TO THE EDITOR:
Clonal hematopoiesis (CH), defined as the clonal expansion of mutated hematopoietic stem and progenitor cells, is associated with the development of hematologic cancers, cardiovascular disease (CVD), and other adverse health outcomes.1, 2, 3 CH is thought to expand with older age resulting in an increasing variant allele frequency (VAF) over time.1 Thus, the frequency of CH detection in a given population is dependent on the age and sensitivity of sequencing techniques, with ultradeep sequencing detecting low-VAF variants.4 CH is also associated with race, smoking, and exposure to oncologic therapy.5, 6, 7, 8 Environmental stressors may increase the likelihood of both mutational acquisition and clonal fitness. Oncologic therapy has been previously shown to increase the fitness advantage of mutant hematopoietic stem and progenitor cells, particularly those bearing mutations in the DNA damage response (DDR) pathway, including TP53, PPM1D, and CHEK2.9,10 However, the extent to which therapy-induced CH expansion persists after the completion of therapy has not been defined.
Survivors of childhood cancer are a growing cohort in whom CH has not been routinely assessed; an estimated 500 000 survivors are currently alive in the United States.11 Despite excellent 5-year survival rates, survivors of childhood cancer remain at a lifelong risk of premature morbidity and mortality, including a 15-fold risk of death due to subsequent malignant neoplasm (SMN) and a sevenfold risk of death due to CVD,12,13 with particularly pronounced risks noted after exposure to chemotherapy and/or radiotherapy, generally in a dose-dependent fashion.14,15 Survivors have also been noted to have higher rates of premature aging.16,17 We hypothesized that CH mutations would be enriched in survivors of childhood cancer previously exposed to cytotoxic therapy because of the increased selection of preexisting CH clones, which may, in part, explain their increased risk of SMN, CVD, and premature aging. Here, we compared the frequency of CH between survivors of childhood cancer and matched controls using error-corrected deep sequencing. We show that survivors have higher frequencies of CH than age-matched controls and that this increased frequency of CH persists for many years after treatment.
This was a retrospective case-control study survivors of childhood, adolescent, and young adult cancer and matched controls. Cases included individuals diagnosed with a solid tumor or lymphoma at ≤28 years of age and treated with systemic chemotherapy and/or radiotherapy who were ≥13 years of age and ≥6 months since the completion of therapy at peripheral blood sample collection during routine clinical visits. Age-matched controls (5-year age groups) with and without a history of cancer were included, given the evidence of shared genomic risk factors between cancer and CH. Controls included 1 group of treatment-naive individuals recently diagnosed with a nonhematologic cancer at Sloan Kettering Cancer Center and another group of healthy adolescents and young adults from Washington University without a cancer history.
Samples collected from all participants were batched and sequenced using a custom targeted amplicon-based UMI sequencing platform (ArcherDX), which included complete exons of DMNT3A, TET2, ASXL1, TP53, CHEK2, and targeted regions of PPM1D, SRSF2, SF3B1, and JAK2.9 The samples were sequenced at an average depth of 19 830 bp. Variant calling was performed using Mutect2, Vardict, and Lofreq2, with additional postvariant calling filters applied to remove artifacts and germline variants. Using this approach, we were able to reliably identify mutations with a VAF >0.1%. CH mutations were annotated as potential drivers using previously published CH annotations and oncology knowledge databases, including the Catalogue of Somatic Mutations in Cancer, Cancer Gene Census, MSK's Oncology Knowledge Base, and Clinvar7,9,18 (supplemental Methods). Logistic regression adjusted for age, sex, and race was used to test for an association between CH and the case-control status. Firth logistic regression was used to test for an association between CH subgroups and case-control status because of the sparse data.
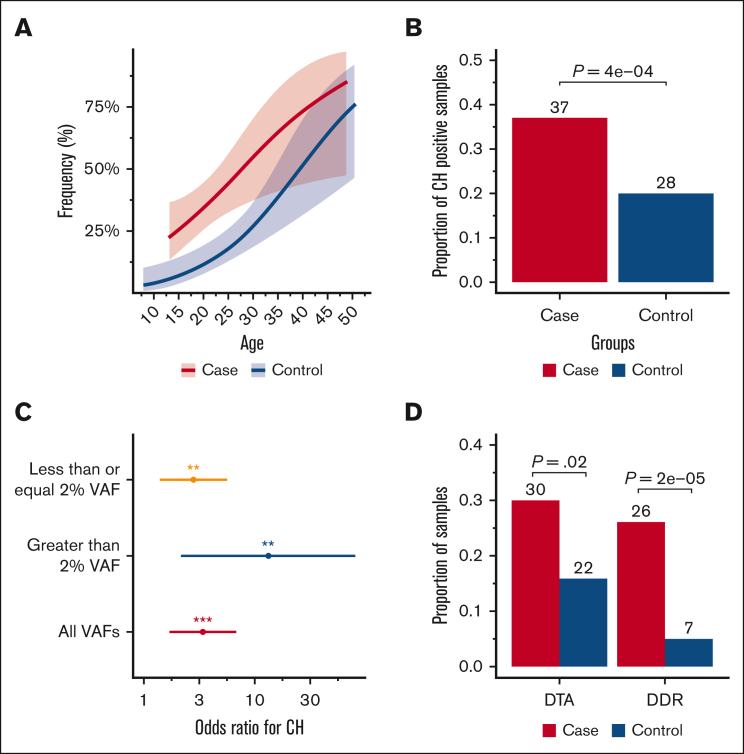
Samples were analyzed from 100 survivors of childhood cancer (median age, 19 years; range, 13-49 years), 71 controls with untreated newly diagnosed cancer (median age, 25 years; range, 8-48 years), and 68 healthy controls (median age, 23 years; range, 15-51 years). Table 1 shows the participant demographics. As expected, the most commonly mutated genes were in the DTA (DNMT3A, TET2, and ASXL1) and DDR (PPM1D, TP53, and CHEK2) classes (supplemental Figure 1). The frequency of CH was higher in survivors of childhood cancer than in controls across the various age groups (Figure 1A), with CH detected in 37.0% of survivors, compared with 20.1% of controls (Figure 1B; OR, 3.5; 95% CI, 1.8-7.1; P = .0004). We observed a trend toward a stronger association for CH mutations with VAF >2% (OR, 12.6; 95% CI, 2.1-74.4; P = .005) compared with VAF ≤2% (OR, 2.8; 95% CI, 1.4-5.5; P = .003; Figure 1C). Sensitivity analyses excluding patients with a history of SMN yielded similar results (supplemental Figure 2). The enrichment of CH in survivors was observed relative to both healthy controls (OR, 2.5; 95% CI, 1.1-5.6; P = .02) and treatment-naive controls with solid tumors (OR, 5.5; 95% CI, 2.2-15.4; P = .0005), suggesting that the enrichment of CH in survivors was driven by selection resulting from prior exposure to cancer therapy (supplemental Figure 3).
Table 1.
Demographic and treatment characteristics of survivors of childhood cancer and of controls
| Cases (n = 100) |
Comparison cohort (n = 139) |
||
|---|---|---|---|
| Surviors of childhood cancer (n = 100) | Treatment-naive controls with solid tumors (n = 71) | Controls without cancer (n = 68) | |
| Age, y | |||
| Mean (SD) | 21.4 (7.2) | 24.8 (8.9) | 24.7 (7.4) |
| Median (range) | 19 (13-49) | 25 (8-48) | 23 (15-51) |
| Sex | |||
| Female | 50 (50.0%) | 39 (54.9%) | 42 (61.8%) |
| Race | |||
| White | 84 (84.0%) | 46 (64.8%) | 43 (63.2%) |
| Black or African American | 6 (6.0%) | 6 (8.5%) | 15 (22.1%) |
| Asian | 8 (8.0%) | 9 (12.7%) | 3 (4.4%) |
| Other | 1 (1.0%) | 5 (7.0%) | 2 (2.9%) |
| Unknown/missing | 1 (1.0%) | 5 (7.0%) | 5 (7.4%) |
| Primary cancer diagnosis, no (%) | |||
| Sarcoma | 51 (51.0%) | ||
| Neuroblastoma | 16 (16.0%) | ||
| Lymphoma | 12 (12.0%) | ||
| CNS tumor | 8 (8.0%) | ||
| Retinoblastoma | 4 (4.0%) | ||
| Germ cell tumor | 3 (3.0%) | ||
| Wilms tumor | 2 (2.0%) | ||
| Thyroid carcinoma | 1 (1.0%) | ||
| Other∗ | 3 (3.0%) | ||
| Chemotherapy, no (%) | 93 (93.0%) | — | — |
| Anthracyclines | 80 (80.0%) | — | — |
| Alkylating agents | 79 (79.0%) | — | — |
| Platinum agents | 45 (45.0%) | — | — |
| Etoposide | 62 (62.0%) | — | — |
| External beam radiotherapy, no (%) | 65 (65.0%) | — | — |
| Radioactive iodine, no. (%) | 6 (6.0%) | — | — |
| Autologous stem cell transplant, no (%) | 11 (11.0%) | ||
| Median time since the completion of therapy, y | 9.7 (0.5-39) | — | — |
One participant was exposed to total body irradiation before autologous stem cell transplant.
CNS, central nervous system.
Other tumors include adrenocortical carcinoma, chordoma, and atypical teratoid rhabdoid tumor.
Figure 1.
Summary of CH findings in survivors of childhood, adolescent, and young adult cancer (cases), compared with controls. (A) CH frequency in survivors vs controls as a function of age; (B) prevalence of CH-positive samples in survivors vs treatment-naive controls with solid tumors; (C) odds ratio of CH on comparing cases with controls for overall CH, CH with a maximum VAF of >2%, and CH with a maximum VAF of ≤ 2%; (D) proportion of samples with DTA vs DDR in cases compared with controls. All analyses were adjusted for age, sex, and race. Fitted curves were generated using a generalized additive model. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Next, we explored CH mutational features between cases and controls. Although survivors had a higher proportion of both DDR and DTA mutations when compared with those in controls (Figure 1D; supplemental Figure 4), the enrichment of DDR mutations was more than that of DTA (OR, 7.1; 95% CI, 2.7-18.6; P = .00002 for DDR; OR, 2.4; 95% CI, 1.2-5.1; P = .02 for DTA). This is consistent with previous studies showing that exposure to cancer-directed therapy is positively associated with DDR and, to a lesser extent, DTA mutations.9,19 Among individuals with CH, there was no significant difference in the VAF or the number of CH mutations between cases and controls (supplemental Figure 5). Given that the enrichment of CH among survivors appeared to be driven by prior therapeutic exposure, we examined the relationship between CH and the time since the completion of therapy. Even among individuals who were at least 5 years from end-of-therapy, a higher frequency of CH was observed compared with that among controls (OR, 2.9; 95% CI, 1.4-6.2; P = .005), suggesting that the impact of oncologic therapy on CH persisted over time (supplemental Figure 6).
Here, we show that survivors of childhood cancer have a markedly higher frequency of CH than age-matched controls. Although exposure to oncologic therapy has been shown to result in a selective advantage of CH mutant clones among patients with ongoing therapy,9 much of the work on CH in the survivorship setting has been limited to survivors of cancers during adulthood.20,21 Conflicting data exist regarding the prevalence of CH among survivors of childhood cancer.22, 23, 24 Here, we observed a substantially higher frequency of CH in slightly older survivors of childhood cancer (median age, 19 years; range, 13-49 years) than that of controls, which persisted among individuals for whom many years had passed since the completion of therapy. This suggests that the impact of oncologic therapy on CH expansion is long-lasting, with the expanded clone persisting for many years after therapeutic exposure.
CH is a prominent risk factor for early onset CVD and hematologic cancers, with emerging data linking it to the risk of nonhematologic cancers. The strong enrichment of CH in survivors of childhood cancer suggests that CH may, in part, drive the long-term high-burden of premature multimorbidity, particularly CVD and SMN, in survivors of childhood cancer.25 These data justify the need for a longitudinal assessment of CH in more diverse cohorts of survivors, including those who do not routinely return for survivorship care, and may provide a rationale for the use of CH to inform treatment modification strategies and risk-based screening. Such efforts will clarify the extent to which CH enrichment may contribute to the mechanistic underpinnings of various late effects and inform the provision of care to survivors of childhood cancer as they age.
Conflict-of-interest disclosure: R.L.L. is on the supervisory board of Qiagen; is a scientific adviser to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics, and Isoplexis; receives research support from Ajax, Zentalis, and AbbVie; has consulted for Incyte, Janssen, and AstraZeneca; and has received honoraria from AstraZeneca for invited lectures. A.L.K. is on the scientific advisory board of Emendo Biotherapeutics, Karyopharm Therapeutics, Imago BioSciences, and DarwinHealth; is a cofounder and on the board directors of Isabl; and has equity interest in Imago BioSciences, Emendo Biotherapeutics, and Isabl. L.A.D.J. is a member of the board of directors of Jounce Therapeutics and Epitope; is a compensated consultant for PetDx, Innovatus CP, Se'er, Delfi, Blackstone, Kinnate, and Neophore; is an inventor on multiple licensed patents related to technology for circulating tumor DNA analyses and mismatch repair deficiency for diagnosis and therapy (some of these licenses and relationships are associated with equity or royalty payments to the inventors); holds equity in Epitope, Jounce Therapeutics, PetDx, Se'er, Delfi, Kinnate, and Neophore; divested his equity from Personal Genome Diagnostics to LabCorp in February 2022 and from Thrive Earlier Detection to Exact Biosciences in January 2021; and his spouse holds equity in Amgen (the terms of all these arrangements are being managed by Memorial Sloan Kettering in accordance with their conflict-of-interest policy). T.E.D. is an employee of Mission Bio, which had no role in the generation, analysis, or interpretation of these data. The remaining authors declare no competing financial interests.
Acknowledgments
Acknowledgments: This work was financially supported by a Memorial Sloan Kettering Society research grant, the Meg Berte Owen Foundation, NCI 2P30CA008748-48 (Memorial Sloan Kettering Cancer Center support grant), MSK Precision Interception Program, Evans Center for MDS, Memorial Sloan Kettering Cancer Center, and Center for Pediatric Immunology, St. Louis Children’s Hospital and Washington University. D.N.F. is supported, in part, by a Clinician Scientist Development grant (133831-CSDG-19-117-01-CPHPS) from the American Cancer Society.
Contribution: D.N.F. and K.L.B. conceived and designed the study; D.N.F., S.L., N.B., N.S., K.T., and M.A.C. collected samples and clinical data; K.L.B., I.C.C., and J.L., called variants and performed post-processing of sequencing data; K.L.B., I.C.C., and C.S.M. performed statistical analyses and/or participated in data interpretation; and D.N.F., I.C.C., C.S.M., S.L., K.T., J.L., N.B., M.F.W., B.S., A.K., M.F.B., M.A.C., I.P., G.U., D.L., T.E.D., L.A.D., R.L.L., N.S., and K.L.B. contributed to the writing of the manuscript and approved it for submission.
Footnotes
This work was presented in oral form at the American Society of Clinical Oncology Annual Meeting on 5 June 2023, Chicago, IL; the International Symposium on Late Complications After Childhood Cancer on 17 June 2023, Atlanta, GA; and will be presented at the Cancer Genomics Consortium Annual Meeting on 13 August 2023, St Louis, MO.
De-identified data are available on request from the corresponding authors, Danielle Novetsky Friedman (friedmad@mskcc.org) and Kelly Bolton (bolton@wustl.edu).
The full-text version of this article contains a data supplement.
Contributor Information
Danielle Novetsky Friedman, Email: friedmad@mskcc.org.
Kelly L. Bolton, Email: bolton@wustl.edu.
Supplementary Material
References
- 1.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7 doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton KL, Gillis NK, Coombs CC, et al. Managing clonal hematopoiesis in patients with solid tumors. J Clin Oncol. 2019;37(1):7–11. doi: 10.1200/JCO.18.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucai L, Falcone J, Ukena J, et al. Radioactive iodine-related clonal hematopoiesis in thyroid cancer is common and associated with decreased survival. J Clin Endocrinol Metab. 2018;103(11):4216–4223. doi: 10.1210/jc.2018-00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombs CC, Gillis NK, Tan X, et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res. 2018;24(23):5918–5924. doi: 10.1158/1078-0432.CCR-18-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374–382.e4. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52(11):1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu JI, Dayaram T, Tovy A, et al. PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell. 2018;23(5):700–713.e6. doi: 10.1016/j.stem.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howlader N, Noone AM, Krapcho M, Miller D, et al., editors. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; Bethesda: MD. 2017. https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 12.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol. 2009;27(14):2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates JE, Howell RM, Liu Q, et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the childhood cancer survivor study. J Clin Oncol. 2019;37(13):1090–1101. doi: 10.1200/JCO.18.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA. 2017;317(8):814–824. doi: 10.1001/jama.2017.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ness KK, Kirkland JL, Gramatges MM, et al. Premature physiologic aging as a paradigm for understanding increased risk of adverse health across the lifespan of survivors of childhood cancer. J Clin Oncol. 2018;36(21):2206–2215. doi: 10.1200/JCO.2017.76.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2013;31(36):4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiley B, Parsons TM, Burkart S, et al. Effect of clonal hematopoiesis on cardiovascular disease in people living with HIV. Exp Hematol. 2022;114:18–21. doi: 10.1016/j.exphem.2022.07.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong TN, Miller CA, Jotte MRM, et al. Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nat Commun. 2018;9(1):455. doi: 10.1038/s41467-018-02858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35(14):1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soerensen JF, Aggerholm A, Rosenberg CA, et al. Clonal evolution in patients developing therapy-related myeloid neoplasms following autologous stem cell transplantation. Bone Marrow Transplant. 2022;57(3):460–465. doi: 10.1038/s41409-022-01567-z. [DOI] [PubMed] [Google Scholar]
- 22.Acuna-Hidalgo R, Sengul H, Steehouwer M, et al. Ultra-sensitive sequencing identifies high prevalence of clonal hematopoiesis-associated mutations throughout adult life. Am J Hum Genet. 2017;101(1):50–64. doi: 10.1016/j.ajhg.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collord G, Park N, Podestà M, et al. Clonal haematopoiesis is not prevalent in survivors of childhood cancer. Br J Haematol. 2018;181(4):537–539. doi: 10.1111/bjh.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagiwara K, Natarajan S, Wang Z, et al. Dynamics of age- versus therapy-related clonal hematopoiesis in long-term survivors of pediatric cancer. Cancer Discov. 2023;13(4):844–857. doi: 10.1158/2159-8290.CD-22-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort study (SJLIFE) Lancet. 2017;390(10112):2569–2582. doi: 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.