TO THE EDITOR:
Glucocorticoids (GCs; ie, steroids) are important chemotherapeutic agents in the treatment of B-cell precursor acute lymphoblastic leukemia (B-ALL),1 and de novo GC resistance predicts relapse and poor clinical outcome.2,3 Glucocorticoids induce B-ALL cell apoptosis through the activation of glucocorticoid receptor (GR), a ligand-induced nuclear receptor transcription factor (TF).4,5 We previously identified disruptions to GR-bound cis-regulatory elements controlling TLE1 expression, a GC-response gene upregulated by steroids, as a novel mechanism affecting GC resistance in primary B-ALL cells from patients.6 Supporting our findings, TLE1 expression was found to be associated with GC resistance in primary B-ALL cells from patients, with 40% of samples of the patients who are GC-resistant harboring low TLE1 expression that further correlates with treatment response (supplemental Figure 1).7,8 In this regard, TLE1 was also identified as an indicator of adverse prognosis in patients with ALL.9 Because TLE1 functions as a repressor of canonical Wnt signaling10 and investigations in other cell systems have suggested interaction between GC and canonical Wnt signaling pathways,11, 12, 13 we investigated mechanisms connecting TLE1 to GC resistance and the extent of cross talk between GC and canonical Wnt signaling pathways in B-ALL.
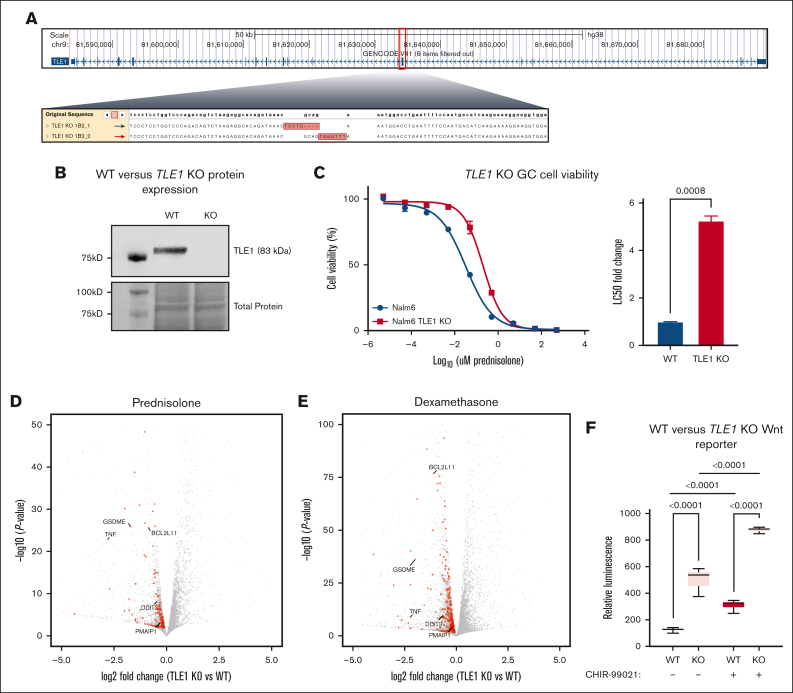
Firstly, we examined the functional effects of a homozygous knockout (KO) of TLE1 on GCs and canonical Wnt signaling using CRISPR-Cas9 genome editing in the Nalm6 human B-ALL cell line (Figures 1A-B). Consistent with our previous observations,6 we found that TLE1 KO led to significantly increased resistance to GCs (ie, prednisolone) with more than a fivefold increase in prednisolone lethal concentration 50% (LC50) compared with that in wild-type (WT) cells (Figure 1C). To better understand the broader effects of TLE1 disruption on GC signaling, we conducted RNA sequencing in WT and TLE1 KO Nalm6 cells treated with prednisolone or vehicle control for 24 hours. Differentially expressed gene analyses between WT and TLE1 KO cells in the presence or absence of prednisolone helped identify an enrichment for apoptotic signaling and cell cycle pathways at TLE1 KO repressed genes and diverse metabolic pathways for TLE1 KO activated genes (Figure 1D; supplemental Figure 2; supplemental Table 1). Similar results were obtained using dexamethasone (Figure 1E). To identify TLE1-dependent changes to canonical Wnt signaling, both WT and TLE1 KO cells were transfected with the M50 Super 8xTOPFlash reporter plasmid14 and treated for 24 hours with a canonical Wnt signaling agonist (CHIR-99021) or vehicle control. This assay measures β-catenin–driven luciferase activity as an indicator of endogenous canonical Wnt signaling. Under both treatment conditions, TLE1 KO cells showed increased canonical Wnt activity compared with WT cells (Figure 1F), which is consistent with the role of TLE1 as a repressor of canonical Wnt signaling.10
Figure 1.
Impact of TLE1 knockout on GC and canonical Wnt signaling. (A) Schematic representation of CRISPR/Cas9 genome editing of exon 7 of the TLE1 gene, with deep sequencing confirmation of the edit to both strands in Nalm6 cells. (B) Western blot showing protein expression of TLE1 (∼83 kD; Abcam ab183742) in both TLE1 KO Nalm6 and WT Nalm6 cells. Ponceau staining for total protein (bottom). (C) Prednisolone drug response curves in TLE1 KO Nalm6 cells (red) and WT Nalm6 cells (blue) after 72 hours of prednisolone treatment (left); n = 3 per group. Concentrations of prednisolone used were 5 pM, 50 pM, 0.5 nM, 5 nM, 50 nM, 0.5 μM, 5 μM, 50 μM, and 500 μM. LC50 fold-change in TLE1 KO Nalm6 cells compared with WT Nalm6 cells (right). (D-E) Volcano plots showing differentially expressed genes between WT and TLE1 KO cells after 24 hours of prednisolone (5 μM) treatment (D) or dexamethasone (100 nM) treatment (E). Genes involved in apoptotic pathways are shown in red, and several notable genes are highlighted. (F) β-Catenin luciferase reporter assay for WT Nalm6 and TLE1 KO cells treated with or without CHIR-99021 (0.5 μM) for 24 hours, n = 6 per group.
Next, we assessed the GC and canonical Wnt signaling pathway cross talk in B-ALL cells independent of TLE1 KO. Two human B-ALL cell lines (Nalm6 and 697) were treated with different prednisolone concentrations for 72 hours in the presence or absence of Wnt agonist, CHIR-99021. At all GC concentrations and in both cell lines, cotreatment with the Wnt agonist significantly increased cellular viability relative to treatment with prednisolone alone (supplemental Figure 3). Similar results were also obtained using dexamethasone (supplemental Figure 4). In addition, we confirmed this effect through ex vivo GC drug viability studies in xenograft cell samples derived from patients with B-ALL (supplemental Figure 5). These findings suggest that Wnt activation opposes GC-induced apoptosis, which is consistent with the role for canonical Wnt signaling in cell proliferation and cancer predisposition15 and prior findings from B-ALL cell viability experiments using Wnt antagonist.16 Concordant with these data, we identified synergy from cotreatment with prednisolone or dexamethasone and canonical Wnt antagonists (supplemental Figure 6) and in TLE1 KO GC-resistant cells (supplemental Figure 7).17 To further determine the extent to which GCs affect canonical Wnt signaling, we measured β-catenin–driven luciferase reporter activity in B-ALL cells treated with prednisolone and/or CHIR-99021. We found that prednisolone treatment significantly dampened canonical Wnt signaling in the presence or absence of Wnt agonist, with consistent results also observed in TLE1 KO cells (supplemental Figure 8). Cross talk between GC and canonical Wnt signaling was also observed in T-cell ALL (T-ALL; supplemental Figures 9-11). Collectively, these data indicate cross talk and antagonism between these 2 signaling pathways. The use of xenograft cell samples derived from patients was approved by the institutional review board at St Jude Children's Research Hospital.
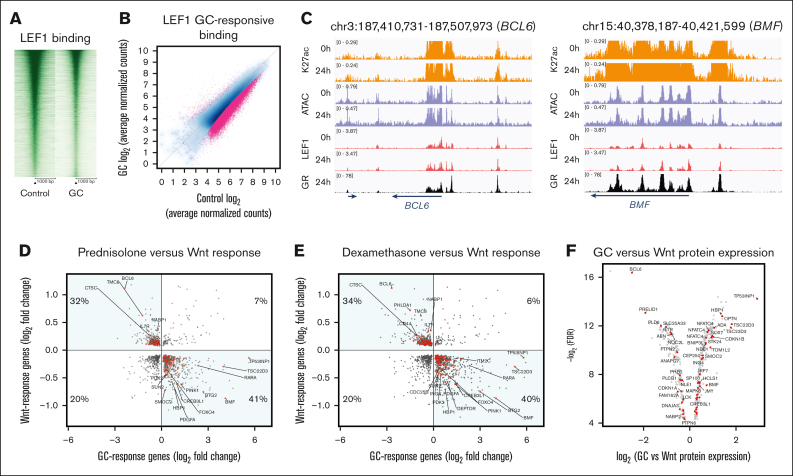
We further investigated genomic mechanisms that promote antagonism between GC and canonical Wnt signaling pathways. Using Nalm6 prednisolone- and dexamethasone-response genes, we found that in addition to upregulation of the TLE1 canonical Wnt repressor gene, both GCs repressed the canonical Wnt activating TF gene LEF1 (supplemental Figure 12). Consistent effects on TLE1 and LEF1 expression were also reported in a panel of 19 human B-ALL cell lines after dexamethasone treatment.18 We therefore asked whether a 24-hour GC treatment resulted in decreased genome occupancy of LEF1. Using the CUT&RUN assay, we determined that LEF1 binding was less at 32 105 (53%) and more at only 730 (1.2%) of the sites after 24 hours of prednisolone treatment (Figures 2A-B). Strikingly, we also uncovered that 58% of GR binding sites (previously mapped in the study by Bergeron et al6) overlap with LEF1 binding sites (examples in Figure 2C), and genes associated with these overlapping sites were significantly enriched for cell proliferation and apoptotic signaling pathways. Consistent results were also observed for the canonical Wnt activating TF TCF7L2 using CUT&RUN, with 91% of differentially bound TCF7L2 sites exhibiting decreased occupancy after prednisolone treatment and 20% of GR binding events overlapping TCF7L2 binding sites at genes enriched for apoptotic signaling (false discovery rate = 1.2 × 10-8; supplemental Figure 13).
Figure 2.
Crosstalk and antagonism between GC and canonical Wnt signaling. (A) CUT&RUN sequencing read enrichment at LEF1 binding sites ± 1 kb (cell signaling antibody #2230). Enrichment is shown for a 24-hour treatment with vehicle control (left) or prednisolone (5 μM; GC) (right). (B) Log2-transformed normalized CUT&RUN sequencing read counts at LEF1 binding sites treated for 24 hours with vehicle control (x-axis) or prednisolone (y-axis). Binding sites exhibiting significant differences in occupancy (false discovery rate <0.05) are shown in pink. (C) Integrative Genomics Viewer (IGV) genome browser images of signal tracks providing examples of GR and LEF1 co-occupancy at BCL6 (left) and BMF (right) gene loci. (D) Log2-transformed fold changes of genes commonly regulated by GC and Wnt signaling pathways in Nalm6 cells after 24 hours of treatment using 5 μM prednisolone (GC-response genes; x-axis) or 0.5 μM CHIR-99021 (Wnt-response genes; y-axis). GC-response and Wnt-response gene log2 fold changes are provided. The percentage of genes in each quadrant is provided, and genes showing opposing effects are highlighted in quadrants 2 and 4. Red denotes genes involved in cell death, cell proliferation, and/or cell cycle pathways and notable genes are labeled. (E) Log2-transformed fold changes of genes commonly regulated by GC and Wnt signaling pathways in Nalm6 cells after 24 hours of treatment using 100 nM dexamethasone (GC-response genes; x-axis) or 0.5 μM CHIR-99021 (Wnt-response genes; y-axis). GC-response and Wnt-response gene log2 fold changes are provided. The percentage of genes in each quadrant is provided, and genes showing opposing effects are highlighted in quadrants 2 and 4. Red denotes genes involved in cell death, cell proliferation, and/or cell cycle pathways and notable genes are labeled. (F) Volcano plot of differential protein expression between prednisolone (5 μM) or CHIR-99021 (0.5 μM) treatment for 24 hours. Plot shows proteins of discordantly regulated prednisolone-response and Wnt-response genes with consistent directionality. Red denotes proteins involved in cell death, cell proliferation, and/or cell cycle pathways, and notable proteins are labeled.
To determine whether overlapping TF occupancy affects cross talk between GC and canonical Wnt signaling transcriptional responses, RNA sequencing was performed using WT Nalm6 cells treated for 24 hours with CHIR-99021 or vehicle control. We identified that 84% of Wnt-response genes were also prednisolone-response genes in Nalm6 cells. Supporting a mutual antagonism between these 2 signaling pathways, 73% of these common response genes exhibited discordant changes in gene expression (Figure 2D) and were enriched in cell death and cell cycle pathways (supplemental Table 2). Consistent patterns were also found using dexamethasone-response genes in Nalm6 cells (Figure 2E) and CEM T-ALL cells (supplemental Figure 14), with most Wnt-response genes overlapping GC-response genes and most of these common response genes being discordantly regulated (Nalm6 = 74% and CEM = 65%) and enriched for similar biological pathways (supplemental Table 2). More than 94% of discordantly regulated prednisolone-response genes were also discordantly regulated dexamethasone-response genes. Most discordantly regulated prednisolone-response genes (58%) were also associated with overlapping GR and LEF1 or GR and TCF7L2 binding events in Nalm6 cells. Sixty-one discordantly regulated prednisolone-response genes were also previously linked to GC resistance in primary B-ALL cells from >200 patients (supplemental Table 3),7 supporting a broader impact on GC resistance, and most of these genes (46 of 61 [75%]) were associated with overlapping GR and LEF1 or GR and TCF7L2 binding events. Most discordantly regulated GC-response genes (≥66%) also exhibited a more blunted GC response in TLE1 KO cells compared with that in WT cells, concordant with enhanced canonical Wnt signaling and reduced cellular apoptosis from TLE1 ablation (supplemental Figure 15). To determine whether this transcriptional antagonism translates to the proteome level, we performed mass spectrometry in Nalm6 cells after a 24-hour treatment with prednisolone or CHIR-99021. We discovered that 50% of discordantly regulated prednisolone-response genes also exhibited significantly different patterns as proteins (supplemental Table 4), and 66% of these proteins displayed consistent directionality (Figure 2F).
Collectively, our data uncovered extensive cross talk and mutual antagonism between GC and canonical Wnt signaling pathways in B-ALL cells, and we confirmed similar effects in T-ALL cells. This antagonism is mediated in part through binding of GC and Wnt TFs to common cis-regulatory elements associated with cell death and cell proliferation genes. This overlap in TF occupancy was further accompanied by overlapping and opposing transcriptional programs that affected protein expression. Overall, these data suggest that cis-regulatory disruptions at TLE1 are linked to GC resistance in primary B-ALL cells from patients through reduced GC-mediated apoptosis via enhanced canonical Wnt signaling. As a result of the deep genomic and gene regulatory connectivity between these 2 signaling pathways, our data support the importance of canonical Wnt signaling in mediating GC resistance in B-ALL and further suggest that treatment with canonical Wnt antagonists may improve GC sensitivity in patients with resistant disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
Acknowledgments: The authors thank the St. Jude Hartwell Center for next-generation sequencing, the St. Jude Center for Advanced Genome Engineering for CRISPR/Cas9 genome editing, and the St. Jude Center for Proteomics and Metabolomics for mass spectrometry. This work was supported by the National Institutes of Health National Cancer Institute grants (R01CA234490 and P30CA021765) and the American Lebanese Syrian Associated Charities. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Contribution: D.S. conceptualized the study; B.P.B., K.R. Barnett, K.R. Bhattarai, R.J.M., A.A.H., J.P., S.M.P.-M., and D.S. developed the methodology; B.P.B., K.R. Barnett, K.R. Bhattarai, R.J.M., B.S.H., A.B., and K.K. carried out investigation; B.P.B., K.R. Barnett, K.R. Bhattarai, R.J.M., A.B., K.K., A.A.H., and D.S. performed formal analysis; S.J. and C.-H.P. were involved in clinical data acquisition; B.P.B. and D.S. contributed to the writing of the original draft; B.P.B., K.R. Barnett, K.R. Bhattarai, R.J.M., B.S.H., A.B., K.K., A.A.H., S.J., C.-H.P., J.P., S.M.P.-M., and D.S. wrote, reviewed, and edited the manuscript; and D.S. acquired funding.
Footnotes
All functional genomic data from cell lines have been deposited in the Gene Expression Omnibus database (accession number GSE215188).
Data are available on request from the corresponding author, Daniel Savic (daniel.savic@stjude.org).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11(11):1096–1106. doi: 10.1016/S1470-2045(10)70114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dordelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94(4):1209–1217. [PubMed] [Google Scholar]
- 3.Pieters R, Huismans DR, Loonen AH, et al. Relation of cellular drug resistance to long-term clinical outcome in childhood acute lymphoblastic leukaemia. Lancet. 1991;338(8764):399–403. doi: 10.1016/0140-6736(91)91029-t. [DOI] [PubMed] [Google Scholar]
- 4.Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat Rev Mol Cell Biol. 2017;18(3):159–174. doi: 10.1038/nrm.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenstein S, Ghias K, Krett NL, Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8(6):1681–1694. [PubMed] [Google Scholar]
- 6.Bergeron BP, Diedrich JD, Zhang Y, et al. Epigenomic profiling of glucocorticoid responses identifies cis-regulatory disruptions impacting steroid resistance in childhood acute lymphoblastic leukemia. Leukemia. 2022;36(10):2374–2383. doi: 10.1038/s41375-022-01685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autry RJ, Paugh SW, Carter R, et al. Integrative genomic analyses reveal mechanisms of glucocorticoid resistance in acute lymphoblastic leukemia. Nat Cancer. 2020;1(3):329–344. doi: 10.1038/s43018-020-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SHR, Yang W, Gocho Y, et al. Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response. Nat Med. 2023;29(1):170–179. doi: 10.1038/s41591-022-02112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brassesco MS, Pezuk JA, Cortez MA, Bezerra Salomao K, Scrideli CA, Tone LG. TLE1 as an indicator of adverse prognosis in pediatric acute lymphoblastic leukemia. Leuk Res. 2018;74:42–46. doi: 10.1016/j.leukres.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Chodaparambil JV, Pate KT, Hepler MR, et al. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J. 2014;33(7):719–731. doi: 10.1002/embj.201387188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meszaros K, Patocs A. Glucocorticoids influencing Wnt/beta-catenin pathway; multiple sites, heterogeneous effects. Molecules. 2020;25(7) doi: 10.3390/molecules25071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugali CK, Rayana NP, Dai J, et al. The Canonical Wnt signaling pathway inhibits the glucocorticoid receptor signaling pathway in the trabecular meshwork. Am J Pathol. 2021;191(6):1020–1035. doi: 10.1016/j.ajpath.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Mehta S, Srivastava SP, et al. Endothelial cell-glucocorticoid receptor interactions and regulation of Wnt signaling. JCI Insight. 2020;5(3) doi: 10.1172/jci.insight.131384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13(8):680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 15.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Dandekar S, Romanos-Sirakis E, Pais F, et al. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2014;167(1):87–99. doi: 10.1111/bjh.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ianevski A, He L, Aittokallio T, Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinformatics. 2020;36(8):2645. doi: 10.1093/bioinformatics/btaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruth KA, Fang M, Shelton DN, et al. Suppression of B-cell development genes is key to glucocorticoid efficacy in treatment of acute lymphoblastic leukemia. Blood. 2017;129(22):3000–3008. doi: 10.1182/blood-2017-02-766204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.