Abstract
Pregnant women are often complicated with diseases that require treatment with medication. Most drugs administered to pregnant women are off-label without the necessary dose, efficacy, and safety information. Knowledge concerning drug transfer across the placental barrier is essential for understanding fetal drug exposure and hence drug safety and efficacy to the fetus. Transporters expressed in the placenta, including adenosine triphosphate (ATP)-binding cassette efflux transporters and solute carrier uptake transporters, play important roles in determining drug transfer across the placental barrier, leading to fetal exposure to the drugs. In this review, we provide an update on placental drug transport, including in vitro cell/tissue, ex vivo human placenta perfusion, and in vivo animal studies that can be used to determine the expression and function of drug transporters in the placenta as well as placental drug transfer and fetal drug exposure. We also describe how the knowledge of placental drug transfer through passive diffusion or active transport can be combined with physiologically based pharmacokinetic modeling and simulation to predict systemic fetal drug exposure. Finally, we highlight knowledge gaps in studying placental drug transport and predicting fetal drug exposure and discuss future research directions to fill these gaps.
Keywords: adenosine triphosphate-binding cassette transporters, fetal drug exposure, physiologically based pharmacokinetic modeling and simulation, placental drug transport, solute carrier transporters
Introduction
Statistics indicate that approximately 80% of pregnant women take at least one medication throughout their pregnancy for treatment of a variety of complications, such as viral infection (e.g., human immunodeficiency virus [HIV]), smoking cessation, or pregnancy-induced conditions like hypertension, depression, preeclampsia, nausea, and gestational diabetes [1], [2], [3]. Some drugs are prescribed to pregnant women to treat both the mother and fetus (e.g., antiretroviral drugs to treat HIV-positive mother and prevent perinatal transmission of HIV [4]) or just the fetus (e.g., digoxin and amiodarone to treat fetal tachyarrhythmia [5]). When pregnant women receive drugs, their fetuses are also exposed to the drugs. However, most drugs are administered to pregnant women off-label without information about the dose, maternal-fetal pharmacokinetics, safety, or efficacy of the drugs [6]. A major concern of drug use by pregnant women is the transfer of drugs across the placental barrier, leading to fetal drug exposure and potential toxicity to the developing fetus. Indeed, 5%–10% of pregnant women receive drugs which are potentially teratogenic [1], and 50% of drugs are used in the first trimester when the fetus is most vulnerable to developmental toxicity [3]. While most drugs can cross the placenta to a certain extent by passive diffusion, the placenta is richly endowed with a variety of adenosine triphosphate (ATP)-binding cassette (ABC) efflux transporters and solute carrier (SLC) uptake transporters [7, 8]. Drug transporters in the placenta play crucial roles in fetal drug exposure. The localization and activity of transporters as well as changes of their expression in the placenta over gestation have a profound impact on fetal drug exposure and hence efficacy or toxicity of the drugs to the fetus. In this review, first, we describe human placenta physiology and transporter expression in the placenta. Second, we provide an overview of in vitro cell/tissue, ex vivo human placenta perfusion, and in vivo systems (animal studies) that can be used to determine the expression and activity of drug transporters in the placenta and transplacental drug transport. Third, we describe how the data obtained from placental transport studies can be combined with physiologically based pharmacokinetic (PBPK) modeling and simulation (M&S) to predict systemic fetal exposure to the drugs that are actively transported across the placenta. Finally, we highlight knowledge gaps to study placental drug transport and predict fetal drug exposure, and discuss future research directions to fill these gaps.
Human placenta physiology
The human placenta connects the fetal compartment and maternal circulation during pregnancy and forms a semipermeable barrier to allow waste elimination, and the exchange of nutrients, gases, endogenous metabolites, and exogenous substances including drugs and metabolites between the mother and fetus, which is essential for fetal growth and development. The human placenta consists of singular epithelia known as the syncytiotrophoblasts arising from the fusion of cytotrophoblasts. This syncytiotrophoblast cell layer separates the maternal and fetal circulations [9]. The apical membrane of the syncytiotrophoblast is in direct contact with the maternal blood and its basal membrane is in contact with the fetal circulation through umbilical vessels into the placental villi [10]. In early pregnancy, the main function of placenta is to mediate the implantation of embryo into the uterus and produce hormones that maintain normal pregnancy [11]. After implantation, the placenta is primarily responsible for the exchange of substances between the maternal and fetal circulations. The syncytiotrophoblast forms a single multinucleated cell layer. Thus, there is no paracellular diffusion of drugs between the syncytiotrophoblasts, and placental drug transfer across the syncytiptrophoblast cell layer occurs only by passive diffusion and/or transporter-mediated transport. In rodents, the placenta consists of a maternal-facing syncytiotrophoblast layer I and a fetal-facing syncytiotrophoblast layer II that are connected by connexin26 gap junctions with efflux transporters (e.g., P-glycoprotein or ABCB1) localized on the apical membrane of the syncytiotrophoblast layer II [12]. More details about species differences in placental physiology between humans and animal models have been reviewed elsewhere [13].
Most drugs can cross the placenta to some extent. Lipophilic, non-ionized small molecule drugs (e.g., < 500 Da) can cross the placenta by passive diffusion. The rate of passive diffusion depends on the concentration gradient across the placental barrier, surface area, and thickness of the placenta, as well as molecular weight and lipophilicity of the drug molecules. The maternal and fetal plasma protein binding, ionization of drug molecules in maternal and fetal blood, and maternal blood flow rate to the placenta can also affect the passive diffusion of drug molecules across the placental barrier [13, 14]. On the other hand, transplacental transfer of hydrophilic drugs with low membrane permeability requires facilitated or active transport mediated by transporter proteins expressed in the placenta (see below). The rate of transporter-mediated drug transfer across the placental barrier depends on the intrinsic activity of transporters for the drug molecules and the abundance of the transporters in the placenta.
Expression, localization, and activity of major placental drug transporters
There are two superfamilies of drug transporters expressed in human placenta, ABC and SLC transporters. Depending on their membrane localization and transport directionality in the syncytiotrophoblast, they transport drugs from the fetal compartment to the maternal circulation or in the opposite direction. For example, P-glycoprotein (P-gp or ABCB1), an efflux transporter on the apical membrane of the syncytiotrophoblast (Figure 1), can actively transport drugs from the fetal compartment to the maternal blood, thus protecting the fetus. Uptake transporters on the apical membrane of the syncytiotrophoblast transport drugs in the opposite direction and may increase fetal drug exposure (Figure 1). Here we briefly describe the major ABC efflux and SLC uptake drug transporters that are expressed in human placenta. More extensive reviews on efflux and/or uptake transporters in the placenta have been published elsewhere [7, 13, 15], [16], [17], [18], [19].
Figure 1:
Localization and direction of transport of major transporters in the syncytiotrophoblast of human placenta. Transporters that have been quantified in protein abundance are shown in green. Transporters that are not quantifiable or have not been quantified in protein abundance are shown in gray. P-gp, P-glycoprotein/ABCB1; BCRP, breast cancer resistance protein/ABCG2; SERT, serotonin transporter/SLC6A4; NET, norepinephrine transporter/SLC6A2; OAT4, organic anion transporter 4/SLC22A11; OATP2B1, organic anion transporting polypeptide 2B1/SLCO2B1; OATP2A1, organic anion transporting polypeptide 2A1/SLCO2A1; OATP1B1, organic anion transporting polypeptide 1B1/SLCO1B1; OATP1B3, organic anion transporting polypeptide 1B3/SLCO1B3; OCT3, organic cation transporter 3/SLC22A3; OCTN1, organic cation transporter novel type 1/SLC22A4; OCTN2, organic cation transporter novel type 2/SLC22A5; MATE1, multidrug and toxin extrusion 1/SLC47A1; MRP1, multidrug resistance protein 1/ABCC1; MRP2, multidrug resistance protein 2/ABCC2; MRP3, multidrug resistance protein 3/ABCC3; MRP4, multidrug resistance protein 4/ABCC4; MRP5, multidrug resistance protein 5/ABCC5; MCT4, monocarboxylate transporter 4/SLC16A3; ENT2; equilibrative nucleoside transporter 2/SLC29A2.
Human P-gp is an ABC efflux transporter highly expressed on the apical membrane of the syncytiotrophoblast (Figure 1) [20]. P-gp mediates active efflux of drugs from the placenta to the maternal circulation, thus reducing fetal exposure to drugs. P-gp has a very broad substrate specificity, and most of its substrates are cationic or amphipathic compounds and are mainly lipophilic [21]. Many drugs administered to pregnant women, such as HIV protease inhibitors (i.e., indinavir, lopinavir) and digoxin, are P-gp substrates [21]. Like P-gp, human breast cancer resistance protein (BCRP or ABCG2) is also an ABC efflux transporter highly expressed on the apical membrane of the syncytiotrophoblast (Figure 1) [22]. BCRP plays a similar role as P-gp in reducing fetal exposure to drugs and xenobiotics. The substrate specificity of BCRP is even broader than that of P-gp, with substrates ranging from hydrophobic anthracyclines (i.e., daunorubicin, doxorubicin) to hydrophilic camptothecins (i.e., topotecan, SN-38), organic anions, and sulfate and glucuronide conjugates of endogenous and exogenous substances [23]. Drugs that may be administered to pregnant women and are BCRP substrates include glyburide used to treat gestational diabetes and nitrofurantoin used to treat urinary tract infections [24, 25]. BCRP and P-gp have a substantial overlap in substrate specificity. P-gp and BCRP are the two most extensively studied placental transporters, and both have been convincingly shown to play a significant role in limiting fetal exposure to drugs and xenobiotics by effluxing these compounds from the fetal compartment back to the maternal circulation [7, 13, 15], [16], [17], [18], [19]. For example, transport studies using plasma microvillus membrane vesicles isolated from human placenta tissues demonstrated P-gp-mediated transport of N-methyl quinidine, a model substrate of P-gp [26], indicating the presence of P-gp in human placenta. Using the Mdr1a/Mdr1b (which are murine homologs of the human ABCB1 gene) knockout mice, Smit et al. showed that the fetal-to-maternal plasma concentration ratios of digoxin, saquinavir, and paclitaxel in the Mdr1a/1b knockout fetuses at a given time point after intravenous administration of these drugs to the dams were 2.2–2.4-fold, 5–7-fold, and 16-fold higher, respectively, compared to wild-type fetuses [27]. Furthermore, the P-gp activity in the placenta was completely abrogated by oral administration of the P-gp inhibitor PSC833 or GF120918 to the dams [27]. Ex vivo placental perfusion studies allowed direct measurement of P-gp-mediated drug transfer across the placenta. For example, with dually perfused human placenta, Molsa et al. showed that the fetal-to-maternal transfer of saquinavir was 108-fold higher than the maternal-to-fetal transfer, and pre-perfusion with the P-gp inhibitors PSC833 or GG918 increased the transplacental transfer of saquinavir from the mother to the fetus 7.9-fold or 6.2-fold, respectively [28]. BCRP has also been shown to limit fetal drug exposure. For instance, we have demonstrated that fetal exposure (the fetal-to-maternal plasma AUC ratios) to the BCRP substrates glyburide and nitrofurantoin in Abcg2 knockout mice is about 2-fold and about 5-fold greater, respectively, than that in wild-type mice [24, 25]. Using ex vivo dual human placenta perfusion, Pollex et al. illustrated that the fetal-to-maternal concentration ratios of glyburide in the presence of nicardipine (a BCRP inhibitor) at all time points were greater than those in the absence of nicardipine, suggesting that BCRP actively transports glyburide in the fetal-to-maternal direction and this transport activity can be inhibited by nicardipine [29].
SLC transporters in the placenta are much less studied compared to ABC transporters. Organic cation transporter 3 (OCT3 or SLC22A3) is a Na+/Cl−-independent, but electrogenic transporter for a variety of organic anions, including 1-methyl-4-phenylpyridinium (MPP+), clonidine, cimetidine, metformin, and tetraethylammonium [30]. OCT3 is expressed on the basal membrane of the syncytiotrophoblast that faces the fetal compartment (Figure 1) [31]. Karahoda et al. performed rat term placenta perfusion and found that OCT3 mediated massive uptake of serotonin from the fetal circulation [32]. A recent study showed that after oral administration of metformin, while the systemic maternal plasma exposure (area under the concentration-time curve, AUC) of metformin was slightly decreased by about 16% in the Oct3 knockout mice, the fetal AUC was reduced by about 47% in Oct3 knockout pregnant mice, compared to wild-type mice [33], suggesting that OCT3 transports metformin from the syncytiotrophoblast to the fetus. Thus, OCT3 is a bidirectional transporter and can facilitate the maternal-to-fetal transfer of metformin in vivo. Organic anion transporter 4 (OAT4 or SLC22A11) is abundantly expressed on the basal membrane of the syncytiotrophoblast (Figure 1) [34, 35]. Typical substrates of OAT4 include sulfate conjugates of steroid hormones, prostaglandins, and a variety of pharmacological agents such as angiotensin II receptor antagonists and leukotriene receptor antagonists [34]. Mechanistically, OAT4 is an organic anion/dicarboxylate exchanger [36]. OAT4 has been shown to transport the fetal-derived estriol precursor 16α-hydroxydehydroepiandrosterone sulfate (16α-OH DHEAS) into the basal plasma membrane vesicles isolated from human placenta [37]. OAT4 also mediates uptake of drugs such as olmesartan into the basal human placental plasma membrane vesicles [38]. Organic anion transporting polypeptide 2B1 (OATP2B1 or SLCO2B1) is also expressed on the basal membrane of the syncytiotrophoblast (Figure 1) [39]. OATP2B1 transports organic anions, sulfated steroid hormones, thyroid hormones, bile salts, and prostaglandins [40]. Its function in the placenta has not been well characterized. It may work in tandem with BCRP on the apical membrane of the syncytiotrophoblast to mediate the fetal-to-maternal transfer of sulfated steroid hormones [41]. The serotonin transporter SERT (SLC6A4) and the norepinephrine transporter NET (SLC6A2) are monoamine transporters and highly expressed on the apical membrane of the syncytiotrophoblast (Figure 1) [42, 43]. While SERT and NET may facilitate the maternal-to-fetal transfer of monoamines including serotonin to support serotonergic neurons in the developing fetal brain [44], their roles in transport of drugs across the placenta are largely unknown [7].
The aforementioned transporters are highly expressed in human placenta and their protein abundance in human placenta has been quantified by LC/MS-based targeted proteomics [45]. It has been shown that the protein abundance of some of the transporters is gestational age dependent [45]. Specifically, from the first trimester to term, the abundance of P-gp and BCRP decreases by 55% and 69%, whereas that of OCT3 and OAT4 increases by 2-fold. The abundance of OATP2B1 decreases by 32% from the first trimester to the second trimester, while no significant change was observed from the second trimester to term. The abundance of NET, and SERT does not change over gestation.
Other transporters, including MRPs 1–5, OATP1B1, OATP1B3, OCTN1, OCTN2, and the multidrug and toxin extrusion 1 (MATE1), can be detected in human placenta at the mRNA levels, but their protein abundance is below the quantifiable levels (see Figure 1) [45]. OATP2A1 protein was recently detected on the apical membrane of human placenta by immunoblotting [46]. Since the function or activity of these transporters in human placenta generally has not been well characterized, these transporters are not further discussed here.
In vitro, ex vivo, and in vivo systems to study placental drug transport
In vitro systems used to study placental drug transport include cell-based models as well as microvillus and basal plasma membrane vesicles isolated from placenta tissues [47]. While the malignant trophoblast cell lines such as BeWo, JAR, and JEG-3 are widely used to study the activity and regulation of placental transporters, these cell lines do not represent the placental syncytiotrophoblast in vivo with respect to the expression profile of placental transporters. For example, BeWo expresses BCRP at high levels and therefore is an excellent cell model to study the function of BCRP and its regulation by pregnancy hormones and xenobiotics [48, 49], whereas P-gp is absent or minimally expressed in BeWo and JEG-3 cell lines [50]. Primary cytotrophoblasts can be isolated from early or term human placenta tissues and cultured for subsequent transport studies [51, 52]. It is worth noting that the expression profiles of transporters in primary cytotrophoblasts may be altered during cell culture due to cytotrophoblast differentiation that can affect transporter gene expression [53, 54]. Recently, trophoblast organoids have been developed using the first trimester human placenta tissue [55]. Trophoblast organoids organize into villous-like structures, secret placental-specific hormones, and differentiate into extravillous trophoblasts which can invade in three-dimensional culture. Thus, this organoid model will be highly valuable for studying human placental development. Whether this organoid model has the same or similar transporter expression profiles as in vivo and can be used to study placental drug transport remains to be investigated. Besides cell models, plasma membrane vesicles can be isolated from the microvillus (apical) and basal plasma membrane fractions of human placental villous tissues [56] and are of high value for direct measurement of transport kinetics of hydrophilic drugs into the vesicles by placental transporters [46, 57].
Ex vivo methods are also widely used to study placental drug transport. Human placental villous tissue fragments or explants can be isolated from placenta villous tissue, cultured, and then used for transport studies [58] as well as regulation of placental transporters by physiological factors (i.e. oxygen tension) and drugs or xenobiotics [59], [60], [61]. In addition, ex vivo placental perfusion can also provide valuable information on transplacental drug transfer clearance [47]. The ex vivo placental perfusion technique is highly useful to study the mechanisms of placental drug transfer and predict the rate of drug transfer across the placenta in vivo [62, 63]. Term human placenta is most frequently used. There are two main perfusion systems, that is, a closed system with recirculation of maternal and fetal perfusates and an open system without recirculation [64]. In the closed system, the drug is mainly added in the maternal reservoir and perfused until the steady state is reached, and then drug concentrations in the maternal and fetal circulations are measured. The fetal-to-maternal drug concentration ratio (ex vivo F/M drug concentration ratio) can be compared with the umbilical vein to the maternal plasma drug concentration ratio (in vivo UV/MP ratio) which is usually determined at a certain time point after delivery in a clinical setting. We want to point out that the UV/MP ratio does not necessarily represent the true fetal drug exposure if the pharmacokinetics of drugs in maternal and fetal circulations does not reach steady state and does not change in parallel at the time of sampling [14]. In an open system, the drug is dually perfused from the maternal effluent container for the maternal-to-fetal perfusion or from the fetal effluent container for the fetal-to-maternal perfusion with separate cotyledons from the same placenta. Since steady state may not be reached, the maternal-to-fetal (CLmf) and the fetal-to-maternal (CLfm) clearances must be calculated [65, 66]. The CLmf/CLfm ratio is equivalent to the F/M ratio in a closed system and compared with the in vivo UV/MP ratio. The CLmf and CLfm clearance values can be combined with PBPK M&S to predict systemic fetal drug exposure (see below). In the open system, the drug is perfused continuously without reservoir. Therefore, the open system would be more rapid to reach steady state than the closed system with reservoir. In addition, reservoir volume in a closed system also affects the time to reach steady state. Thus, an open system is generally better to predict fetal drug exposure in vivo, especially for drugs with poor membrane permeability. However, CLmf and CLfm in the open system must be calculated from separate perfusion experiments, while the F/M concentration ratio at steady state can be obtained from a single perfusion experiment in the closed system. More details about the closed and open systems can be found elsewhere [64].
In vivo systems such as chronically catheterized pregnant macaque, baboon or sheep have been used to study placental drug transfer [16]. For example, the placental and non-placental clearances of drugs can be determined using an in vivo catheterized sheep model, and this model is relevant to humans [67]. A typical experiment involves intravenous infusion of the drug to the mother and her fetus separately to steady state, and then the maternal and fetal blood concentrations are measured [68]. The fetal-to-maternal blood concentration ratios of drugs can be calculated to represent fetal drug exposure. More recently, transporter knockout mice have been used to investigate the roles of specific placental transporters in determining fetal drug exposure [24, 25, 27]. Typically, maternal plasma and fetuses are collected after administration of drugs to pregnant wild-type and transporter knockout mice. Then, drug concentrations in maternal plasma and fetus are determined, and the fetal-to-maternal plasma drug concentration ratios or area under the drug concentration-time curve (AUC) ratios are calculated and compared between wild-type and transporter knockout mice. With such in vivo animal models, Smit et al. showed that the fetal-to-maternal plasma concentration ratios of the P-gp substrate drugs digoxin, saquinavir, and paclitaxel in the P-gp knockout fetuses are significantly increased several-fold as compared to those in wild-type fetuses, suggesting a protective role of P-gp for the fetus [27]. Likewise, we have demonstrated that the fetal-to-maternal plasma AUC ratios of the Bcrp substrates nitrofurantoin and glyburide in Bcrp knockout mice are 5 and 2 times greater, respectively, than those in wild-type mice [24, 25]. Caution should be taken when using transporter knockout mice. We have shown that P-gp does not play a role in limiting fetal exposure to norbuprenorphine which is an excellent P-gp substrate [69]. A recent study suggests that this is likely due to the presence of connexin26 gap junctions that connect the syncytiotrophoblast cell layers I and II in mouse placenta. Small molecules such as norbuprenorphine and digoxin may pass the channels and hence bypass the efflux by P-gp [70]. Therefore, caution should be taken when extrapolating rodent data to humans. Positron emission tomography (PET) imaging has been used to examine the role of P-gp in determining fetal drug exposure in pregnant nonhuman primates [71]. PET imaging is non-invasive and can quantify drug distribution in the whole body over time, and hence tissue distribution of drugs in both the mother and fetus can be evaluated simultaneously with high sensitivity. However, technical challenges such as the fundamental trade-offs between resolution and noise and the quantitative accuracy of the measurements along with the expensive data-acquisition system of PET imaging are hurdles to massive application of this technology [72].
Factors that determine fetal drug exposure
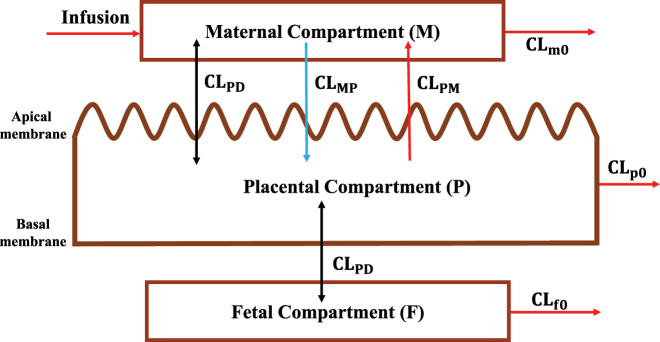
Placental drug transport is one of the most important factors that determine fetal drug exposure. Other factors that may also influence fetal drug exposure include irreversible clearances of the drug by the placenta and fetus, such as placental metabolism and fetal hepatic clearance (e.g., fetal liver metabolism). Zhang et al. [14] systematically analyzed all potential factors that determine fetal drug exposure. Here we provide a brief overview of these factors. Fetal drug exposure can be defined as the ratio (Kp,uu) of the steady-state unbound plasma concentration (or AUC) in the fetal circulation to the corresponding maternal unbound plasma concentration (or AUC). Kp,uu is independent of drug dose or maternal plasma concentration as long as the placental and fetal drug disposition follows linear kinetics. Zhang et al. [14] derived the relationship Equation (1) of Kp,uu with factors that determine fetal drug exposure according to a three-compartment model with the maternal (M), placental (P) and fetal (F) compartments (Figure 2).
| (1) |
Where CLPD is unbound placental passive diffusion clearance; CLPM is unbound active placental efflux in the placental-to-maternal direction; CLMP is the unbound active placenta influx in the maternal-to-placental direction; CLp0 is unbound placental metabolic clearance; and CLf0 is unbound fetal clearance (e.g., fetal hepatic metabolism).
Figure 2:
A maternal-fetal three-compartment model. M, P, and F represent the maternal, placental, and fetal compartments, respectively. Drug is infused at a constant rate to the maternal compartment. CLPD represents passive diffusion clearance. CLPM and CLMP are unidirectional transporter-mediated placental-to-maternal and maternal-to-placenta clearances, respectively. CLm0, CLp0, and CLf0 are irreversible elimination clearances from the maternal, placental, and fetal compartments, respectively.
To illustrate the role of placental efflux transporters conceptually more clearly, Equation (1) can be simplified. The placenta does not highly express enzymes (e.g., cytochrome P450 enzymes or CYPs) that can extensively metabolize drugs [73]. To date, few studies have shown that drugs are transported from the maternal circulation into the placenta by influx transporters. Thus, CLMP and CLp0 can be assumed to be zero. In contrast, the placenta highly expresses efflux transporters such as P-gp and BCRP on the apical membrane of the syncytiotrophoblast, limiting fetal drug exposure (see above). While the fetal liver expresses some enzymes (primarily CYP3A7 [74]), due to its small size and limited expression of these enzymes, the fetal liver usually is not considered a significant contributor to fetal drug exposure relative to other factors such as passive diffusion. Thus, CLf0 can also be negligible. Collectively, Equation (1) can then be simplified to Equation (2).
| (2) |
When there is no active placental efflux, CLPM = 0 and Kp,uu = 1, indicating that fetal drug exposure will be solely determined by placental passive diffusion clearance. In this case, fetal exposure (unbound AUC or concentration) will be equal to maternal exposure (unbound AUC or concentration). When there is active efflux of drug from the placenta to the maternal circulation, CLPM > 0 and Kp,uu < 1, indicating that fetal exposure will be less than maternal exposure.
PBPK modeling and simulation to predict systemic fetal drug exposure
Since Kp,uu is the ratio of the steady-state unbound plasma concentration in the fetal circulation to the corresponding maternal unbound plasma concentration, once Kp,uu is predicted (see below), the fetal unbound steady state drug concentration can be calculated from its corresponding unbound maternal drug concentration. However, Kp,uu itself does not provide a dynamic fetal plasma drug concentration time profile. Dynamic fetal drug exposure can be predicted by determining the absolute values of in vivo CLPM and CLPD according to the three-compartment model shown in Figure 2 and populating these clearance values into the maternal-fetal PBPK models. Here we provide a brief overview of two such approaches.
The first approach is the ER-REF approach developed in the laboratory of Dr. Anoshchenko et al. [75]. ER stands for efflux ratio of any drug transported by efflux transporters (e.g., P-gp or BCRP) and can be determined in the Transwell® assay using transporter overexpressing cell lines. REF stands for relative expression factor which is the ratio of the abundance of the transporter of interest (e.g., P-gp) in human placenta to that in the cell lines. REF is used to extrapolate the ER measured in the cell lines to that in vivo by considering the difference in abundance of the transporter between the cell lines and human placenta. Using this approach, Anoshchenko et al. [75] derived Equation (3) to scale ER determined in a cell line by in vitro to in vivo extrapolation (IVIVE) using REF to predict in vivo Kp,uu (Kp,uu IVIVE) as follows.
| (3) |
Where ERInh(+) and ERInh(−) are the efflux ratio in the presence and absence of complete inhibition of the transporter of interest, respectively. Since the ERs and REF can be determined experimentally, Kp,uu, IVIVE can also be experimentally determined. The cell line used to determine the ERs can be Madin-Darby canine kidney II (MDCKII) cells that overexpress a placental efflux transporter of interest such as P-gp. To perform PBPK M&S, the exact values of all transplacental clearances are needed. In vivo passive diffusion clearance CLPD can be estimated as follows. CLPD of any drug can be estimated using the in vivo midazolam CLPD as a calibrator [76] (Equation [4]).
| (4) |
Where Papp is the apparent membrane permeability across the MDCKII or another mammalian cell monolayer (e.g., Caco-2) by passive diffusion only. Once in vivo CLPD has been estimated, in vivo CLPM can be calculated from Kp,uu,IVIVE using Equation (2). The in vivo CLPD and CLPM values are then populated into the maternal-fetal (m-f) PBPK models to predict dynamic fetal drug exposure. This ER-REF plus m-f PBPK approach has been successfully validated for four P-gp substrate drugs dexamethasone, betamethasone, darunavir, and lopinavir [75]. Given the P-gp abundance in early placenta has already been quantified [45], this ER-REF plus m-f PBPK approach in principle can also predict fetal exposure to P-gp substrate drugs in early pregnancy if relevant systems parameters (e.g., fetal physiology in early gestation) and clinical data are available to validate model predictions. Contributions of placental and/or fetal metabolism of drugs to fetal drug exposure should also be considered based on Equation (1) if these contributions are significant. More details of this approach have been reviewed recently elsewhere [77].
The second approach is the ex vivo perfused human placenta plus m-f PBPK approach. As aforementioned, unbound transplacental transfer clearances from the CLmf and CLfm directions of a drug can be determined in dually perfused human placenta studies with individual cotyledons. The clearance values determined should be scaled to the whole placenta by considering the average number of cotyledons in the placenta or taking into account the cotyledon weight or volume vs. the total placenta weight or volume, respectively. The scaled transplacental clearance values are populated into m-f PBPK models to predict dynamic fetal drug exposure. This approach has been successfully validated for drugs that passively cross the placenta [78], [79], [80], [81], [82], [83], [84], but has not yet been tested for drugs that are actively transported across the placenta by drug transporters. It is worth noting that predicting fetal exposure to the drugs that passively cross the placenta does not need placental perfusion data as demonstrated by Zhang et al. [76]. Also, this approach may only predict fetal drug exposure at term because most placental perfusion studies are performed with human term placenta. Again, placental and/or fetal metabolism of drugs should be considered in PBPK M&S if they are significant contributors to fetal drug exposure.
Summary and future directions
The placenta and transporters expressed in the placenta are crucial in determining fetal drug exposure. Understanding placental drug transport and its relevance to fetal drug exposure are critical for optimizing therapeutic use of drugs for pregnant women and their fetuses with better efficacy and lower toxicity. We have seen significant progress in this research area in the last 5–10 years. In Table 1, we have summarized the advantages and disadvantages of in vitro, ex vivo, and in vivo models as well as PBPK M&S currently used in determining and understanding placental drug transport and fetal drug exposure. Future studies may focus on the following aspects. (1) Current studies are primarily focused on ABC transporters in the placenta. We need more studies to investigate the roles of SLC uptake transporters in determining fetal drug exposure. (2) Further studies are needed to determine whether and how placental transporters are altered through induction or down-regulation by drugs, xenobiotics, genetics (e.g., single nucleotide polymorphisms), gestational age, and diseases (e.g., viral and bacterial infections), leading to altered fetal drug concentrations and hence efficacy or toxicity for the fetus. In this regard, novel placental cell systems that truly represent the placental physiology in vivo such as trophoblast organoids may be explored. (3) In the past 5 years, much progress has been made in the quantitative prediction of systemic fetal exposure to drugs that passively cross the placenta or actively transported by the placenta through PBPK M&S. However, the latter has been validated only for four P-gp substrate drugs using the ER-REF plus m-f PBPK approach [75]. The ER-REF plus m-f PBPK approach has yet to be validated for more P-gp substrate drugs and for drugs that are transported by other placental transporters such as BCRP and SLC transporters. It is also necessary to develop approaches to predict fetal exposure to drugs that are transported by multiple transporters in the placenta. (4) To date, PBPK M&S has only been used to predict fetal drug exposure at term. In the future, we should also predict fetal drug exposure in early gestation (e.g., < 15 weeks). To this end, it is critical that we must better understand fetal physiology in early gestation, which is currently lacking. (5) Fetal drug exposure is driven by maternal exposure. Maternal exposure will be altered by changes in the expression or activity of drug metabolizing enzymes or transporters in organs important for drug disposition (e.g., liver, kidney, and small intestine). While the impact of pregnancy on CYPs (e.g., CYP3A) in maternal organs (e.g., liver) has been well documented, we know little about the effects of pregnancy on non-CYP enzymes and transporters. More studies are needed in this regard. (6) Further clinical studies with established enzyme and transporter probe drugs in pregnant women (hopefully at all gestational ages including early gestation) are needed. Clinical pharmacokinetic data from such studies are essential for accurate predication and validation of the prediction of both maternal and fetal drug exposure so that we do not need to conduct clinical studies for every drug administered to pregnant women that is metabolized or transported by the same enzymes or transporters. Collectively, to optimize dosing regimens of drugs administered to pregnant women with better efficacy and lower toxicity for both the mother and her fetus, a thorough understanding and accurate prediction of maternal and fetal drug exposure as well as their determining factors, as outlined above, are urgently needed.
Table 1:
Comparison of in vitro, ex vivo, in vivo, and virtual models for studying placental drug transport and fetal drug exposure.
| Category | Model | Pros | Cons |
|---|---|---|---|
| In vitro | Malignant trophoblast cell lines | Wide application | Lack of in vivo placental transporter expression representation |
| Primary cytotrophoblasts | Primary samples | Potentially altered transporter expression during cell culture | |
| Trophoblast organoids | Three-dimensional structure | Unclear transporter expression profiles | |
| Plasma membrane vesicles isolated from human placenta | Direct measurement of transport kinetics | Not practical for drugs with high membrane permeability | |
| Ex vivo | Human placenta perfusion | Primary samples | Steady-state may not be reached in the open system; usually performed with term placenta only. |
| In vivo | Chronically catheterized animals (macaque, baboon, sheep) | Continuous sampling within the same animal | High cost; interspecies variability |
| Transporter knockout mice | Low cost; rodent transporters highly homolgous to human transporters | Small molecules may bypass transporter-mediated transplacental transport and give false negative data | |
| Positron emission tomography imaging | Non-invasive; whole body quantification | High cost; trade-offs between resolution and noise | |
| Virtual | Physiologically based pharmacokinetic modeling and simulation | Fast; no actual experiments; generic (not drug specific) models | Limited validation so far; still under development |
Footnotes
Research funding: This work was supported by the National Institute on Drug Abuse (Grant P01DA032507) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant R01HD102786). The funding organizations played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Author contributions: The author wrote this review and revised it critically for important intellectual content, and finally approves the version to be published. The author agrees to be accountable for all aspects of the work in ensuring the accuracy or integrity of any part of this work.
Competing interests: The author declares no competing interests.
References
- 1.Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188:1039–45. doi: 10.1067/mob.2003.223. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S. National Birth Defects Prevention S: medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205:51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mofenson LM. Centers for disease C, prevention USPHSTF: U. S. public health service task force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep (Morb Mortal Wkly Rep) 2002;51:1–38. quiz CE31–4. [PubMed] [Google Scholar]
- 5.Gozar L, Gabor-Miklosi D, Toganel R, Fagarasan A, Gozar H, Toma D, et al. Fetal tachyarrhythmia management from digoxin to amiodarone-A review. J Clin Med. 2022;11:804. doi: 10.3390/jcm11030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaffidi J, Mol BW, Keelan JA. The pregnant women as a drug orphan: a global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG. 2017;124:132–40. doi: 10.1111/1471-0528.14151. [DOI] [PubMed] [Google Scholar]
- 7.Mao Q, Ganapathy V, Unadkat JD. Drug transport in the placenta. In: You G, Morris ME, editors. Drug transporters: molecular characterization and role in drug disposition. New Jersey, USA: John Wiley & Sons; 2014. pp. 341–53. [Google Scholar]
- 8.Yamashita M, Markert UR. Overview of drug transporters in human placenta. Int J Mol Sci. 2021;22:13149. doi: 10.3390/ijms222313149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev. 1999;38:3–15. doi: 10.1016/s0169-409x(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 10.Kliman HJ, Quaratella SB, Setaro AC, Siegman EC, Subha ZT, Tal R, et al. Pathway of maternal serotonin to the human embryo and fetus. Endocrinology. 2018;159:1609–29. doi: 10.1210/en.2017-03025. [DOI] [PubMed] [Google Scholar]
- 11.Myren M, Mose T, Mathiesen L, Knudsen LE. The human placenta-an alternative for studying foetal exposure. Toxicol Vitro. 2007;21:1332–40. doi: 10.1016/j.tiv.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Shin BC, Suzuki T, Matsuzaki T, Tanaka S, Kuraoka A, Shibata Y, et al. Immunolocalization of GLUT1 and connexin 26 in the rat placenta. Cell Tissue Res. 1996;285:83–9. doi: 10.1007/s004410050623. [DOI] [PubMed] [Google Scholar]
- 13.Han LW, Gao C, Mao Q. An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expet Opin Drug Metabol Toxicol. 2018;14:817–29. doi: 10.1080/17425255.2018.1499726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Imperial MZ, Patilea-Vrana GI, Wedagedera J, Gaohua L, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model I: insights into factors that determine fetal drug exposure through simulations and sensitivity analyses. Drug Metab Dispos. 2017;45:920–38. doi: 10.1124/dmd.117.075192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni Z, Mao Q. ATP-binding cassette efflux transporters in human placenta. Curr Pharmaceut Biotechnol. 2011;12:674–85. doi: 10.2174/138920111795164057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unadkat JD, Dahlin A, Vijay S. Placental drug transporters. Curr Drug Metabol. 2004;5:125–31. doi: 10.2174/1389200043489171. [DOI] [PubMed] [Google Scholar]
- 17.Ganapathy V, Prasad PD, Ganapathy ME, Leibach FH. Placental transporters relevant to drug distribution across the maternal-fetal interface. J Pharmacol Exp Therapeut. 2000;294:413–20. [PubMed] [Google Scholar]
- 18.Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol. 2009;158:665–78. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker N, Filis P, Soffientini U, Bellingham M, O’Shaughnessy PJ, Fowler PA. Placental transporter localization and expression in the Human: the importance of species, sex, and gestational age differencesdagger. Biol Reprod. 2017;96:733–42. doi: 10.1093/biolre/iox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFarland A, Abramovich DR, Ewen SW, Pearson CK. Stage-specific distribution of P-glycoprotein in first-trimester and full-term human placenta. Histochem J. 1994;26:417–23. doi: 10.1007/bf00160054. [DOI] [PubMed] [Google Scholar]
- 21.Bikadi Z, Hazai I, Malik D, Jemnitz K, Veres Z, Hari P, et al. Predicting P-Glycoprotein-Mediated drug transport based on support vector machine and three-dimensional crystal structure of P-glycoprotein. PLoS One. 2011;6:e25815. doi: 10.1371/journal.pone.0025815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- 23.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport-an update. AAPS J. 2015;17:65–82. doi: 10.1208/s12248-014-9668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an obstetric-fetal pharmacology research unit network and university of Washington specialized center of research study. Mol Pharmacol. 2008;73:949–59. doi: 10.1124/mol.107.041616. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang H, Unadkat JD, Mao Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos. 2007;35:2154–8. doi: 10.1124/dmd.107.018044. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Song Y, Wu D, Li Q, Lu S, Li X, et al. Inter-individual variations and modulators of MDR1 transport activity in human placenta. Placenta. 2020;97:46–50. doi: 10.1016/j.placenta.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Smit JW, Huisman MT, van Tellingen O, Wiltshire HR, Schinkel AH. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J Clin Invest. 1999;104:1441–7. doi: 10.1172/jci7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molsa M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, et al. Functional role of P-glycoprotein in the human blood-placental barrier. Clin Pharmacol Ther. 2005;78:123–31. doi: 10.1016/j.clpt.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Pollex E, Lubetsky A, Koren G. The role of placental breast cancer resistance protein in the efflux of glyburide across the human placenta. Placenta. 2008;29:743–7. doi: 10.1016/j.placenta.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Martel F, Azevedo I. An update on the extraneuronal monoamine transporter (EMT): characteristics, distribution and regulation. Curr Drug Metabol. 2003;4:313–8. doi: 10.2174/1389200033489433. [DOI] [PubMed] [Google Scholar]
- 31.Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, et al. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Therapeut. 2005;315:888–95. doi: 10.1124/jpet.105.086827. [DOI] [PubMed] [Google Scholar]
- 32.Karahoda R, Horackova H, Kastner P, Matthios A, Cerveny L, Kucera R, et al. Serotonin homeostasis in the materno-foetal interface at term: role of transporters (SERT/SLC6A4 and OCT3/SLC22A3) and monoamine oxidase A (MAO-A) in uptake and degradation of serotonin by human and rat term placenta. Acta Physiol. 2020;229:e13478. doi: 10.1111/apha.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee N, Hebert MF, Wagner DJ, Easterling TR, Liang CJ, Rice K, et al. Organic cation transporter 3 facilitates fetal exposure to metformin during pregnancy. Mol Pharmacol. 2018;94:1125–31. doi: 10.1124/mol.118.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–12. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 35.Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab. 2003;284:E390–8. doi: 10.1152/ajpendo.00257.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, et al. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94:297–304. doi: 10.1254/jphs.94.297. [DOI] [PubMed] [Google Scholar]
- 37.Tomi M, Eguchi H, Ozaki M, Tawara T, Nishimura S, Higuchi K, et al. Role of OAT4 in uptake of estriol precursor 16alpha-hydroxydehydroepiandrosterone sulfate into human placental syncytiotrophoblasts from fetus. Endocrinology. 2015;156:2704–12. doi: 10.1210/en.2015-1130. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi S, Nishimura T, Fujibayashi A, Maruyama T, Tomi M, Nakashima E. Organic anion transporter 4-mediated transport of olmesartan at basal plasma membrane of human placental barrier. J Pharmacol Sci. 2015;104:3128–35. doi: 10.1002/jps.24434. [DOI] [PubMed] [Google Scholar]
- 39.Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–51. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinzi J, Grube M, Meyer Zu Schwabedissen HE. OATP2B1 - the underrated member of the organic anion transporting polypeptide family of drug transporters? Biochem Pharmacol. 2021;188:114534. doi: 10.1016/j.bcp.2021.114534. [DOI] [PubMed] [Google Scholar]
- 41.Grube M, Reuther S, Meyer Zu Schwabedissen H, Kock K, Draber K, Ritter CA, et al. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos. 2007;35:30–5. doi: 10.1124/dmd.106.011411. [DOI] [PubMed] [Google Scholar]
- 42.Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–6. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramamoorthy S, Prasad PD, Kulanthaivel P, Leibach FH, Blakely RD, Ganapathy V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncytiotrophoblast. Biochemistry. 1993;32:1346–53. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
- 44.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–50. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anoshchenko O, Prasad B, Neradugomma NK, Wang J, Mao Q, Unadkat JD. Gestational age-dependent abundance of human placental transporters as determined by quantitative targeted proteomics. Drug Metab Dispos. 2020;48:735–41. doi: 10.1124/dmd.120.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fokina VM, Patrikeeva S, Wang XM, Noguchi S, Tomi M, Konig J, et al. Role of uptake transporters OAT4, OATP2A1, and OATP1A2 in human placental bio-disposition of pravastatin. J Pharmacol Sci. 2022;111:505–16. doi: 10.1016/j.xphs.2021.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sastry BV. Techniques to study human placental transport. Adv Drug Deliv Rev. 1999;38:17–39. doi: 10.1016/s0169-409x(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Lee EW, Zhou L, Leung PC, Ross DD, Unadkat JD, et al. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol Pharmacol. 2008;73:845–54. doi: 10.1124/mol.107.041087. [DOI] [PubMed] [Google Scholar]
- 49.Neradugomma NK, Liao MZ, Mao Q. Buprenorphine, norbuprenorphine, R-methadone, and S-methadone upregulate BCRP/ABCG2 expression by activating aryl hydrocarbon receptor in human placental trophoblasts. Mol Pharmacol. 2017;91:237–49. doi: 10.1124/mol.116.107367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol. 2003;285:C584–91. doi: 10.1152/ajpcell.00418.2002. [DOI] [PubMed] [Google Scholar]
- 51.Stenqvist AC, Chen T, Hedlund M, Dimova T, Nagaeva O, Kjellberg L, et al. An efficient optimized method for isolation of villous trophoblast cells from human early pregnancy placenta suitable for functional and molecular studies. Am J Reprod Immunol. 2008;60:33–42. doi: 10.1111/j.1600-0897.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 52.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 53.Karahoda R, Zaugg J, Fuenzalida B, Kallol S, Moser-Haessig R, Staud F, et al. Trophoblast differentiation affects crucial nutritive functions of placental membrane transporters. Front Cell Dev Biol. 2022;10:820286. doi: 10.3389/fcell.2022.820286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berveiller P, Degrelle SA, Segond N, Cohen H, Evain-Brion D, Gil S. Drug transporter expression during in vitro differentiation of first-trimester and term human villous trophoblasts. Placenta. 2015;36:93–6. doi: 10.1016/j.placenta.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564:263–7. doi: 10.1038/s41586-018-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fokina V, Patrikeeva S, Wang X, Shah M, Shah P, Russell WK, et al. Physicochemical and biological properties of membrane vesicles derived from human term placentas. J Biomed Nanotechnol. 2022;18:589–99. doi: 10.1166/jbn.2022.3255. [DOI] [PubMed] [Google Scholar]
- 57.Ruzycki SM, Kelley LK, Smith CH. Placental amino acid uptake. IV. Transport microvillous membrane vesicles. Am J Physiol. 1978;234:C27–35. doi: 10.1152/ajpcell.1978.234.1.c27. [DOI] [PubMed] [Google Scholar]
- 58.Vaidya SS, Walsh SW, Gerk PM. Formation and efflux of ATP-binding cassette transporter substrate 2,4-dinitrophenyl-S-glutathione from cultured human term placental villous tissue fragments. Mol Pharm. 2009;6:1689–702. doi: 10.1021/mp900019z. [DOI] [PubMed] [Google Scholar]
- 59.Gorczyca L, Du J, Bircsak KM, Wen X, Vetrano AM, Aleksunes LM. Low oxygen tension differentially regulates the expression of placental solute carriers and ABC transporters. FEBS Lett. 2021;595:811–27. doi: 10.1002/1873-3468.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szilagyi JT, Composto-Wahler GM, Joseph LB, Wang B, Rosen T, Laskin JD, et al. Anandamide down-regulates placental transporter expression through CB2 receptor-mediated inhibition of cAMP synthesis. Pharmacol Res. 2019;141:331–42. doi: 10.1016/j.phrs.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bircsak KM, Gupta V, Yuen PY, Gorczyca L, Weinberger BI, Vetrano AM, et al. Genetic and dietary regulation of glyburide efflux by the human placental breast cancer resistance protein transporter. J Pharmacol Exp Therapeut. 2016;357:103–13. doi: 10.1124/jpet.115.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuntland T, Odinecs A, Pereira CM, Nosbisch C, Unadkat JD. In vitro models to predict the in vivo mechanism, rate, and extent of placental transfer of dideoxynucleoside drugs against human immunodeficiency virus. Am J Obstet Gynecol. 1999;180:198–206. doi: 10.1016/s0002-9378(99)70175-4. [DOI] [PubMed] [Google Scholar]
- 63.Kraemer J, Klein J, Lubetsky A, Koren G. Perfusion studies of glyburide transfer across the human placenta: implications for fetal safety. Am J Obstet Gynecol. 2006;195:270–4. doi: 10.1016/j.ajog.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Kurosawa K, Chiba K, Noguchi S, Nishimura T, Tomi M. Development of a pharmacokinetic model of transplacental transfer of metformin to predict in vivo fetal exposure. Drug Metab Dispos. 2020;48:1293–302. doi: 10.1124/dmd.120.000127. [DOI] [PubMed] [Google Scholar]
- 65.Brownbill P, Sebire N, McGillick EV, Ellery S, Murthi P. Ex vivo dual perfusion of the human placenta: disease simulation, therapeutic pharmacokinetics and analysis of off-target effects. Methods Mol Biol. 2018;1710:173–89. doi: 10.1007/978-1-4939-7498-6_14. [DOI] [PubMed] [Google Scholar]
- 66.Ceckova M, Reznicek J, Ptackova Z, Cerveny L, Muller F, Kacerovsky M, et al. Role of ABC and solute carrier transporters in the placental transport of lamivudine. Antimicrob Agents Chemother. 2016;60:5563–72. doi: 10.1128/aac.00648-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong H, Kumar S, Riggs KW, Rurak DW. Pharmacokinetics and renal excretion of diphenhydramine and its metabolites, diphenylmethoxyacetic acid and diphenhydramine-N-oxide, in developing lambs. J Pharmacol Sci. 2000;89:1362–70. doi: 10.1002/1520-6017(200010)89:10<1362::aid-jps14>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 68.Tuntland T, Odinecs A, Nosbisch C, Unadkat JD. In vivo maternal-fetal-amniotic fluid pharmacokinetics of zidovudine in the pigtailed macaque: comparison of steady-state and single-dose regimens. J Pharmacol Exp Therapeut. 1998;285:54–62. [PubMed] [Google Scholar]
- 69.Liao MZ, Gao C, Shireman LM, Phillips B, Risler LJ, Neradugomma NK, et al. P-gp/ABCB1 exerts differential impacts on brain and fetal exposure to norbuprenorphine. Pharmacol Res. 2017;119:61–71. doi: 10.1016/j.phrs.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujita A, Noguchi S, Hamada R, Inoue S, Shimada T, Katakura S, et al. Limited impact of murine placental MDR1 on fetal exposure of certain drugs explained by bypass transfer between adjacent syncytiotrophoblast layers. Pharm Res. 2022;39:1645–58. doi: 10.1007/s11095-022-03165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eyal S, Chung FS, Muzi M, Link JM, Mankoff DA, Kaddoumi A, et al. Simultaneous PET imaging of P-glycoprotein inhibition in multiple tissues in the pregnant nonhuman primate. J Nucl Med. 2009;50:798–806. doi: 10.2967/jnumed.108.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaquero JJ, Kinahan P. Positron emission tomography: current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annu Rev Biomed Eng. 2015;17:385–414. doi: 10.1146/annurev-bioeng-071114-040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Myllynen P, Pasanen M, Vahakangas K. The fate and effects of xenobiotics in human placenta. Expet Opin Drug Metabol Toxicol. 2007;3:331–46. doi: 10.1517/17425255.3.3.331. [DOI] [PubMed] [Google Scholar]
- 74.Li H, Lampe JN. Neonatal cytochrome P450 CYP3A7: a comprehensive review of its role in development, disease, and xenobiotic metabolism. Arch Biochem Biophys. 2019;673:108078. doi: 10.1016/j.abb.2019.108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anoshchenko O, Storelli F, Unadkat JD. Successful prediction of human fetal exposure to P-glycoprotein substrate drugs using the proteomics-informed relative expression factor Approach and PBPK modeling and simulation. Drug Metab Dispos. 2021;49:919–28. doi: 10.1124/dmd.121.000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model II: verification of the model for passive placental permeability drugs. Drug Metab Dispos. 2017;45:939–46. doi: 10.1124/dmd.116.073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Storelli F, Yin M, Kumar AR, Ladumor MK, Evers R, Chothe PP, et al. The next Frontier in ADME science: predicting transporter-based drug disposition, tissue concentrations and drug-drug interactions in humans. Pharmacol Ther. 2022;238:108271. doi: 10.1016/j.pharmthera.2022.108271. [DOI] [PubMed] [Google Scholar]
- 78.De Sousa Mendes M, Hirt D, Urien S, Valade E, Bouazza N, Foissac F, et al. Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women. Br J Clin Pharmacol. 2015;80:1031–41. doi: 10.1111/bcp.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mian P, Allegaert K, Conings S, Annaert P, Tibboel D, Pfister M, et al. Integration of placental transfer in a fetal-maternal physiologically based pharmacokinetic model to characterize acetaminophen exposure and metabolic clearance in the fetus. Clin Pharmacokinet. 2020;59:911–25. doi: 10.1007/s40262-020-00861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freriksen JJM, Schalkwijk S, Colbers AP, Abduljalil K, Russel FGM, Burger DM, et al. Assessment of maternal and fetal dolutegravir exposure by integrating ex vivo placental perfusion data and physiologically-based pharmacokinetic modeling. Clin Pharmacol Ther. 2020;107:1352–61. doi: 10.1002/cpt.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abduljalil K, Ning J, Pansari A, Pan X, Jamei M. Prediction of maternal and fetoplacental concentrations of cefazolin, cefuroxime, and amoxicillin during pregnancy using bottom-up physiologically based pharmacokinetic models. Drug Metab Dispos. 2022;50:386–400. doi: 10.1124/dmd.121.000711. [DOI] [PubMed] [Google Scholar]
- 82.Abduljalil K, Pansari A, Ning J, Jamei M. Prediction of maternal and fetal acyclovir, emtricitabine, lamivudine, and metformin concentrations during pregnancy using a physiologically based pharmacokinetic modeling approach. Clin Pharmacokinet. 2022;61:725–48. doi: 10.1007/s40262-021-01103-0. [DOI] [PubMed] [Google Scholar]
- 83.De Sousa Mendes M, Hirt D, Vinot C, Valade E, Lui G, Pressiat C, et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81:646–57. doi: 10.1111/bcp.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu XI, Momper JD, Rakhmanina N, van den Anker JN, Green DJ, Burckart GJ, et al. Physiologically based pharmacokinetic models to predict maternal pharmacokinetics and fetal exposure to emtricitabine and acyclovir. J Clin Pharmacol. 2020;60:240–55. doi: 10.1002/jcph.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]