibition were 0.072 μg/mL and 0.652 μg/mL, respectively, while the for PI3K- inhibition was 2.33 μg/mL (Figure 2B). During cycle 1, eganelisib doses of 30–60 mg resulted in plasma concentrations above the for PI3K- inhibition and below the for PI3K- inhibition for the majority of the dosing interval. Measurements at the beginning of cycle 2 indicated that doses of 15–40 mg were above the for PI3K- inhibition and below the for PI3K- , whereas the 60-mg dose exceeded the for PI3K- inhibition for part of the dosing interval. These results suggest the optimal doses for selective inhibition of PI3K- by eganelisib are 30 or 40 mg daily.
Figure 2:
PK/PD results in the monotherapy dose-escalation and expansion cohort. (A) Mean PI3K- pathway dependent pAKT measured in whole blood on day 1 cycle 1 and cycle 2. (B) Mean plasma eganelisib concentrations during the cycle 1 and cycle 2 dosing intervals with dotted lines for the of PI3K- inhibition and the EC50 of PI3K- inhibition.
Abbreviations: , half maximal effective concentration; , 90% maximal effective concentration; pAKT, phosphorylated AKT; PD, pharmacodynamic; PI3K-, phosphoinositide-3-kinase gamma; PI3K-, phosphoinositide-3-kinase delta; PK, pharmacokinetic.
Error bars show standard deviation.
Estimated eganelisib for PI3K- inhibition is 0.652 μg/mL, and for PI3K- inhibition is 2.33 μg/mL.
Safety
Eganelisib monotherapy
During monotherapy dose escalation (Part A; n=19), no DLTs were observed over the 28-day observation period. Based on an aggregate assessment of PK/PD and available safety data, 60 mg was chosen as the highest dose in the dose escalation and was selected for monotherapy dose expansion.
Treatment-related AEs for monotherapy are summarized in Table 1. In dose escalation (Part A; n=19), the most common events, occurring in ≥2 patients, were aspartate aminotransferase (AST) increased in 4 (21%) patients; alanine aminotransferase (ALT) increased and rash in 3 (16%) patients each; and fatigue and headache in 2 (11%) patients each. Grade ≥3 treatment-related AEs occurred in 2 (11%) patients: ALT and AST increased in 1 patient and hypercalcemia in 1 patient; both at the 60-mg dose level. In the 60-mg monotherapy dose expansion (n=20), the most common treatment-related AEs, occurring in ≥2 patients, were AST increased in 9 (45%) patients; ALT increased and rash in 8 (40%) patients each; fatigue in 3 (15%) patients; and blood alkaline phosphatase (ALP) increased and dyspnea in 2 (10%) patients each. The grade ≥3 treatment-related AEs in the 60-mg dose expansion were AST and ALT increased, each occurring in the same 6 (30%) patients; rash and blood ALP increased in 2 (10%) patients each; and blood bilirubin increased and dyspnea in 1 (5%) patient each. Of these, the only grade 4 treatment-related AEs were single instances of ALT and bilirubin increased that occurred in the same patient. There were no grade 5 treatment-related AEs.
Table 1.
Treatment-related AEs occurring in at least 5% of patients or with any event of grade 3 or highera in the eganelisib monotherapy cohort
| n (%) | Eganelisib dose escalation (Part A) | Eganelisib dose expansion (Part D: 60 mg) (n=20) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 10–30 mg (n=12) | 40 mg (n=4) | 60 mg (n=3) | ||||||
|
| ||||||||
| Any grade | G≥3 | Any grade | G≥3 | Any grade | G≥3b | Any grade | G≥3 | |
| Any treatment-related TEAE | 6 (50) | - | 3 (75) | - | 2 (67) | 2 (67) | 14 (70) | 8 (40) |
| AST increased | 1 (8) | - | 2 (50) | - | 1 (33) | 1 (33) | 9 (45) | 6 (30) |
| ALT increased | 2 (50) | - | 1 (33) | 1 (33) | 8 (40) | 6 (30) | ||
| Rashc | 1 (8) | - | 2 (50) | - | 8 (40) | 2 (10) | ||
| Fatigue | 1 (8) | - | 1 (25) | - | 3 (15) | - | ||
| Headache | 2 (17) | - | 1 (5) | - | ||||
| Blood ALP increased | 1 (33) | - | 2 (10) | 2 (10) | ||||
| Dyspnea | 2 (10) | 1 (5) | ||||||
| Amylase increased | 1 (8) | - | 1 (5) | - | ||||
| Lipase increased | 1 (8) | - | 1 (5) | - | ||||
| WBC decreased | 1 (25) | - | 1 (5) | - | ||||
| Blood bilirubin increased | 1 (5) | 1 (5) | ||||||
| Hypercalcemia | 1 (33) | 1 (33) | ||||||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; DLT, dose-limiting toxicity; G≥3, grade 3 or higher; TEAE, treatment-emergent adverse event; WBC, white blood cell count.
All events were grade 3 except for grade 4 increases in ALT and bilirubin that both occurred in the same patient.
No grade ≥3 events occurred during the DLT observation period (first treatment cycle).
Includes preferred terms pruritis, rash, and rash maculopapular.
Across all eganelisib monotherapy patients, 2 (5%) patients experienced treatment-related serious AEs (SAEs), which were grade 3 ALT and AST increases in 1 patient, and grade 4 bilirubin and ALT increased and grade 3 AST increased in the other patient; both patients received the 60-mg dose. One patient (3%) had a dose reduction of eganelisib for a treatment-related AE: grade 2 fatigue and headache at the 15-mg dose. Three (8%) patients discontinued eganelisib for a treatment-related AE: grade 3 ALT and AST increases in 1 patient at the 60-mg dose, grade 4 AST increased in 1 patient at the 60-mg dose, and grade 2 pruritus and maculopapular rash in 1 patient at the 40-mg dose. Although no dose of eganelisib monotherapy met MTD criteria, the aggregate of PK/PD and safety data did not support exploring daily doses exceeding 60 mg.
Combination therapy
During the combination therapy dose-escalation phase (Part C; n=31), DLTs occurred in 2 (6%) patients in the 30-mg group (grade 3 maculopapular rash), and in 2 (6%) patients in the 40-mg group (grade 3 rash and grade 3 ALT/AST increase). Based on an aggregate assessment of the PK/PD and safety profile of the 60-mg monotherapy dose, and a review of the treatment-related AEs observed in Part C, an eganelisib dose of 40 mg daily was chosen for combination treatment with nivolumab in the combination expansion cohorts.
A total of 149 patients received 40 mg eganelisib in combination with nivolumab in the expansion cohorts. A total of 110 (74%) patients experienced at least one treatment-related AE (Table 2), the most common of which (occurring in >10% of patients) were rash in 77 (52%) patients; AST increased in 39 (26%); ALT increased in 36 (24%); fatigue in 28 (19%); and nausea and pyrexia in 21 (14%) patients each. Grade ≥3 treatment-related AEs occurred in 58 (39%) patients, the most common of which (occurring in more than one patient) were rash and AST increased in 21 (14%) patients each; ALT increased in 16 (11%); blood ALP increased in 6 (4%) patients; lipase increased in 3 (2%) patients; and nausea, pyrexia, and vomiting in 2 (1%) patients each. Grade 4 treatment-related AEs occurred in 3 (2%) patients in the eganelisib plus nivolumab dose expansion (AST increased and transaminase increased, and lymphocyte count decreased in 1 patient each). There were no treatment-related grade 5 AEs.
Table 2:
Treatment-related AEs occurring in at least 5% of patients in the eganelisib + nivolumab combination therapy cohorta
| n (%) | Eganelisib dose escalation + nivolumab (Part C) | Eganelisib + nivolumab dose expansion (Parts E-H: eganelisib 40 mg) (n=149) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 20 mg (n=7) | 30 mg (n=12) | 40 mg (n=12) | ||||||
|
| ||||||||
| Any grade | G≥3 | Any grade | G≥3b | Any grade | G≥3c | Any grade | G≥3d | |
| Any treatment-related TEAE | 4 (57) | 1 (14) | 9 (75) | 4 (33) | 9 (75) | 5 (42) | 110 (74) | 58 (39) |
| Rashe | 1 (14) | 1(14) | 5 (42) | 1 (8) | 8 (67) | 3 (25) | 77 (52) | 21 (14) |
| AST increased | 1 (8) | - | 5 (42) | 5 (42) | 39 (26) | 21 (14) | ||
| ALT increased | 1 (8) | - | 5 (42) | 4 (33) | 36 (24) | 16 (11) | ||
| Fatigue | 2 (17) | - | 28 (19) | - | ||||
| Nausea | 1 (14) | - | 1 (8) | 21 (14) | 2 (1) | |||
| Pyrexia | 1 (8) | - | 1 (8) | - | 21 (14) | 2 (1) | ||
| Blood ALP increased | 2 (17) | - | 13 (9) | 6 (4) | ||||
| Decreased appetite | 12 (8) | - | ||||||
| Diarrhea | 2 (17) | - | 1 (8) | - | 9 (6) | 1 (1) | ||
| Vomiting | 1 (14) | - | 1 (8) | - | 1 (8) | - | 9 (6) | 2 (1) |
| Chills | 10 (7) | 1 (1) | ||||||
| Arthralgia | 9 (6) | - | ||||||
| Myalgia | 9 (6) | - | ||||||
| Lipase increased | 1 (8) | 1 (8) | 1 (8) | - | 8 (5) | 2 (1) | ||
Abbreviations: ALT, alanine aminotransferase; AST, alanine aminotransferase; ALP, alkaline phosphatase; G≥3, grade 3 or higher; Q2W, every 2 weeks; Q4W, every 4 weeks; TEAE, treatment-emergent adverse event.
Eganelisib was administered once daily and nivolumab as 240 mg Q2W or 480 mg Q4W.
Grade 3 events of dermatitis acneiform (n=1) and joint effusion (n=1) not shown.
All events were grade 3 except for grade 4 ALT increased (n=1). Grade 3 event of abdominal pain (n=1) not shown.
All events were grade 3 except for grade 4 AST increased (n=1), transaminases increased (n=1), and lymphocyte count decreased (n=1).
Includes preferred terms pruritis, rash, rash macular, and rash maculopapular.
A total of 19 (13%) patients in the combination therapy expansion cohorts experienced treatment-related SAEs, the most common of which were pyrexia in 8 (5%) patients; rash in 6 (4%); and maculopapular rash, cytokine release syndrome, and infusion-related reaction in 2 (1%) each. A total of 14 (9%) patients in the combination expansion cohorts had a dose reduction of eganelisib because of treatment-related AEs, the most common of which were ALT increased in 5 (3%) patients; AST increased and rash in 3 (2%) patients each; and dermatitis acneiform in 2 (1%) patients. A total of 13 (9%) patients in the combination expansion cohorts discontinued treatment because of treatment-related AEs, the most common of which were AST increased and rash in 2 (1%) patients each.
Anti-tumor activity
Eganelisib monotherapy
In the monotherapy cohorts, the only formal RECIST response was observed in a patient with heavily pretreated peritoneal mesothelioma with multiple sites of metastatic disease. This was observed at the eganelisib 20-mg dose level in Part A. This patient demonstrated a PR after 11.1 months of treatment, and the response was maintained for 9.5 months.
Combination treatment
Two of the 31 patients who received escalating doses of eganelisib with nivolumab (Part C) experienced a PR. One patient had metastatic ACC and received eganelisib 30 mg and demonstrated a PR for 11.4 months, and the other patient had metastatic gallbladder adenocarcinoma and received eganelisib 40 mg, experiencing a PR of 9.3 months’ duration.
According to cohort-specific eligibility criteria (see Supplementary Materials and Methods), all patients in Part E were previously treated with a PD-1/PD-L1 inhibitor, all patients in Part F were not previously treated with a PD-1/PD-L1 inhibitor, and patients in Parts G and H may or may not have previously received a PD-1/PD-L1 inhibitor. Of the 29 anti-PD-1/PD-L1-naïve patients with TNBC in Part F, the ORR was 7% (1 CR of 34.6 months and 1 PR of 7.5 months) and the DCR was 30%. The ORR for the 11 patients with mesothelioma in Part G was 9% based on 1 PR of 10.9 months, and the DCR was 55%. No RECIST response was observed in any patients with ACC (n=5 in Part G) or tumors with high-circulating MDSCs (n=23 in Part H).
Results for the disease-specific expansion cohort in which patients were refractory to a prior PD-1/PD-L1 inhibitor immediately before study enrollment (Part E) are summarized in Table 3 (complete anti-tumor activity results are provided in Supplemental Tables S2 and S3). Among patients with anti-PD-1/PD-L1-resistant melanoma (n=40), the ORR was 10%. The DOR for the 4 responders was 4.1, 8.9, 19, and 26.5 months, respectively. The ORR for patients with ICI-resistant SCCHN (n=21) was 10%, with 2 PRs of 3.8 and 9.2 months duration, respectively; the median PFS was 3.7 months. The ORR for the 20 patients with anti-PD-1/PD-L1-resistant NSCLC was 5%, with 1 patient experiencing a PR for 8.0 months. The DCR for the all patients progressing on a PD-1/PD-L1-inhibitor prior to treatment initiation was 36% to 43%.
Table 3:
Anti-tumor activity in patients in combination expansion cohorts and previously treated with PD-1/PD-L1 inhibitors (Part E)
| Population | ORRa n (%) | DCRb n (%) | Median PFSc Months [95% CI] |
|---|---|---|---|
| Melanoma (n=40) | 4 (10) | 14 (36) | 1.9 [1.8, 3.7] |
| SCCHN (n=21) | 2 (10) | 9 (43) | 3.7 [1.9, 5.5] |
| NSCLC (n=20) | 1 (5) | 8 (40) | 2.1 [1.8, 3.9] |
Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate; ORR, overall response rate; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; PR, partial response; SCCHN, squamous cell cancer of the head and neck.
Best response of CR or PR
Best response of CR, PR, or stable disease.
Based on Kaplan-Meier estimates for all treated patients.
Discussion
The results of this first-in-human, phase 1/1b clinical trial of eganelisib include PK/PD findings, a comprehensive safety characterization, and preliminary anti-tumor activity of this first-in-class PI3K- inhibitor, both as a monotherapy and in combination with the ICI nivolumab. During monotherapy dose escalation, the most common treatment-related AEs were elevation of hepatic transaminases and skin rash, which were generally dose-dependent. For example, hepatic AEs were reported in 33% of all monotherapy patients, most of whom received the highest dose of eganelisib (60 mg). No grade ≥3 hepatic events occurred in patients who received doses lower than 60 mg daily. A similar pattern was observed for skin AEs, as no patients experienced grade ≥3 skin AEs at eganelisib doses of 10–40 mg daily. Based on the PK/PD data, eganelisib concentrations at the 60-mg dose (and to a lesser degree, the 40-mg dose) resulted in plasma concentrations that could also partially inhibit PI3K-, which is also associated with hepatic and skin AEs (13). The relatively small sample sizes and PK variability between patients limited the ability to make definitive conclusions about the relative contributions of PI3K- and PI3K- inhibition to hepatic and skin-related AEs at these doses. Nonetheless, despite some overlap in the hepatic and skin organ classes, the overall safety profile of eganelisib appeared distinct from that of approved PI3K- inhibitors, for which serious and sometimes fatal AEs related to diarrhea/colitis, pneumonitis, and infection have been reported (13). Given the absence of DLTs in our study, eganelisib 40 mg was the dose chosen for further study in combination with nivolumab in the cohort expansion phase.
When eganelisib was administered with nivolumab, the overall safety profile was consistent with that observed with eganelisib monotherapy and the previously described AE profile of nivolumab (14), which included hepatic and skin-related AEs among the most common events. Consistent with the monotherapy phase, a trend suggesting eganelisib dose dependency for treatment-related hepatic AEs was detected. For example, treatment-related hepatic events (most commonly AST and ALT increases) occurred in 54 (29%) patients, 53 of whom received eganelisib 40 mg in combination with nivolumab. In the combination expansion cohort with 40 mg eganelisib plus nivolumab, treatment-related grade ≥3 hepatic events occurred in 19.5% of patients, but tended to occur early, generally resolved quickly, usually without corticosteroids, and were not associated with criteria for drug-induced liver injury, such as Hy’s Law. For patients in the 60 mg single-agent dose expansion (n=20), the median time to onset of the first treatment-related grade ≥3 hepatic event was 32 days (measured from cycle 1 day 1) and resolved to grade 1 or baseline with a median of only 8.5 days. For patients who received any dose of eganelisib in combination with nivolumab (n=185), the median time to onset for first treatment-related grade ≥3 hepatic event was 43 days, with a median time for resolution to grade 1 or baseline of 14 days. Although the mechanism of hepatic AEs with eganelisib is incompletely understood, its relatively rapid reversal with dose modification suggests some mechanistic differentiation from hepatic toxicity typically associated with ICI administration. Hepatic enzyme elevations have been reported with inhibitors of the macrophage colony stimulating factor (15), suggesting the possibility that alterations in monocyte/macrophage signaling could affect Kupffer cell homeostasis in the liver.
In general, skin rashes observed with eganelisib plus nivolumab were clinically indistinguishable from those commonly observed with ICIs. The spectrum of AEs in other organ systems was generally consistent with PD-1/PD-L1 inhibitors like nivolumab, with pneumonitis occurring in 2% of patients (all grade 1 or 2) and myocarditis (grade 3), colitis (grade 3), hyperthyroidism (grade 2), hypothyroidism (grade 2), and adrenal insufficiency (grade 2) each occurring in 1% of patients (data on file). The frequency of pyrexia (14% overall; 1% grade 3) among patients who received 40 mg eganelisib with nivolumab in the dose-expansion phase was higher than anticipated with nivolumab alone, and thus may be related to the mechanism of action of eganelisib in reprograming macrophages to a more pro-inflammatory phenotype. Nonetheless, the overall safety profile of eganelisib in combination with nivolumab was acceptable and manageable.
Based on preclinical studies showing modest growth inhibition in syngeneic tumor models as monotherapy, we anticipated that targeting PI3K- alone might have limited anti-tumor activity as monotherapy. Indeed, eganelisib demonstrated only modest evidence of single-agent activity at concentrations exceeding the of PI3K- in whole blood assays. Interestingly, the only RECIST response among patients who received monotherapy occurred in a patient with mesothelioma, for which a high proportion of tumor M2 macrophages has been implicated with inferior overall survival (16).
The cohort expansions of eganelisib in combination with nivolumab focused on tumor types with historically limited responses to single-agent PD-1/PD-L1 inhibitors and included patients previously treated with these agents. To test the clinical relevance of preclinical reports implicating the PI3K- pathway in ICI resistance (6), these cohorts included the strict requirement that patients must have progressed on a PD-1/PD-L1 inhibitor as their immediate prior therapy. Although the overall ORR was modest (5–10%), 15–24% of patients experienced disease control (CR, PR, stable disease) for at least 24 weeks with nivolumab and eganelisib, unexpected for a patient population that had progressed on anti-PD-1/PD-L1 monotherapy just prior to study entry. Given the heterogeneous nature of anti-PD-1/PD-L1 resistance, further work will be required to identify patient subsets for which targeting PI3K- might enrich patient benefit.
In summary, in this report we provide the initial PK/PD, safety, and efficacy characterization of eganelisib, a first-in-class highly specific PI3K- inhibitor suitable for daily oral administration. Relatively little was known about the effects of PI3K- inhibition in humans prior to this study, and a full safety characterization will require further study. Nonetheless, it appears that a blockade of this myeloid checkpoint signaling pathway, alone or in combination with a PD-1 inhibitor, is associated with hepatic, skin, and pyrexia-related events that are consistent with an immune-related mechanism. The precise mechanism(s) underlying the hepatic events will require further investigation, but they mainly consisted of abnormalities on liver function tests, with relatively infrequent abnormalities in serum bilirubin. The general rapid reversibility of these events when holding or reducing administered doses of eganelisib, with or without corticosteroid administration, may be related to the reversibility of macrophage reprogramming effects, facilitated by the relatively short terminal half-life of eganelisib compared to the antibody nivolumab. Although the overall efficacy of eganelisib in combination with nivolumab was fairly modest, this was confounded by the heterogeneity of tumor types explored, the heavy weighting towards ICI-refractory patients (100 of the 149 patients in combination dose expansion cohorts), and the heavily pre-treated nature of the study patient population, which has been associated with inferior immunotherapy outcomes (17). The favorable PK/PD margin of eganelisib on PI3K- versus PI3K- signaling at the 40-mg and 30-mg dose levels supported Phase 2 exploration of eganelisib in combination with nivolumab in patients with ICI-naïve platinum-resistant urothelial cancer (NCT03980041) and in a triplet regimen with nab-paclitaxel and atezolizumab in patients with first-line metastatic TNBC (NCT03961698), respectively.
Supplementary Material
Statement of translational relevance.
Eganelisib (IPI-549) is a first-in-class, potent, and highly selective phosphoinositide-3-kinase (PI3K)-γ inhibitor. Based on preclinical studies, PI3K-γ blockade reprograms myeloid cells to a pro-inflammatory phenotype, leading to immune activation in the tumor microenvironment and synergistic anti-tumor activity in combination with checkpoint inhibitors. Here, we present the data from MAcrophage Reprogramming in Immuno-Oncology-1 (MARIO-1), the first-in-human study of eganelisib as monotherapy and in combination with nivolumab in patients with advanced solid tumors. The overall safety profile was manageable both as monotherapy and combination therapy. Based on safety, preliminary clinical activity, pharmacokinetic, and pharmacodynamic results of this study, eganelisib doses of 30 mg and 40 mg daily are being investigated in combination with immune checkpoint inhibitors in phase 2 clinical trials.
Acknowledgments
The authors would like to thank the participating patients and their families, and the clinical staff, research coordinators, and study management team. The clinical trial was supported by Infinity Pharmaceuticals, Inc. and funded in part through the NIH/NCI Cancer Center Support Grant P30CA008748 (M.A. Postow). Editorial assistance with the preparation of this manuscript was provided by Nate Connors, PhD, CMPP of Acumen Medical Communications, Boston MA, with funding from Infinity Pharmaceuticals, Inc. and by Jacqueline Campbell, an employee of Infinity Pharmaceuticals, Inc.
Financial support
This study was supported by Infinity Pharmaceuticals, Inc. and funded in part through the NIH/NCI Cancer Center Support Grant P30CA008748 (M.A. Postow). D.S. Hong acknowledges support from the MD Anderson Cancer Center Support Grant (P30 CA016672) and from the Center for Clinical and Translational Sciences (1UL1 TR003167).
Disclosures/conflicts of interest
DSH reports grants and research funding from AbbVie, Adaptimmune, Adlai-Nortye, Amgen, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Deciphera, Endeavor, Erasca, F. Hoffmann-La Roche, Fate Therapeutics, Genentech, Genmab, Immunogen, Infinity Pharmaceuticals, Inc., Merck, Mirati, Navier, NCI-CTEP, Novartis, Numab, Pfizer, Pyramid Bio, Revolution Medicine, SeaGen, ST-Cube, Takeda, TCR2, Turning Point Therapeutics, and VM Oncology; travel funding from Bayer, Genmab, AACR, ASCO, SITC, and Telperian; speaking and/or consulting fees from Adaptimmune, Alpha Insights, Acuta, Alkermes, Amgen, Aumbiosciences, Axiom, Baxter, Bayer, Boxer Capital, BridgeBio, COR2ed, COG, Cowen, Ecor1, F. Hoffmann-La Roche, Gennao Bio, Genentech, Gilead, GLG, Group H, Guidepoint, HCW Precision, Immunogen, Janssen, Liberium, MedaCorp, Medscape, Numab, Oncologia Brasil, Orbi Capital, Pfizer, Pharma Intelligence, POET Congress, Prime Oncology, RAIN, Seattle Genetics, ST Cube, Takeda, Tavistock, Trieza Therapeutics, Turning Point, WebMD, YingLing Pharma, Ziopharm; and other ownership interests with Molecular Match (Advisor), OncoResponse (Founder, Advisor), and Telperian (Founder, Advisor).
MP reports institutional support from RGenix, Infinity Pharmaceuticals, Inc., Bristol-Myers Squibb, Merck, Array BioPharma, Novartis, and AstraZeneca and personal consulting fees from Bristol-Myers Squibb, Merck, Array BioPharma, Novartis, Eisai, and Pfizer.
BC reports serving on advisory boards for IDEAYA Biosciences, OncoSec, Genentech, Novartis, Delcath Systems, and Instil Bio; data monitoring committees for Nektar; and providing clinical trial support for Bristol-Myers Squibb, Macrogenics, Merck, Karyopharm Therapeutics, Infinity Pharmaceuticals, Inc., Advenchen Laboratories, Idera, Xencor, Compugen, Iovance Biotherapeutics, PACT Pharma, RAPT Therapeutics, Immunocore, IDEAYA Biosciences, Ascentage Pharma, Novartis, Atreca, Replimune, Instil Bio, Adagene, and TriSalus Life Sciences.
RS reports consulting fees from Bristol-Myers Squibb, Merck, Novartis, and Pfizer; and research support from Merck.
AP reports honoraria from the Texas Society of Clinical Oncology (TxSCO); serving in a consulting or advisory role for Bayer, Novartis, Merck, Seattle Genetics, Silverback Therapeutics, Shenzhen IONOVA Life Science Co., Ltd., Gilead, Daiichi-Sankyo, HalioDx, and Janssen; research funding from Merck, Pfizer, Lilly, Plexxikon, Corvus Pharmaceuticals, Tesaro, Abbvie, Forty Seven, Inc., Five Prime Therapeutics, Infinity Pharmaceuticals, Inc., Pieris Pharmaceuticals, Surface Oncology, Livzon Pharmaceutical Group Inc., Vigeo Therapeutics, Astellas Pharma, KLUS Pharma, Inc., Symphogen, Syndax Pharmaceuticals, Inc., Arcus Biosciences, Fochon Biosciences, Upsher-Smith Laboratories, LLC, Exelixis Inc., Seattle Genetics, Bolt Biotherapeutics, Shenzhen IONOVA Life Science Co., Ltd, Daiichi-Sankyo, Sanofi, Gilead Sciences, Seagen Inc., Pionyr Immunotherapeutics, Inc., Loxo Oncology, Inc. on behalf of Eli Lilly and Company, Nektar Therapeutics, Alpine Immune Science, Inc., Amgen, Institut de Researches Internationales Servier (I.R.I.S.), 100 Pharma, Arcus Biosciences, Genentech, Aadi Bioscience, Prelude Therapeutics, and KSQ Therapeutics, Inc.; and has immediate family members serving in a consulting or advisory role for Genentech/Roche, Merck, and Bristol-Myers Squibb.
EC reports consulting fees from Axelia, Eisai, Hoopika, ImmunoSensor, lstari, Janssen, Kahr Medical, Mana Therapeutics, Merck, Mirati, MSD, Nectin Tx, Pangea Therapeutics, and Roche; performing data safety monitoring for Ayala and Kura; serving on boards of directors for NCCN and Psioxus Therapuetics; advisory boards for Plant Therapeutics, Inc.; scientific advisory board for Kinnate Biopharma, and has stock/equity in Kinnate Biopharma and Primmune Therapeutics.
JW reports consulting fees from Amgen, Apricity, Ascentage Pharma, Astellas, AstraZeneca, Bicara Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, CellCarta, Chugai, Daiichi Sankyo, Dragonfly, Georgiamune, Idera, Imvaq, Larkspur, Maverick Therapeutics, Merck, Psioxus, Recepta, Tizona, Trishula, Sellas, Surface Oncology, and Werewolf Therapeutics; grant/research support from Bristol-Myers Squibb and Sephora; and has ownership/equity in Apricity, Arsenal IO, Ascentage, Beigene, Imvaq, Linneaus, Georgiamune, Maverick, Tizona Pharmaceuticals, and Trieza
BO is an employee of Infinity Pharmaceuticals, Inc. and reports a patent for 63/122,892.
NZ and RI are employees of Infinity Pharmaceuticals, Inc.
GP and JP are paid consultants of Infinity Pharmaceuticals, Inc.
GS, CS, MG, HY-R, and AT have no disclosures to report.
References
- 1.Awad RM, De VY, Maebe J, Goyvaerts C, Breckpot K. Turn back the TIMe: targeting tumor infiltrating myeloid cells to revert cancer progression. Front Immunol. 2018;9:1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16(7):447–62. [DOI] [PubMed] [Google Scholar]
- 3.Berry S, Giraldo NA, Green BF, Cottrell TR, Stein JE, Engle EL, et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade. Science. 2021;372(6547). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19(6):715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De HO, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539(7629):443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foubert P, Kaneda MM, Varner JA. PI3Kgamma activates integrin alpha4 and promotes immune suppressive myeloid cell polarization during tumor progression. Cancer Immunol Res. 2017;5(11):957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans CA, Liu T, Lescarbeau A, Nair SJ, Grenier L, Pradeilles JA, et al. Discovery of a selective phosphoinositide-3-kinase (PI3K)-gamma inhibitor (IPI-549) as an immuno-oncology clinical candidate. ACS Med Chem Lett. 2016;7(9):862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeHenau O, Merghoub T, Winkler D, Sharma S, Pink M, Tchaicha J, et al. Checkpoint blockade therapy is improved by altering the immune-suppressive microenvironment with IPI-549, a potent and selective inhibitor of PI3K- [ [Google Scholar]
- 10.Kutok J, Ali J, Brophy E, Castro A, DiNitto J, Evans C, et al. , editors. The potent and selective phosphoinositide-3-kinase-gamma inhibitor, IPI-549, inhibits tumor growth in murine syngeneic solid tumor models through alterations in the immune suppressive microenvironment. Poster presented at CRI-CIMT-EATI-AACR - The Inaugural International Cancer Immunotherapy Conference, 2015 Sept 16–19; New York, NY: 2015. [Google Scholar]
- 11.McGovern K, Ali J, Brophy E, Castro A, DiNitto J, Evans C, et al. The potent and selective phosphoinositide-3-kinase (PI3K)-. 2015. [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 13.Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: clinical implications and adverse effects. Int J Mol Sci. 2021;22(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2018. [ [Google Scholar]
- 15.Radi ZA, Koza-Taylor PH, Bell RR, Obert LA, Runnels HA, Beebe JS, et al. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am J Pathol. 2011;179(1):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen R, Lievense LA, Maat AP, Hendriks RW, Hoogsteden HC, Bogers AJ, et al. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PLoS One. 2014;9(9):e106742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyein AF, Bari S, Hogue S, Zhao Y, Maller B, Sha S, et al. Effect of prior antibiotic or chemotherapy treatment on immunotherapy response in non-small cell lung cancer. BMC Cancer. 2022;22(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.