Abstract
Necrotizing Enterocolitis (NEC) is a common and devastating gastrointestinal emergency that primarily affects premature infants. The incidence of necrotizing enterocolitis is 6–10% among infants with birth weight less than 1500 grams. The mortality due to NEC has not improved significantly despite advances in neonatal care and better understanding of clinical and basic sciences. The pathogenesis of NEC is not well understood and several factors such as prematurity, abnormal colonization with pathogenic bacteria, feeding practices, blood transfusion and altered intestinal barrier function may be involved. The clinical presentation of NEC could be sudden and the treatment plan could vary with the stage and type of presentation. Further research is needed to better understand the pathophysiology of NEC and, biomarkers for prediction, prevention and treatment need to be developed. Further clinical trials are needed to determine prevention and treatment modalities for this devastating disease.
Introduction:
Necrotizing enterocolitis (NEC) is a common and devastating gastrointestinal emergency that primarily affects premature infants in neonatal intensive care units worldwide. Despite major advances in the medical care of premature infants, the etiology of NEC is still incompletely understood, and has become a research priority [1].
Epidemiology:
The incidence of NEC is 6 to 10% among infants with birth weight less than 1500 gms and varies among different US centers and across the world [2, 3] The disease is primarily seen in premature infants, however some term infants may also develop NEC. NEC in term infants is usually associated with predisposing factors such as asphyxia, polycythemia, congenital heart disease and congenital gastro-intestinal anomalies, and the disease pathophysiology in term infants is different from that in preterm infants. In the long term, survivors of NEC are at an increased risk of neurodevelopmental delays [4, 5]. Due to technological advances in the recent years, the mortality in preterm infants due to pulmonary causes, infection and central nervous system injury have been on the decline but NEC related deaths have steadily increased [6]. Over the past three decades, the NEC related mortality (up to 40%) has not improved significantly despite advances in neonatal care and better understanding of clinical and basic sciences [4]. The annual cost of managing NEC infants in United States is between $500 million and $ 1 billion. The mean cost of care over 5 year’s period for a child with short bowel syndrome has been estimated to be around $1.5 million [7]
Pathogenesis:
Prematurity:
Prematurity is the single most important risk factor for NEC. 90% of the cases occur in very low birth weight infants although the exact underlying mechanisms responsible for NEC, still remain unknown. Animal and human studies have identified alterations in the intestinal host immune [8] defense[9], bacterial colonization[10], blood flow regulation[11], motility[12] and inflammatory response[13, 14] to be responsible for gut inflammation and injury. Low gastric acid secretion in preterm infants and its inhibition by use of H2 blockers has also been linked to development of NEC [8].
Role of Feeding:
Delayed introduction of enteral feeding has been associated with the development of NEC. Total parental nutrition leads to intestinal mucosal atrophy and increases the risk of NEC [15] and recent data suggests that early enteral feeding may reduce the risk of NEC [16, 17]. Preterm infants given exclusive diets of preterm formula had a significantly greater duration of parenteral nutrition, and higher incidence of surgical NEC compared to infants who were administered human milk [18, 19]. In preterm and low birth weight infants, feeding with formula milk compared with donor breast milk resulted in a higher rate of short-term growth but also a higher risk of developing NEC [20]. Larger randomized control trials are currently underway to provide insights into the long term outcomes of donor human milk on preterm infants.
Role of Inflammation and Immunological Factors:
Inflammatory and immunological factors play a vital role in the pathogenesis of NEC. A large body of data suggests that imbalance between Toll like receptors 4 (TLR) and TLR 9 is responsible for development of the disease. TLR4 activation leads to apoptosis and effects enterocyte proliferation and migration[21]. Some studies have implicated the role of Paneth cells (PCs) in NEC. Cooperation between intestinal stem cells and Paneth cells in a niche localized to the crypts is essential for normal renewal of intestinal surface. Moreover, after the intestine is seriously damaged by infection or other insults, Paneth cell dysfunction leads to NEC [22].
Intestinal macrophages progressively acquire a non-inflammatory profile during gestational development. Transforming Growth Factor β (TGF-β), particularly the TGF-β2 isoform, suppresses macrophage inflammatory responses in the developing intestine and protects against inflammatory mucosal injury. Enterally administered TGF-β2 protects mice from experimental NEC like injury [23]. The infants who developed NEC have low levels of circulating TGF-β on postnatal day one as compared to non-NEC controls [24]. Data suggests there are low levels of TGF-β, and increased level of in the tumor necrosis factor and IL-1beta in surgically resected human NEC tissue. Some studies suggest that an increased IL-8 secretion by fetal enterocytes may trigger an excessive inflammatory response responsible for intestinal injury [25, 26].
Role of Blood Transfusion:
In the recent years, packed red blood cell (PRBC) transfusions have been associated with onset of NEC. Transfusion associated NEC (TANEC) has been reported in multiple studies, although the causality between PRBC transfusion and NEC has not yet been established [27, 28, 29]. Majority of very low birth weight (VLBW) infants require one or more PRBC transfusions during their stay in the neonatal intensive care unit (NICU [30], thus making TANEC, an important entity to consider. Some studies although show contradictory results and indicate that blood transfusions are not a risk factor for NEC [31]
Preterm splanchnic tissue undergoes a large degree of tissue oxygenation variability during RBC transfusions and does not change with increasing maturity. This combined with lower average tissue oxygenation, may explain susceptibility of the preterm gut to transfusion- related acute gut injury (TRAGI) [32]. PRBC transfusion in enterally fed preterm lambs promotes mesenteric vasoconstriction and impairs vasorelaxation by reducing mesenteric arterial endothelial NO synthase (eNOS) [33]. In addition stored PRBC may be less deformable and may not pass well through the gut microcirculation.
Feeding during blood transfusion is controversial and different neonatal units have different practices [34]. Some investigators have suggested that withholding feeds during transfusion could prevent NEC [35], and few believe that the infant remain on small volume feeds during transfusion. Overall, the current body of literature suggests that transfusion may be ‘associated’ with severe form of NEC (stage 3, Surgical NEC) in the extremely premature (less than 28 weeks), and low birth weight infants. In our view, TANEC may represent a different subset of NEC, and more evidence is needed before making any major practice changes.
Role of Intestinal Barrier:
Experimental NEC been has associated with disruption of the intestinal barrier. Epithelial growth factor helps in intestinal integrity at the site of injury by accelerating goblet cell maturation and mucin production and normalizing expression of tight junction proteins [36]. The administration of probiotics (B. bifidum) protects against NEC in the neonatal rat model. This protective effect was associated with reduction of inflammatory reaction in the ileum, regulation of main components of mucus layer, and improvement of intestinal integrity [37, 38]
Intestinal barrier function can be assessed by urinary measurement of Biomarkers such as intestinal fatty acid-binding protein (I-FABP) and fecal measurement of calprotectin [39, 40] and optimising intestinal barrier function may be important in preventing NEC [41].
Altered Microbial Colonization:
‘Microbiota’ can be defined as the microbes associated with a particular context. Unlike traditional microbiological approaches that aim to identify individual pathogens, microbiome analysis characterizes all of the bacterial species present both in terms of their identities and relative abundance[42]. Technological advances have revolutionized the efforts to understand the role played by microbes in disease physiology in recent years. High-throughput sequencing of 16S rRNA gene generated from bacteria-containing samples yields a large number of short sequences that can be subsequently aligned and sorted according to a predefined level of homology. Then, these are classified according to publicly available taxonomic databases [43, 44]. Recent evidence suggests, that alteration in gut microbiome may contribute to intestinal injury and NEC in preterm infants. Hospitalized premature infants intestines are colonized with less diverse bacterial species as compared to healthy breast fed infants which are colonized predominantly with anaerobic species of Bifidobacteria and Lactobacilli [45, 46]. Data suggests that preterm infants are colonized with less diverse bacterial population, more predominantly with Proteobacterial species and less Clostridia species [10, 47, 48]. Nevertheless, a recent study indicates that no consistent pattern in the microbiome composition was seen in infants who developed NEC and strains colonizing each baby were typically distinct [49]. In animal models, investigators have examined the correlation of mucosa-associated microbiome abundance with the severity of intestinal injury, and found that animals developing NEC have higher quantities of bacterial micro colonies along the villi and in the intestinal crypts compared to healthy animals [50]. Another recent study, supports this hypothesis and indicates that depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical NEC [51].
The different etiological factors have been summarized in figure 1.
Figure 1-.
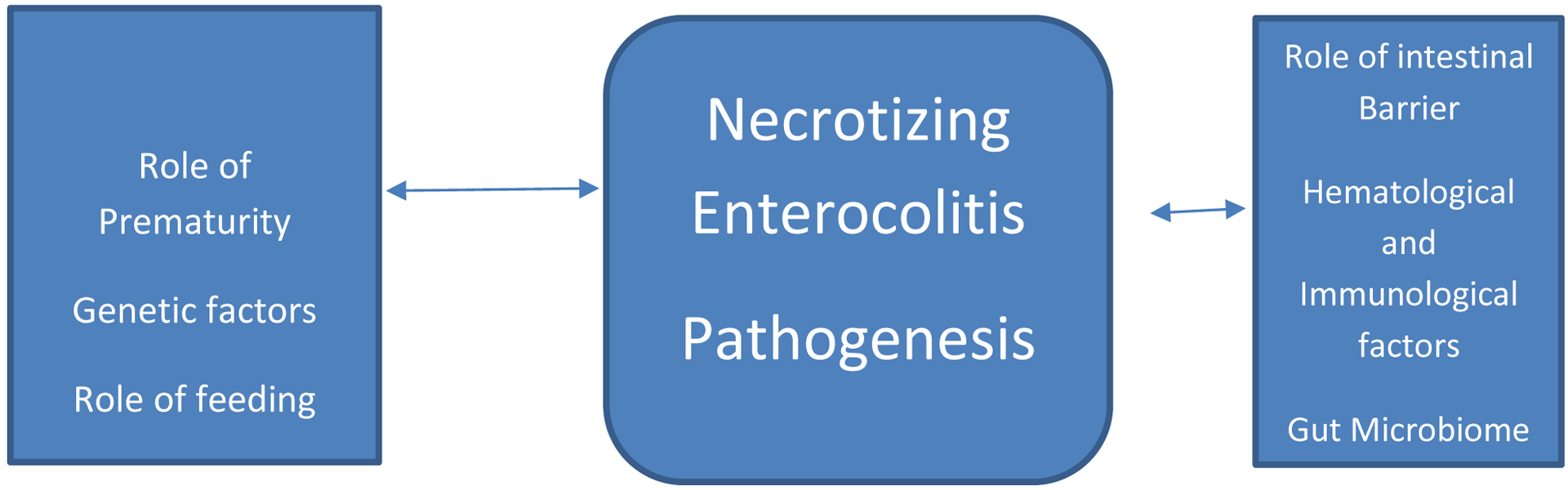
NEC pathogenesis
The study of systems biology of NEC is a fascinating new field of research and includes employing microbiomics, metabolomics, transcriptomics and other ‘OMIC’ strategies. As our understanding about host-microbiome interactions and the development of NEC moves forward, we must be mindful of an extremely complex ecology that houses 10 times more cells and 100 times more genes than the human host. Continued awareness of underlying processes and recognition that associations are not the same as mechanistic explanations, remain essential [52]
Clinical Presentation:
The clinical presentation of NEC may be extremely variable. NEC may present with symptoms of increased gastric residuals, feeding intolerance, bradycardia, tachycardia, apnea , vomiting, abdominal distension, blood in stools, abdominal discoloration, metabolic acidosis and if severe, with the clinical picture of shock and sepsis. The hematological abnormalities seen in NEC are anemia, thrombocytopenia, disseminated intravascular coagulopathy, significant decrease in the number of absolute monocyte count, increased or decreased level of neutrophils, eosinophilia [53]. A recent study has shown that NEC leads to renal dysfunction, metabolic acidosis through disruption of tight junction proteins [54, 55].
Diagnosis:
Clinical and radiological features are primarily employed for the diagnosis of NEC. Bell and colleagues suggested a classification scheme that differentiates suspected NEC (stage 1) from proven (stage 2) and advanced NEC (stage 3). Stage 1 presents with nonspecific symptoms and stage 2 is definitive NEC with radiological evidence of pneumatosis intestinalis and/or portal venous gas. Stage 3 NEC is presents with systemic signs with radiographic evidence of intestinal perforation (pneumoperitoneum) [56]. The Bell’s staging is shown in table 1.
Table 1:
NEC Staging
| Staging | Common Clinical findings | Common Radiological findings | Common Gastrointestinal findings |
|---|---|---|---|
| Stage 1 | Apnea and bradycardia, temperature instability | Normal gas pattern or mild ileus | Gastric residuals, occult blood in stool, mild abdominal distention |
| Stage II | Apnea and bradycardia, temperature instability, Thrombocytopenia and mild metabolic acidosis | Ileus gas pattern with one or more dialated loops and pneumatosis, ascites, portal-venous gas | Grossly bloody stools, prominent abdominal distention, absent bowel sounds, abdominal wall edema with palpable loops and tenderness |
| Stage III | Worsening metabolic acidosis, oliguria, hypotension, coagulopathy, shock, deterioration in laboratory values and vital signs | Prominent bowel loops, worsening ascites, Pneumoperitoneum | Worsening wall edema, erythema and induration, Perforated bowel |
A very close differential of NEC is spontaneous intestinal perforation (SIP) as shown in table 2 which is usually seen first few days of life in extremely preterm infants. In SIP, the inflammatory response is less marked as compared to NEC, and both diseases have completely different pathophysiological mechanisms [57]. Indomethacin and early use of steroids have been shown to increase the incidence of SIP [58, 59].
Table 2:
Difference between NEC and SIP in preterm infants
| NEC | SIP | |
|---|---|---|
| Age | >2 weeks | First 2 weeks |
| Bloody stools | Uncommon | No |
| Ileus | Yes | Variable |
| Pneumoperitoneum | In advanced NEC | Always |
| Pneumatosis Intestinalis | Yes | No |
| Inflammatory response | Yes | Variable/No |
| Prognosis | Variable-Worse if surgical NEC | Relatively better than NEC |
Patients with NEC stage 1 and 2 are usually managed conservatively, while infants with stage 3 NEC may require surgical intervention, which may include laparotomy or a peritoneal drain placement. A multicenter randomized trial comparing outcomes of primary peritoneal drainage with laparotomy in preterm infants with perforated NEC found no significant differences and hence, further larger studies are underway [60]
Recent evidence suggests that, fecal volatile organic compounds (VOCs) analysis by an electronic nose (eNose) may allow for an early detection of NEC [61]. Due to low specificity, immunologic and hematological abnormalities are likely to be less useful as diagnostic tests and may be a “early-warning” systems that indicate a need for clinical monitoring and/or imaging [53, 62]
Prevention:
Breast milk contains multiple factors, which may improve the preterm intestinal host defenses, and early human milk feedings have been shown to be protective against NEC [17, 63]. Donor breast milk has also shown promise in the prevention of NEC compared to formula feeds [64], although larger long term studies are still underway. In addition, standardized feeding protocols may help in decreasing the incidence of NEC [65]. Although probiotics may decrease the incidence of NEC, they have not been shown to change NEC related mortality [66–68]. Most reports support routine administration of probiotics as safe and efficacious in the preterm population, however the long-term effects such as neurodevelopmental outcomes and growth patterns, are yet to be investigated. The FDA has not yet approved the routine use of probiotics in preterm infant and further trials are needed before their routine usage. Some data suggests that supplementing feeds with prebiotics such as oligosaccharides, which increases the growth of beneficial intestinal flora may be beneficial [69, 70]. Very few studies have examined the effects of synbiotics (combination of prebiotic and probiotic preparations) in the preterm population. Although the use a symbiotic combination (Lactobacilli + Fructooligosaccharides + Bifidobacteria) resulted in an increased stool content of Bifidobacteria in preterm infants, no differences in stool short chain fatty acids content, weight gain, or incidence of NEC were observed when compared to placebo [71].
Liberal use of antibiotics in premature infants is associated with an increased incidence of NEC [72]. With the mounting evidence regarding the role of the gut microbiome dysbiosis in NEC pathogenesis, excessive use of antibiotics and H2 blocker in preterm infants, should be discouraged. Antibiotic stewardship in preterm infants is the need of the hour.
Future Directions:
The understanding of cellular processes in the pathogenesis of NEC is still in its nascent phases. Hence, more translational and basic science studies are required to further enhance our understanding about innate immune, barrier function and inflammatory response in the premature gut. Sensitive and specific biomarkers using newer systems biology approaches need to be developed, for the prediction, early diagnosis and progression of NEC. Moreover, well-designed prospective clinical trials with long term outcome data are needed for the prevention and treatment of this devastating disease.
Table 4:
NEC: Summary of risk factors and prevention stratergies
| Risk Factors for Necrotizing Enterocolitis : |
| Prematurity[8–14] |
| Role of inflammation/immunological fcators [21–26] |
| Role of feeding[15–20] |
| Role of intestinal barrier[36–41] |
| Role of hematological factors[27–35] |
| Gut microbiome [42–51] |
| Possible prevention stratergies : |
| Breast milk [17,63] |
| Donor breast milk [64] |
| Standardized feeding protocol[65] |
| Probiotics[66–68] |
| Prebiotics[69,70] |
| Synbiotics[71] |
| Minimal use of antibiotics[72] |
Funding:
The article is supported by NIH funding K08HL141652 awarded to CVL.
References
- 1.Grave GD, et al. , New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res, 2007. 62(4): p. 510–4. [DOI] [PubMed] [Google Scholar]
- 2.Holman RC, et al. , Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol, 2006. 20(6): p. 498–506. [DOI] [PubMed] [Google Scholar]
- 3.Horbar JD, et al. , Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics, 2002. 110(1 Pt 1): p. 143–51. [DOI] [PubMed] [Google Scholar]
- 4.Hintz SR, et al. , Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics, 2005. 115(3): p. 696–703. [DOI] [PubMed] [Google Scholar]
- 5.Salhab WA, et al. , Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants <1000 g. J Perinatol, 2004. 24(9): p. 534–40. [DOI] [PubMed] [Google Scholar]
- 6.Patel RM, et al. , Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med, 2015. 372(4): p. 331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer AU, et al. , Pediatric short–bowel syndrome: the cost of comprehensive care. Am J Clin Nutr, 2008. 88(6): p. 1552–9. [DOI] [PubMed] [Google Scholar]
- 8.Guillet R, et al. , Association of H2–blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics, 2006. 117(2): p. e137–42. [DOI] [PubMed] [Google Scholar]
- 9.Walker WA, Role of nutrients and bacterial colonization in the development of intestinal host defense. J Pediatr Gastroenterol Nutr, 2000. 30 Suppl 2: p. S2–7. [PubMed] [Google Scholar]
- 10.Wang Y, et al. , 16S rRNA gene–based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J, 2009. 3(8): p. 944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowicki PT, Effects of sustained flow reduction on postnatal intestinal circulation. Am J Physiol, 1998. 275(4 Pt 1): p. G758–68. [DOI] [PubMed] [Google Scholar]
- 12.Berseth CL, Gut motility and the pathogenesis of necrotizing enterocolitis. Clin Perinatol, 1994. 21(2): p. 263–70. [PubMed] [Google Scholar]
- 13.Dvorak B, et al. , Comparison of epidermal growth factor and heparin–binding epidermal growth factor–like growth factor for prevention of experimental necrotizing enterocolitis. J Pediatr Gastroenterol Nutr, 2008. 47(1): p. 11–8. [DOI] [PubMed] [Google Scholar]
- 14.Nanthakumar N, et al. , The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One, 2011. 6(3): p. e17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niinikoski H, et al. , Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN–fed neonatal piglets. J Nutr, 2004. 134(6): p. 1467–74. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenkranz RA, et al. , Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res, 2011. 69(6): p. 522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisk PM, et al. , Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol, 2007. 27(7): p. 428–33. [DOI] [PubMed] [Google Scholar]
- 18.Cristofalo EA, et al. , Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr, 2013. 163(6): p. 1592–1595.e1. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan S, et al. , An exclusively human milk–based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk–based products. J Pediatr, 2010. 156(4): p. 562–7.e1. [DOI] [PubMed] [Google Scholar]
- 20.Quigley M and McGuire W, Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev, 2014. 4: p. Cd002971. [DOI] [PubMed] [Google Scholar]
- 21.Fusunyan RD, et al. , Evidence for an innate immune response in the immature human intestine: toll–like receptors on fetal enterocytes. Pediatr Res, 2001. 49(4): p. 589–93. [DOI] [PubMed] [Google Scholar]
- 22.McElroy SJ, Underwood MA, and Sherman MP, Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology, 2013. 103(1): p. 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maheshwari A, et al. , TGF–beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology, 2011. 140(1): p. 242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maheshwari A, et al. , Cytokines associated with necrotizing enterocolitis in extremely–low–birth–weight infants. Pediatr Res, 2014. 76(1): p. 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanthakumar NN, et al. , Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A, 2000. 97(11): p. 6043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin CR and Walker WA, Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med, 2006. 11(5): p. 369–77. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed A and Shah PS, Transfusion associated necrotizing enterocolitis: a meta–analysis of observational data. Pediatrics, 2012. 129(3): p. 529–40. [DOI] [PubMed] [Google Scholar]
- 28.Paul DA, et al. , Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics, 2011. 127(4): p. 635–41. [DOI] [PubMed] [Google Scholar]
- 29.Christensen RD, et al. , Is “transfusion–associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion, 2010. 50(5): p. 1106–12. [DOI] [PubMed] [Google Scholar]
- 30.Strauss RG, Practical issues in neonatal transfusion practice. Am J Clin Pathol, 1997. 107(4 Suppl 1): p. S57–63. [PubMed] [Google Scholar]
- 31.Sharma R, et al. , Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J Perinatol, 2014. 34(11): p. 858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey SM, Hendricks-Munoz KD–, and Mally PV, Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion–related acute gut injury . Blood Transfus, 2015: p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair J, et al. , Packed red cell transfusions alter mesenteric arterial reactivity and nitric oxide pathway in preterm lambs. Pediatr Res, 2013. 74(6): p. 652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parige R, et al. , Enteral feeding during packed red blood cell transfusion in English neonatal units. Arch Dis Child Fetal Neonatal Ed, 2014. 99(2): p. F173. [DOI] [PubMed] [Google Scholar]
- 35.El–Dib M, et al. , Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol, 2011. 31(3): p. 183–7. [DOI] [PubMed] [Google Scholar]
- 36.Clark JA, et al. , Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol, 2006. 291(5): p. G938–49. [DOI] [PubMed] [Google Scholar]
- 37.Khailova L, et al. , Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol, 2009. 297(5): p. G940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergmann KR, et al. , Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol, 2013. 182(5): p. 1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reisinger KW, et al. , Noninvasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid–binding protein in necrotizing enterocolitis. J Pediatr Surg, 2012. 47(9): p. 1640–5. [DOI] [PubMed] [Google Scholar]
- 40.Evennett NJ, et al. , Urinary intestinal fatty acid–binding protein concentration predicts extent of disease in necrotizing enterocolitis. J Pediatr Surg, 2010. 45(4): p. 735–40. [DOI] [PubMed] [Google Scholar]
- 41.Halpern MD and Denning PW, The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers, 2015. 3(1–2): p. e1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers GB, et al. , Respiratory microbiota: addressing clinical questions, informing clinical practice. Thorax, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, et al. , Accurate taxonomy assignments from 16S rRNA sequences produced by highly parallel pyrosequencers. Nucleic Acids Res, 2008. 36(18): p. e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schloss PD, et al. , Introducing mothur: open–source, platform–independent, community–supported software for describing and comparing microbial communities. Appl Environ Microbiol, 2009. 75(23): p. 7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gewolb IH, et al. , Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed, 1999. 80(3): p. F167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacquot A, et al. , Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr, 2011. 158(3): p. 390–6. [DOI] [PubMed] [Google Scholar]
- 47.Mai V, et al. , Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One, 2011. 6(6): p. e20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMurtry VE, et al. , Bacterial diversity and Clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome, 2015. 3: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raveh–Sadka T, et al. , Gut bacteria are rarely shared by co–hospitalized premature infants, regardless of necrotizing enterocolitis development. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoy AC, et al. , Necrotizing Enterocolitis in Preterm Pigs Is Associated with Increased Density of Intestinal Mucosa–Associated Bacteria Including Clostridium perfringens. Neonatology, 2015. 108(3): p. 188–95. [DOI] [PubMed] [Google Scholar]
- 51.Remon JI, et al. , Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J Perinatol, 2015. 35(9): p. 755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McElroy SJ, The Role of Bacteria in Necrotizing Enterocolitis: Understanding the Forest for the Trees. Neonatology, 2015. 108(3): p. 196–7. [DOI] [PubMed] [Google Scholar]
- 53.Maheshwari A, Immunologic and Hematological Abnormalities in Necrotizing Enterocolitis. Clin Perinatol, 2015. 42(3): p. 567–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garg PM, et al. , Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatric research, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg PM, et al. , Necrotizing enterocolitis leads to renal abnormality mediated through tight junction proteins (60.8). The FASEB Journal, 2014. 28(1 Supplement): p. 60.8. [Google Scholar]
- 56.Bell MJ, et al. , Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg, 1978. 187(1): p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan KY, et al. , Genome–wide expression profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in human intestinal tissues: dysregulation of functional pathways. Ann Surg, 2014. 260(6): p. 1128–37. [DOI] [PubMed] [Google Scholar]
- 58.Stark AR, et al. , Adverse effects of early dexamethasone in extremely–low–birth–weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med, 2001. 344(2): p. 95–101. [DOI] [PubMed] [Google Scholar]
- 59.Attridge JT, et al. , New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol, 2006. 26(2): p. 93–9. [DOI] [PubMed] [Google Scholar]
- 60.Moss RL, et al. , Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med, 2006. 354(21): p. 2225–34. [DOI] [PubMed] [Google Scholar]
- 61.de Meij TG, et al. , Early Detection of Necrotizing Enterocolitis by Fecal Volatile Organic Compounds Analysis. J Pediatr, 2015. 167(3): p. 562–567.e1. [DOI] [PubMed] [Google Scholar]
- 62.Asmerom M, Crowe L, and Marin T, Understanding the Biologic Therapies of Probiotics, Prebiotics, and Synbiotics: Exploring Current Evidence for Use in Premature Infants for the Prevention of Necrotizing Enterocolitis. J Perinat Neonatal Nurs, 2015. 29(3): p. 240–7. [DOI] [PubMed] [Google Scholar]
- 63.Berseth CL, Bisquera JA, and Paje VU, Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics, 2003. 111(3): p. 529–34. [DOI] [PubMed] [Google Scholar]
- 64.Quigley MA, et al. , Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev, 2007(4): p. Cd002971. [DOI] [PubMed] [Google Scholar]
- 65.Kamitsuka MD, Horton MK, and Williams MA, The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics, 2000. 105(2): p. 379–84. [DOI] [PubMed] [Google Scholar]
- 66.Lin HC, et al. , Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics, 2005. 115(1): p. 1–4. [DOI] [PubMed] [Google Scholar]
- 67.Bin–Nun A, et al. , Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr, 2005. 147(2): p. 192–6. [DOI] [PubMed] [Google Scholar]
- 68.Lin HC, et al. , Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics, 2008. 122(4): p. 693–700. [DOI] [PubMed] [Google Scholar]
- 69.Gibson GR and McCartney AL, Modification of the gut flora by dietary means. Biochem Soc Trans, 1998. 26(2): p. 222–8. [DOI] [PubMed] [Google Scholar]
- 70.Sherman PM, et al. , Potential roles and clinical utility of prebiotics in newborns, infants, and children: proceedings from a global prebiotic summit meeting, New York City, June 27–28, 2008. J Pediatr, 2009. 155(5): p. S61–70. [DOI] [PubMed] [Google Scholar]
- 71.Underwood MA, et al. , A randomized placebo–controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short–chain fatty acids. J Pediatr Gastroenterol Nutr, 2009. 48(2): p. 216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cotten CM, et al. , Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics, 2009. 123(1): p. 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


