Abstract
Adiponectin is an adipokine predominantly produced by fat cells, circulates and exerts insulin-sensitizing, cardioprotective and anti-inflammatory effects. Dysregulation of adiponectin and/or adiponectin signaling is implicated in a number of metabolic diseases such as obesity, insulin resistance, diabetes, and cardiovascular diseases. However, while the insulin-sensitizing and cardioprotective effects of adiponectin have been widely appreciated in the field, the obesogenic and anti-inflammatory effects of adiponectin are still of much debate. Understanding the physiological function of adiponectin is critical for adiponectin-based therapeutics for the treatment of metabolic diseases.
Keywords: adipogenesis, adiponectin, inflammation, insulin sensitivity, obesity
Introduction
Adipose tissue plays a central role in the maintenance of whole-body energy and metabolic homeostasis at both organ and system levels [1], by serving as a passive fuel reservoir, an endocrine organ and thermogenic effector. Adiponectin, the most representative adipokine, is a 30 kDa protein predominantly secreted by adipocytes and targets a variety of cell types or tissue/organs by binding to its own receptors, protecting against obesity [2], [3], [4], [5], insulin resistance [6, 7], and inflammation-related diseases [5, 8]. As a circulating protein, adiponectin accumulates in heart, vasculature, and skeletal muscles through interaction with T-cadherin, which is essential for adiponectin-mediated cardiovascular protection [9], [10], [11]. Since obesity increases the risk of developing serious health problems including metabolic diseases [12, 13], there is an urgent need for the advanced understanding of the physiological function of adiponectin for the therapeutic purpose [14].
Despite well-accepted beneficial effects in metabolism, adiponectin-based treatment is still challenging given its high abundance in circulation and the potential adverse effects, including increased food intake, elevated adipogenesis, decreased energy expenditure, and substantial adiposity [15], [16], [17], [18].
In this review, we have discussed the physiological roles of adiponectin, not only its beneficial properties but also the unfavorable effects related to its therapeutic potential for the treatment of obesity and its related diseases. We also summarized the advances in the understanding of adiponectin action in the regulation of metabolism and inflammation, highlighting the obesogenic and pro-inflammatory effects of adiponectin.
Adiponectin and adiponectin signaling
Adiponectin was discovered as an adipocyte-enriched protein highly induced during adipogenesis [2, 3, 5, 19] and cloned in 1995 [5]. The full-length adiponectin protein contains a signal peptide, a variable region, a collagen-like domain, and a C-terminal globular domain [20, 21]. Adiponectin circulates in multiple forms: low-molecular weight (LMW) trimers, medium-molecular weight (MMW) hexamers, and high-molecular weight (HMW) multimers [22], [23], [24], [25], and globular adiponectin (gAd) which is a proteolytic adiponectin globular domain at very low concentrations in human plasma [26, 27]. Each form exerts distinct biological effects due to their divergent affinity to their receptors and various cellular targets [20, 28], [29], [30]. The HMW form presents most biological effects of adiponectin [24, 25, 30], [31], [32]. However, it is still a challenge to measure the levels of individual isoform of adiponectin and enrich a particular isoform in vivo [33].
The pleiotropic actions of adiponectin are mediated by its receptors including AdipoRs (AdipoR1 and AdipoR2) and T-cadherin [34]. Adiponectin receptors present in metabolically active cell types such as adipocytes, hepatocytes, and muscle cells as well as immune cells and neuronal cells in the different brain regions [18, 35], [36], [37], [38]. AdipoR1 is abundantly expressed in skeletal muscle, while AdipoR2 is enriched in liver. Adiponectin binds to AdipoRs and exerts antidiabetic effect through activation of two critical downstream factors: 5′-adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α (PPARα) [37, 39], [40], [41]. APPL1 binds to AdipoRs and mediates adiponectin signaling and its downstream events, promoting glucose uptake and insulin receptor substrate-1 (IRS-1) action to sensitize insulin signaling [42, 43]. Adiponectin homolog osmotin activates AdipoR1/R2 to suppress abdominal fat accumulation in mice on high-fat diet (HFD) [44, 45], and prevents obesity-caused NAFLD by upregulating AdipoRs/APPL1/PPAR-α/AMPK/SIRT1 pathway [46]. The additional downstream pathways of adiponectin, including MAPK, mTOR, STAT3, PI3K/Akt and NF-κB pathways, are tissue- and cell-specific [47], [48], [49], [50]. However, while the recombinant globular adiponectin has been wide used to study its action in various cells, HMW multimer of adiponectin has been considered as most biologically active form [24, 25, 30], [31], [32]. Whereas the mechanism underlying the binding of HMW adiponectin to its receptor and subsequent signaling transduction are poorly defined. One of existing challenge is that HMW form 16–18mer is over 400 kDa, about 10 times larger than the receptors themselves. The structural analysis using current state-of-the-art-technology may provide information on how HMW multimer works.
Furthermore, ceramidase-activating effect of AdipoRs is critical for the clearance of cellular ceramides which was considered an important mechanism by which adiponectin improves glucose and lipid metabolism [51], [52], [53], [54], [55], [56]. AdipoRs are also required for adiponectin to suppress macrophage lipid accumulation and foam cell formation via an APPL1-dependent mechanism [57]. In an AdipoR2-dependent manner, adiponectin promotes M2 macrophage polarization [58, 59], whereas the anti-inflammatory effects of gAd in brain are mediated by AdipoR1 to limit the M1 activation state of microglia [60]. Moreover, Smad1/5/8 acts as a novel intracellular partner of adiponectin/AdipoR1 signaling in osteoblasts and mesenchymal progenitor cells that is parallel to APPL1 [61]. In addition, the physiological role of AdipoRs beyond adiponectin signaling have been recognized. For instance, the insulin sensitiving effects of adiponectin are partially mediated by AdipoR1 and AdipoR2 in mice [34, 62], [63], [64]. In adiponectin deficient mice, pioglitazone is able to improve insulin sensitivity by stimulation of AdipoR2 pathway in skeletal muscle [65].
T-cadherin is a glycosylphosphatidylinositol-anchored (GPI-anchored) cadherin without the intracellular or transmembrane domain required for intracellular signaling. It selectively binds to MMW and HMW adiponectin and impacts circulating adiponectin by regulating its binding to cardiovascular tissues, and adiponectin positively regulates T-cadherin abundance on endothelial cells via attenuating phosphatidylinositol-specific phospholipase C-mediated T-cadherin release from the cell surface [66, 67]. Furthermore, adiponectin was reported to be endocytosed into multivesicular bodies with T-cadherin and enhances exosome biogenesis and release dependently on T-cadherin, leading to reduction of cellular ceramides in endothelial cells [68]. Single nucleotide polymorphism of T-cadherin strongly correlates with plasma adiponectin level and cardiovascular diseases in human [69], [70], [71], [72], [73], [74]. It has been demonstrated that adiponectin/T-cadherin axis protects against vascular injury related to atherosclerosis and cardiac stress [9, 10, 75]. Besides, T-cadherin is also required for tethering of adiponectin to M2 macrophages during cold-induced browning in subcutaneous white adipose tissue (WAT) [76]. Later, adiponectin was shown to be accumulated in CD63-positive endosomes of regenerating myotubes and promote muscle regeneration in a T-cadherin-dependent manner [77]. This ability of T-cadherin in adiponectin sequestration to cell surface is responsible for adiponectin signal transduction pathway, and could be disrupted by an increased specific phospholipase D (GPI-PLD)-mediated cleavage of T-cadherin during diet-induced obesity or insulin resistance [78], ultimately leading to a state of adiponectin resistance in metabolic diseases [79]. Taken together, these findings supported adiponectin/T-cadherin interaction interprets the cardioprotective effects of adiponectin.
Insulin sensitizing effect of adiponectin
Adiponectin is an insulin-sensitizing hormone. The circulating levels of adiponectin are inversely correlated with obesity, and such downregulation has been considered as a mechanism mediating obesity-induced insulin resistance and diabetes in mice and humans [41, 80], [81], [82], [83], [84]. Congenital deletion of adiponectin led to insulin resistance in mice fed a high calorie diet [40, 85], and acute depletion of adiponectin resulted in more severe systemic insulin resistance, hyperlipidemia and dramatic reduction in survival rate in obese mice [86]. Conversely, enhanced adiponectin expression improves insulin resistance and other metabolic parameters in ob/ob mice [16]. Administration of adiponectin or pharmaceutically enhancing adiponectin signaling ameliorates insulin resistance and hyperglycemia in several mouse models [16, 34, 39, 40, 65, 84, 87, 88]. Furthermore, adiponectin has been shown to mediate insulin sensitizing effects of FGF21 on maintaining a “healthy-obese” status, a factor that contributes to prolonged healthspan and lifespan [89], [90], [91].
Suppressing gluconeogenesis has been suggested as a primary mechanism by which adiponetin enhances insulin sensitivity in liver [92, 93]. In addition, other mechanisms include stimulating fatty acid oxidation via AdipoRs-mediated activation of AMPK and PPAR-α in the liver and skeletal muscle [7, 34, 84, 94, 95], and suppressing hepatic SREBP1c, the master regulator in fatty acid synthesis, through the AdipoR1/LKB1/AMPK pathway [96]. Moreover, adiponectin via signal transducer and activator of transcription-3 (STAT3) upregulates hepatic IRS-2 and enhances insulin signaling, which is linked to adiponectin-increased NFκB activity and subsequently macrophage IL-6 production [97]. Ceramides-lowing effect of adiponectin and the ceramidase activity of AdipoRs provide new insight into insulin sensitizing effects of adiponectin [52, 55, 56]. Adiponectin stimulates the ceramidase activity of AdipoRs to improve insulin action by disruption of ceramide accumulation and decrease apoptosis via formation of sphingosine-1-phosphate (S1P) which does not require AMPK [53]. Overexpression of AdipoRs in adipocyte or hepatocyte enhances ceramidase activity and results in adiponectin-dependent improvement of insulin sensitivity and hepatic steatosis [54]. In line with this, an FGF21-adiponectin-ceramide axis was shown to exert its glycemic and insulin sensitizing effects in mice [89]. The adiponectin-increased S1P secretion attenuated insulin resistance in HFD mice or palmitate-induced cardiomyocyte lipotoxicity model, another mechanism mediating insulin sensitizing and cardioprotective effects of adiponectin [51]. Contradictory to this, a recent study showed that the total ceramide content may not reflect the insulin-sensitizing effect of adiponectin in liver and muscle under obesity conditions [98].
More recently, skeletal muscle has been identified as a source of adiponectin [99, 100], fueling interest in the role of adiponectin as both a circulating adipokine and a locally expressed paracrine/autocrine factor [101, 102]. Within skeletal muscle, adiponectin stimulates phosphorylation of IRS1 at Ser 636/639 and activates of Akt by inhibiting mTOR signaling [100]. It also improves insulin signal transduction by decreasing fatty-acid transporter CD36 and subsequent triglyceride accumulation in muscle and liver [84]. In addition, adiponectin regulates mitochondrial biogenesis and insulin sensitivity via AdipoR1-mediated CaMKKβ/AMPK/SIRT1 pathway to induce PGC-1α deacetylation and further regulating the expression of mitochondrial-derived peptide MOTS-c [103, 104]. What’s more, adiponectin activates skeletal muscle autophagy and reduces oxidative stress by which enhances insulin sensitivity in HFD-feeding mice [105, 106]. Along this line, suppressing endoplasmic reticulum (ER) stress mediates adiponectin-induced autophagy in skeletal muscle cells [107]. Consistently, syringin improve HFD-induced insulin resistance through adiponectin-mediated attenuation of ER stress in skeletal muscle [108]. Interestingly, adiponectin and its signaling are improved in skeletal muscle and may explain the beneficial effects of exercise [109], [110], [111], [112]. Characterization of the role of exercise-activated adiponectin signaling in muscle or system metabolism may provide mechanistic insights into how exercise promotes metabolic health and improves physical fitness.
By targeting adipocytes, adipoenctin induces glucose transporter 4 (GLUT4) gene expression and improve insulin sensitivity [15]. Consistently, HMW adiponectin has been reported to enhance insulin action by regulation of GLUT4 in 3T3-L1 adipocytes [113]. Overexpression of AdipoRs in adipocytes suppresses the levels of ceramide in both fat and liver and improves whole-body insulin sensitivity [54]. To date, multiple mechanisms have been suggested to contribute to the insulin sensitizing effects of adiponectin (Figure 1), which are critical for the development of adiponectin-based therapeutic strategies.
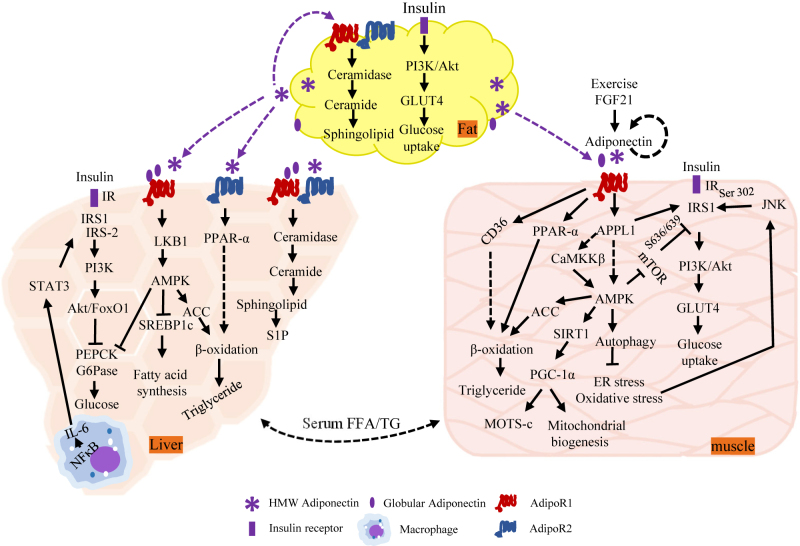
Figure 1:
Insulin-sensitizing effects of adiponectin. Upon release from adipocytes, adiponectin circulates and targets three metabolic tissue/organs liver, muscle and adipose tissue, by binding to its own receptors AdipoR1 and AdipoR2, adiponectin activates AMPK and PPARα and subsequently sensitizes insulin signaling, promoting glucose update and inhibiting glucose production in liver and muscle respectively, in addition to enhanced fatty acid oxidation in both tissues. Adiponectin via APPL1 facilitates the binding of IRS1/2 to insulin receptor through which enhances insulin receptor downstream signaling. In addition, adiponectin stimulates the activation of ceramidase and reduces intracellular and circulating levels of ceramide, improving insulin resistance. IR, insulin receptor; IRS, insulin receptor substrate; PI3K, phosphoinositide 3-kinase; FoxO1, Forkhead Box O1; PEPCK, phosphoenolpyruvate carboxykinase; G6Pase, glucose-6-phosphatase; IL-6, interleukin-6; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of transcription 3; LKB1, liver kinase B1; AMPK, AMP-activated protein kinase; PPAR-α, peroxisome proliferator-activated receptor-α; SREBP1c, sterol regulatory element-binding protein 1c; ACC, acetyl coenzyme A carboxylase; S1P, sphingosine-1-phosphate; FFA/TG, free fatty acid/triglycerides; APPL1, adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1; CaMBBβ, Calcium/calmodulin-dependent protein kinase kinase β; SIRT1, sirtuin 1; PGC-1α, Peroxisome proliferator-activated receptor-gamma coactivator-1α; ER, endoplasmic reticulum; JNK, c-Jun N-terminal kinase.
Adiponectin serves as a starvation mediator
Adiponectin is induced by fasting [114] and calorie restriction [83, 115, 116], and elevated adiponectin by intermittent fasting (IF) [117] may mediate the beneficial effects of IF on cardioprotection and metabolic stress [118], [119], [120]. In agreement with this, circulating levels of adiponectin were markedly increased in patients with anorexia nervosa [121]. Nutritional stress also explains the increased adiponectin levels in the circulation of heart failure patients [122]. Consistent with this, the inducing effects on adiponectin/adiponectin signaling components in muscle, adipose and brain are also applied under nutritional stress conditions [123], [124], [125], suggesting the involvement of adiponectin/adiponectin signaling in fasting adaptation.
Furthermore, adiponectin contributes to fasting adaptation through its central and peripheral action. Infusion of adiponectin increased food intake of mice on the high fat diet [84]. Adiponectin targets hypothalamus and stimulates food intake but reduces energy expenditure via AMPK-dependent mechanism [17]. In line with this, activating adiponectin-AMPK signaling in the hypothalamus has been shown to enhance food intake, a mechanism that thiazolidinediones (TZDs) increase body mass [126]. As a peripheral “starvation” signal, overexpressing adiponectin promotes the storage of triglycerides in adipose tissue with an improved metabolic profile [16]. Thus, the character of adiponectin as a starvation hormone and its related positive energy balance has became a concern for adiponectin-based therapeutics. However, the role of adiponectin in regulating food intake is of much debate [124]. Some studies showed that adiponectin deficiency had little effect on food intake in response to fasting or caloric restriction [116, 127, 128]. Adiponectin was also shown to decrease food intake and increase energy expenditure by directly regulating the cellular activity of arcuate Pomc and NPY/AgRP neurons [129]. In support of this, adiponectin enhances energy expenditure and suppresses ceramide by which it mediates the beneficial effects of FGF21, another well-known fasting hormone [89]. Although adiponectin regulation of appetite mainly depends on its central action [130], whether adiponectin moderates energy-expenditure through central nervous system remains incompletely understood. Only LMW form of adiponectin was shown to be able to cross through the brain blood barrier [131]. Little is known whether LMW multimer directly binds to adiponectin receptor in specific neuron or needs to be assembled to MMW and HMW before its action.
Obesogenic effects of adiponectin
It has been debated for many years on the bona fide role of adiponectin in the regulation of energy homeostasis and thermogenesis [18]. Several studies show that adiponectin promotes energy expenditure and the cold-induced browning effect through its central and peripheral actions [76, 85, 89, 128, 132], [133], [134]. However, other studies have suggested that adiponectin may be a negative regulator of energy expenditure and thermogenesis [16], [17], [18, 126, 135], [136], [137], [138]. These controversies may be partly due to the difference in the adiponectin isoform, dose, genetic background, administration approach or animal tools. It is worth noticing that although adiponectin deficiency does not appear to overtly alter insulin sensitivity under normal chow diet conditions [85, 139], adiponectin has been established to boost appetite, slow energy metabolism, and promote adipogenesis; all well-defined factors promoting obesity development [17, 135, 137]. This effect of adiponectin is similar to that of PPARγ agonists thiazolidinediones (TZDs) that improve insulin sensitivity but cause severe obesity [16, 140, 141]. The recent study using two adiponectin-deficient mouse models suggests that adiponectin inhibits energy expenditure and exerts pro-obesogenic effect by regulating cold-induced type 2 immunity in adipose tissue [18]. In support of this, adiponectin inhibits thermogenesis gene expression under cold stress by suppressing β3-adrenergic receptor expression in brown adipocytes independent of AdipoRs [137]. Kajimura et al. also reported that adiponectin suppresses energy expenditure, but their data suggest that the suppressing effect of adiponectin is mediated by the central nervous system [135]. Collectively, alteration of sympathetic tone, type 2 immunity, and β3-adrenergic signaling in adipose tissue may be responsible for adiponectin suppression of thermogenesis and energy expenditure. Adiponectin-based therapeutics may bring potential side effects such as anti-thermogenic and pro-obesity effects.
On the other hand, adiponectin is a well-accepted biomarker of adipogenesis and adipocyte differentiation in human mesenchymal stromal cells [142, 143]. It is induced during adipogenesis and has been suggested to facilitate adipogenesis [15, 144]. Consistently, adiponectin exhibits an ability to promote the adipogenesis of marrow osteoblasts [145] and hepatic stellate cells [146]. The inducing effect of adiponectin on adipogenesis is likely mediated by its feedforward activation of PPARγ, a master regulator of adipogenesis [144, 147], [148], [149]. The supporting evidence also include adiponectin-mediated induction of preadipocyte factor Pref-1 as well assuppression of C/EBPα, leading to the proliferation of pre-adipocytes rather than to promote adipocyte differentiation [132]. Given the adverse effect of PPARγ agonists TZDs as potent anti-diabetic drugs [84, 150], whether adiponectin-based therapeutics causes the similar side effects as TZD remains to be determined.
Dual role of adiponectin in inflammation
Besides the insulin sensitizing effect, the anti-inflammatory effect of adiponectin has gained lot of attention [28, 151], [152], [153], [154]. Adiponectin modulates inflammatory response, mainly by targeting various types of immune cell [155], [156], [157], [158], [159], [160], involving macrophage [161, 162], eosinophil [159], and mast cell [38]. A recent study reported a novel mechanism for the anti-inflammatory effect of adiponectin by promoting IL-10 release in human Tregs via adiponectin/AdipoR1 axis [163]. Besides, adiponectin in myotubes suppresses Toll-Like Receptor 4 (TLR4) signaling, leading to alleviated skeletal muscle inflammation [164]. Adiponectin inhibits diet-induced liver inflammation by suppressing MCP-1 expression and macrophage infiltration [165]. These findings suggest adiponectin signaling as a suitable therapeutic option for the treatment of inflammatory conditions.
However, there is a controversy as to whether adiponectin acts as a pro-inflammatory mediator [29, 59, 85, 97, 166], [167], [168], [169], [170], [171]. Several studies showed the pro-inflammatory properties of adiponectin in cells such as macrophages [29, 97, 172, 173], monocytes [174, 175], synovial fibroblasts [169, 176], endothelial cells [115, 177] and osteoblasts [115]. Cot/tpl2 was reported to participate in the production of inflammatory mediators upon stimulation of macrophages with adiponectin [178]. Besides, adiponectin-induced pro-inflammatory response was also found in human astrocytes [179], human microglia [175] and isolated human macrophages and T cells [166]. However, Surendar et al. reported that adiponectin reduces pro-inflammatory CD4+ T cells from HFD mouse via restraining cell intrinsic glycolysis during obesity [180], suggesting the context-specific and cell-dependent manner of adiponectin pro-inflammatory properties [60]. In support of this, elevated adiponectin levels in children with multiple sclerosis enhances pro-inflammatory activation of innate and adaptive peripheral immune cells [175]. Consistently, increased levels of adiponectin in muscle occurs 2 h post exercise and is required for the acute exercise-induced inflammatory response in muscle [181]. More importantly, it remains largely unknown whether adiponectin regulates thermogenesis and energy expenditure via inflammatory response, a key factor of WAT browning [182], [183], [184], [185]. By using adiponectin knockout (KO) mice, Hui et al. found that adiponectin promotes WAT browning by direct stimulation of anti-inflammatory M2 macrophage proliferation with little effect on group 2 innate lymphoid cells (ILC2s) mediated pro-inflammatory effects [76]. This mouse model was shown to display a rather moderate phenotype in insulin sensitivity [139]. By employing the adiponectin KO mice which displayed magnified insulin resistance compared to the controls, when dietarily challenged [40], it was observed that adiponectin plays an inhibitory role in regulating adipose-resident ILC2s, type 2 immunity and energy expenditure [18], suggesting that the inhibitory effect of adiponectin on thermogenesis may be due to its pro-inflammatory property.
The dual role of adiponectin in the regulation of inflammation seems contrary but connected. Adiponectin isoforms may contribute to dual effects of adiponectin on inflammation as the full-length adiponectin was found to inhibit inflammation response in cells, while gAd promoted inflammation reaction in macrophage through activation of NF-κB signaling [179, 186, 187]. In support of this, gAd was found to activate NF-κB and promote pro-inflammatory cytokine production in macrophage, but full-length adiponectin exerts PI3K mediated anti-inflammatory effects to promote macrophage migration [188]. Kyoung-Hee et al. verified dual anti- and pro-inflammatory effects of adiponectin in macrophages, depending on divergent stimulation term [29, 58, 189]. It was suggested that the anti-inflammatory action of adiponectin in macrophage is mediated by its initial induction on inflammatory response and the subsequent tolerance to itself and to other pro-inflammatory signals [29, 172, 173]. In line with this, short-term treatment of macrophage with adiponectin initially increases TNF-alpha production, which in turn leads to increased expression of interleukin-10, autophagy induction, and an eventual decreasing of LPS-mediated production of inflammatory cytokine [29, 190], [191], [192]. Additional mechanism was proposed that adiponectin induced IRAK-M, an inactive isoform of the IRAK family of kinases, suppressing the production of proinflammatory mediators that are controlled by IRAK/TRAF6 signals [193]. A recent study showed that a lower concentration of adiponectin in women with the metabolic syndrome but not in men, suggesting that sex-specific regulation of adiponectin in systemic low-grade inflammation [194, 195].
Despite the downregulated adiponectin/adiponectin signaling in many metabolic diseases [196], higher adiponectin levels has been observed and could enhance inflammatory disorders such as preeclampsia [197], inflammatory bowel disease [198], rheumatoid arthritis [199, 200], multiple sclerosis [175], and chronic obstructive pulmonary disease [201], [202], [203], [204], [205] and heart failure with reduced ejection fraction (HFrEF) [206, 207]. Clinic studies revealed that increased serum adiponectin predicts the development of rheumatoid arthritis, especially in subjects with obesity [208], [209], [210]. In these inflammatory diseases, whether adiponectin persistently acts as a pro-inflammation factor or controls immune tolerance is still ambiguous [28, 211, 212]. The limitation of the studies on the pro-inflammatory action and immune tolerance of adiponectin is highly context-dependent and lack of establishment in vivo relevant in vitro systems. It is still not clear whether adiponectin exerts proinflammatory effects orchestrating the obesogenic function on the development of metabolic diseases. Thus, the detailed mechanisms underlying pro-inflammatory effects of adiponectin and its physiological significance under certain circumstances need to be further investigated (Figure 2).
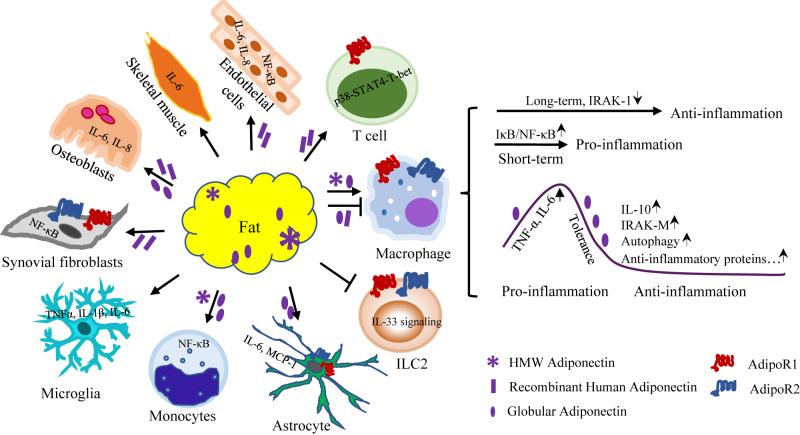
Figure 2:
Pro-inflammatory properties of adiponectin under certain circumstances. Adiponectin level in circulation is positively correlated with a number of inflammatory disorders such as preeclampsia, rheumatoid arthritis and chronic obstructive pulmonary disease. αUnder certain circumstances, adiponectin induces the expression of inflammatory mediators in skeletal muscle, immune cells and non-immune cells, via various mechanisms. In macrophage, adiponectin acts as anti- and pro-inflammatory factor, which is mediated by its initial induction on inflammatory response and the subsequent tolerance to its own stimulation and/or to other pro-inflammatory signals. NF-κB, nuclear factor-κB; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IL-33, interleukin-33; ILC2, group 2 innate lymphoid cells; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-α; IRAK-1, interleukin-1 receptor-associated kinase-1. ↑ refers to increase, while ↓ means decrease.
Summary
Adiponectin is an adipose tissue-derived hormone that mediates inter-organ communication [213]. Dysregulation of adiponectin production has been implicated in the development and progression of metabolic diseases. As an insulin sensitizer, adiponectin has been proved to improve insulin resistance and protect against metabolic syndrome. The inverse correlation of adiponectin with metabolic diseases in humans emerges adiponectin as a noninvasive biomarker for disease state, indicative of that upregulating adiponectin could be an effective approach to prevent and treat hypoadiponectinemia-associated diseases such as obesity and diabetes [214]. However, the obesogenic effect of adiponectin, reflecting the induction of adipogenesis and food intake and the inhibition of thermogenesis, has gained increased attention (Figure 3). Similar to PPARγ agonists TZDs, adiponectin likely drives the development of healthy obesity with improved adipose tissue fibrosis and insulin sensitivity [16] through distinct mechanisms which necessitate to be further clarified. The accumulated evidence also raises a concern that adiponectin-based therapeutics may bring adverse effects such as obesity and inflammation. On the other hand, the positive correlation between adiponectin and inflammatory disorders suggests that adiponectin is a diversified player in immune system. Upon binding to its receptors AdipoR1, AdipoR2, and T-cadherin, adiponectin targets a variety of immune cells and acts on both innate and acquired immunity. However, while it is well documented that adiponectin talks to metabolically active tissue-resident immune cells, whether and how adiponectin controls the development and function of immune organ remain largely unknown. Therefore, the study on adiponectin regulation of inflammation and energy metabolism may be a key, given the emerging recognition of the interplay between immune system and metabolism in health and disease.
Figure 3:
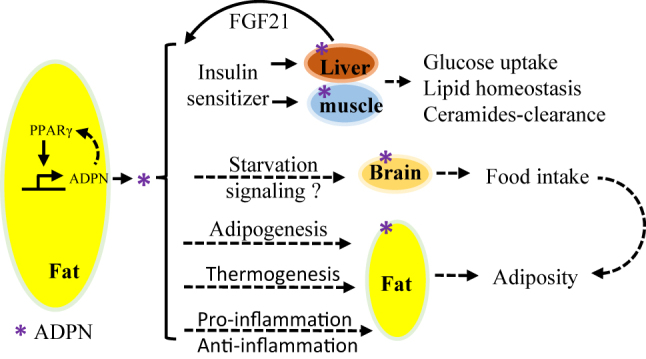
Adiponectin controls metabolic homeostasis via a variety of mechanisms. In addition to the insulin-sensitizing effect, adiponectin plays a critical role in regulating energy balance and inflammation. As a starvation signal, adiponectin promotes food intake and suppresses cold-induced thermogenesis, leading to positive energy balance and enhanced adipogenesis. This may interpret in part the obesogenic effect of adiponectin and potential side effects of adiponectin-based therapeutics. PPARγ, peroxisome proliferator-activated receptor-γ; ADPN, adiponectin; FGF21, fibroblast growth factor 21.
Footnotes
Research funding: This work was supported by an R01 Award (DK110439 PI: M.L.) from NIDDK, a grant (82100914) from National Natural Science Foundation of China, and a grant (2021M703639) from China Postdoctoral Science Foundation. The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Author contributions: LL drafted manuscript. LL and ML revised it. ML supervise this work. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: Not applicable.
Contributor Information
Liping Luo, Email: llp3253@163.com.
Meilian Liu, Email: meilianliu@salud.unm.edu.
References
- 1.Luo Y, Liu M. Adiponectin: a versatile player of innate immunity. J Mol Cell Biol. 2016;8:120–8. doi: 10.1093/jmcb/mjw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 4.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.m311113200. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 6.Haluzik M, Parizkova J, Haluzik MM. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res. 2004;53:123–9. [PubMed] [Google Scholar]
- 7.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang C, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargolzaei J, Chamani E, Kazemi T, Fallah S, Soori H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clin Biochem. 2018;54:1–10. doi: 10.1016/j.clinbiochem.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–52. doi: 10.1172/jci43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujishima Y, Maeda N, Matsuda K, Masuda S, Mori T, Fukuda S, et al. Adiponectin association with T-cadherin protects against neointima proliferation and atherosclerosis. FASEB J. 2017;31:1571–83. doi: 10.1096/fj.201601064r. [DOI] [PubMed] [Google Scholar]
- 11.Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, et al. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288:24886–97. doi: 10.1074/jbc.m113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–84. doi: 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parida S, Siddharth S, Sharma D. Adiponectin, obesity, and cancer: clash of the Bigwigs in health and disease. Int J Mol Sci. 2019;20:2519. doi: 10.3390/ijms20102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–79. doi: 10.1194/jlr.m400373-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/jci31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Luo Y, Luo L, Wu D, Ding X, Zheng H, et al. Adiponectin restrains ILC2 activation by AMPK-mediated feedback inhibition of IL-33 signaling. J Exp Med. 2021;218:e20191054. doi: 10.1084/jem.20191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab. 2014;28:25–31. doi: 10.1016/j.beem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 23.Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun. 2007;356:487–93. doi: 10.1016/j.bbrc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.m207198200. [DOI] [PubMed] [Google Scholar]
- 25.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.m300365200. [DOI] [PubMed] [Google Scholar]
- 26.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–10. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–6. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 28.Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci. 2020;21:1219. doi: 10.3390/ijms21041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282:21695–703. doi: 10.1074/jbc.m701419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–7. doi: 10.1074/jbc.m309469200. [DOI] [PubMed] [Google Scholar]
- 31.Arroyo-Jousse V, Jaramillo A, Castano-Moreno E, Lepez M, Carrasco-Negue K, Casanello P. Adipokines underlie the early origins of obesity and associated metabolic comorbidities in the offspring of women with pregestational obesity. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165558. doi: 10.1016/j.bbadis.2019.165558. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, He L, Gao M, Xiao F, Zhang F, Wang S, et al. Collagen beta(1-O) galactosyltransferase 2 deficiency contributes to lipodystrophy and aggravates NAFLD related to HMW adiponectin in mice. Metabolism. 2021;120:154777. doi: 10.1016/j.metabol.2021.154777. [DOI] [PubMed] [Google Scholar]
- 33.van Andel M, Heijboer AC, Drent ML. Adiponectin and its isoforms in Pathophysiology. Adv Clin Chem. 2018;85:115–47. doi: 10.1016/bs.acc.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 35.Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–702. doi: 10.1523/jneurosci.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang TT, Narendran P. The distribution of adiponectin receptors on human peripheral blood mononuclear cells. Ann N Y Acad Sci. 2008;1150:143–5. doi: 10.1196/annals.1447.021. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15–23. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Zelechowska P, Brzezinska-Blaszczyk E, Wiktorska M, Rozalska S, Wawrocki S, Kozlowska E, et al. Adipocytokines leptin and adiponectin function as mast cell activity modulators. Immunology. 2019;158:3–18. doi: 10.1111/imm.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–60. doi: 10.1074/jbc.m505311200. [DOI] [PubMed] [Google Scholar]
- 41.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 42.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–23. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 43.Ryu J, Galan AK, Xin X, Dong F, Abdul-Ghani MA, Zhou L, et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 2014;7:1227–38. doi: 10.1016/j.celrep.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anil Kumar S, Hima Kumari P, Shravan Kumar G, Mohanalatha C, Kavi Kishor PB. Osmotin: a plant sentinel and a possible agonist of mammalian adiponectin. Front Plant Sci. 2015;6:163. doi: 10.3389/fpls.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo MG, Kim MW, Jo MH, Bin Abid N, Kim MO. Adiponectin homolog osmotin, a potential anti-obesity compound, suppresses abdominal fat accumulation in C57BL/6 mice on high-fat diet and in 3T3-L1 adipocytes. Int J Obes. 2019;43:2422–33. doi: 10.1038/s41366-019-0383-3ra. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad A, Ali T, Kim MW, Khan A, Jo MH, Rehman SU, et al. Adiponectin homolog novel osmotin protects obesity/diabetes-induced NAFLD by upregulating AdipoRs/PPARalpha signaling in ob/ob and db/db transgenic mouse models. Metabolism. 2019;90:31–43. doi: 10.1016/j.metabol.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghoshal K, Bhattacharyya M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J Diabetes. 2015;6:151–66. doi: 10.4239/wjd.v6.i1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozaki KI, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016;310:E643–51. doi: 10.1152/ajpendo.00445.2015. [DOI] [PubMed] [Google Scholar]
- 50.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Basel) 2012;11:8–20. doi: 10.1007/bf03401534. [DOI] [PubMed] [Google Scholar]
- 51.Botta A, Liu Y, Wannaiampikul S, Tungtrongchitr R, Dadson K, Park TS, et al. An adiponectin-S1P axis protects against lipid induced insulin resistance and cardiomyocyte cell death via reduction of oxidative stress. Nutr Metab. 2019;16:14. doi: 10.1186/s12986-019-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–94. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, Sharma AX, et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab. 2017;6:267–75. doi: 10.1016/j.molmet.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, et al. CerS2 Haploinsufficiency inhibits beta-oxidation and Confers Susceptibility to diet-induced Steatohepatitis and insulin resistance. Cell Metab. 2014;20:919. doi: 10.1016/j.cmet.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Reibe-Pal S, Febbraio MA. Adiponectin serenades ceramidase to improve metabolism. Mol Metab. 2017;6:233–5. doi: 10.1016/j.molmet.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian L, Luo N, Zhu X, Chung BH, Garvey WT, Fu Y. Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis. 2012;221:66–75. doi: 10.1016/j.atherosclerosis.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–9. doi: 10.1074/jbc.m110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–60. doi: 10.1074/jbc.m109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicolas S, Cazareth J, Zarif H, Guyon A, Heurteaux C, Chabry J, et al. Globular adiponectin limits microglia pro-inflammatory phenotype through an AdipoR1/NF-kappaB signaling pathway. Front Cell Neurosci. 2017;11:352. doi: 10.3389/fncel.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu L, Tu Q, Han Q, Zhang L, Sui L, Zheng L, et al. Adiponectin regulates bone marrow mesenchymal stem cell niche through a unique signal transduction pathway: an approach for treating bone disease in diabetes. Stem Cell. 2015;33:240–52. doi: 10.1002/stem.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, et al. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–92. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- 64.Parker-Duffen JL, Nakamura K, Silver M, Zuriaga MA, MacLauchlan S, Aprahamian TR, et al. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. J Biol Chem. 2014;289:16200–13. doi: 10.1074/jbc.m114.548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Mendonca M, Dos Santos BAC, de Sousa E, Rodrigues AC. Adiponectin is required for pioglitazone-induced improvements in hepatic steatosis in mice fed a high-fat diet. Mol Cell Endocrinol. 2019;493:110480. doi: 10.1016/j.mce.2019.110480. [DOI] [PubMed] [Google Scholar]
- 66.Fukuda S, Kita S, Obata Y, Fujishima Y, Nagao H, Masuda S, et al. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J Biol Chem. 2017;292:7840–9. doi: 10.1074/jbc.m117.780734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuda K, Fujishima Y, Maeda N, Mori T, Hirata A, Sekimoto R, et al. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology. 2015;156:934–46. doi: 10.1210/en.2014-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M, et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight. 2018;3:e99680. doi: 10.1172/jci.insight.99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung CM, Lin TH, Chen JW, Leu HB, Yang HC, Ho HY, et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes. 2011;60:2417–23. doi: 10.2337/db10-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fava C, Danese E, Montagnana M, Sjogren M, Almgren P, Guidi GC, et al. A variant upstream of the CDH13 adiponectin receptor gene and metabolic syndrome in Swedes. Am J Cardiol. 2011;108:1432–7. doi: 10.1016/j.amjcard.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 71.Gao H, Kim YM, Chen P, Igase M, Kawamoto R, Kim MK, et al. Genetic variation in CDH13 is associated with lower plasma adiponectin levels but greater adiponectin sensitivity in East Asian populations. Diabetes. 2013;62:4277–83. doi: 10.2337/db13-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jee SH, Sull JW, Lee JE, Shin C, Park J, Kimm H, et al. Adiponectin concentrations: a genome-wide association study. Am J Hum Genet. 2010;87:545–52. doi: 10.1016/j.ajhg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morisaki H, Yamanaka I, Iwai N, Miyamoto Y, Kokubo Y, Okamura T, et al. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum Mutat. 2012;33:402–10. doi: 10.1002/humu.21652. [DOI] [PubMed] [Google Scholar]
- 74.Wu Y, Li Y, Lange EM, Croteau-Chonka DC, Kuzawa CW, McDade TW, et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet. 2010;19:4955–64. doi: 10.1093/hmg/ddq423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/hypertensionaha.107.099424. [DOI] [PubMed] [Google Scholar]
- 76.Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22:279–90. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka Y, Kita S, Nishizawa H, Fukuda S, Fujishima Y, Obata Y, et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci Rep. 2019;9:16. doi: 10.1038/s41598-018-37115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masuda S, Fujishima Y, Maeda N, Tsugawa-Shimizu Y, Nakamura Y, Tanaka Y, et al. Impact of glycosylphosphatidylinositol-specific phospholipase D on hepatic diacylglycerol accumulation, steatosis, and insulin resistance in diet-induced obesity. Am J Physiol Endocrinol Metab. 2019;316:E239–50. doi: 10.1152/ajpendo.00319.2018. [DOI] [PubMed] [Google Scholar]
- 79.Kalkman HO. An Explanation for the adiponectin paradox. Pharmaceuticals. 2021;14:1266. doi: 10.3390/ph14121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Banerjee A, Khemka VK, Roy D, Poddar J, Roy TKS, Karnam SA. Role of serum adiponectin and Vitamin D in Prediabetes and diabetes Mellitus. Can J Diabetes. 2017;41:259–65. doi: 10.1016/j.jcjd.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Kashiwagi R, Yamada Y, Ito Y, Mitsui Y, Sakaue T, Iwamoto R, et al. Increase in adiponectin level prevents the development of type 2 diabetes in Japanese men with low adiponectin levels. J Endocr Soc. 2018;2:753–64. doi: 10.1210/js.2018-00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/s0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 83.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51:1884–8. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 84.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 85.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 86.Xia JY, Sun K, Hepler C, Ghaben AL, Gupta RK, An YA, et al. Acute loss of adipose tissue-derived adiponectin triggers immediate metabolic deterioration in mice. Diabetologia. 2018;61:932–41. doi: 10.1007/s00125-017-4516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 88.Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, et al. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358–70. doi: 10.2337/diabetes.54.12.3358. [DOI] [PubMed] [Google Scholar]
- 89.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–7. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B, et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9:272. doi: 10.1038/s41467-017-02677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li N, Zhao S, Zhang Z, Zhu Y, Gliniak CM, Vishvanath L, et al. Adiponectin preserves metabolic fitness during aging. Elife. 2021;10:e65108. doi: 10.7554/elife.65108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 93.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–81. doi: 10.1172/jci14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 95.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.m209033200. [DOI] [PubMed] [Google Scholar]
- 96.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51–6. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- 97.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–12. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Li X, Zhang D, Vatner DF, Goedeke L, Hirabara SM, Zhang Y, et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci USA. 2020;117:32584–93. doi: 10.1073/pnas.1922169117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, et al. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol. 2008;295:C203–12. doi: 10.1152/ajpcell.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y, Sweeney G. Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab. 2014;28:33–41. doi: 10.1016/j.beem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Krause MP, Milne KJ, Hawke TJ. Adiponectin-consideration for its role in skeletal muscle health. Int J Mol Sci. 2019;20:1528. doi: 10.3390/ijms20071528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2009;425:41–52. doi: 10.1042/bj20091045. [DOI] [PubMed] [Google Scholar]
- 103.Guo Q, Chang B, Yu QL, Xu ST, Yi XJ, Cao SC. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1alpha. Diabetologia. 2020;63:2675–88. doi: 10.1007/s00125-020-05269-3. [DOI] [PubMed] [Google Scholar]
- 104.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 105.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64:36–48. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- 107.Ahlstrom P, Rai E, Chakma S, Cho HH, Rengasamy P, Sweeney G. Adiponectin improves insulin sensitivity via activation of autophagic flux. J Mol Endocrinol. 2017;59:339–50. doi: 10.1530/jme-17-0096. [DOI] [PubMed] [Google Scholar]
- 108.Kim B, Kim MS, Hyun CK. Syringin attenuates insulin resistance via adiponectin-mediated suppression of low-grade chronic inflammation and ER stress in high-fat diet-fed mice. Biochem Biophys Res Commun. 2017;488:40–5. doi: 10.1016/j.bbrc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 109.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–5. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rocchi A, He C. Regulation of exercise-induced autophagy in skeletal muscle. Curr Pathobiol Rep. 2017;5:177–86. doi: 10.1007/s40139-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity. 2008;16:241–56. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- 112.Yang W, Liu L, Wei Y, Fang C, Zhou F, Chen J, et al. Exercise ameliorates the FGF21-adiponectin axis impairment in diet-induced obese mice. Endocr Connect. 2019;8:596–604. doi: 10.1530/ec-19-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pandey GK, Vadivel S, Raghavan S, Mohan V, Balasubramanyam M, Gokulakrishnan K. High molecular weight adiponectin reduces glucolipotoxicity-induced inflammation and improves lipid metabolism and insulin sensitivity via APPL1-AMPK-GLUT4 regulation in 3T3-L1 adipocytes. Atherosclerosis. 2019;288:67–75. doi: 10.1016/j.atherosclerosis.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 114.Steinberg GR, Kemp BE. Adiponectin: starving for attention. Cell Metab. 2007;6:3–4. doi: 10.1016/j.cmet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 115.Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014;15:149–56. doi: 10.1007/s11154-013-9283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–59. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 117.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feizollahzadeh S, Rasuli J, Kheirouri S, Alizadeh M. Augmented plasma adiponectin after prolonged fasting during ramadan in men. Health Promot Perspect. 2014;4:77–81. doi: 10.5681/hpp.2014.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. 2010;21:188–95. doi: 10.1016/j.jnutbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–7. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dostalova I, Smitka K, Papezova H, Kvasnickova H, Nedvidkova J. Increased insulin sensitivity in patients with anorexia nervosa: the role of adipocytokines. Physiol Res. 2007;56:587–94. doi: 10.33549/physiolres.931089. [DOI] [PubMed] [Google Scholar]
- 122.Agra RM, Fernandez-Trasancos A, Diaz-Rodriguez E, Cordero A, Varela-Roman A, Gomez-Otero I, et al. Nutrients restriction upregulates adiponectin in epicardial or subcutaneous adipose tissue: impact in de novo heart failure patients. Int J Med Sci. 2018;15:417–24. doi: 10.7150/ijms.22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnea M, Madar Z, Froy O. High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue. Obesity. 2010;18:230–8. doi: 10.1038/oby.2009.276. [DOI] [PubMed] [Google Scholar]
- 124.Tang N, Zhang X, Chen D, Li Z. The controversial role of adiponectin in appetite regulation of animals. Nutrients. 2021;13:3387. doi: 10.3390/nu13103387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–22. doi: 10.1074/jbc.m402367200. [DOI] [PubMed] [Google Scholar]
- 126.Quaresma PG, Reencober N, Zanotto TM, Santos AC, Weissmann L, de Matos AH, et al. Pioglitazone treatment increases food intake and decreases energy expenditure partially via hypothalamic adiponectin/adipoR1/AMPK pathway. Int J Obes. 2016;40:138–46. doi: 10.1038/ijo.2015.134. [DOI] [PubMed] [Google Scholar]
- 127.Kmiec Z, Pokrywka L, Kotlarz G, Kubasik J, Szutowicz A, Mysliwski A. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology. 2005;51:357–62. doi: 10.1159/000088698. [DOI] [PubMed] [Google Scholar]
- 128.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 129.Sun J, Gao Y, Yao T, Huang Y, He Z, Kong X, et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol Metab. 2016;5:882–91. doi: 10.1016/j.molmet.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suyama S, Maekawa F, Maejima Y, Kubota N, Kadowaki T, Yada T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci Rep. 2016;6:30796. doi: 10.1038/srep30796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA. 2014;111:15810–5. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bauche IB, El Mkadem SA, Pottier AM, Senou M, Many MC, Rezsohazy R, et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology. 2007;148:1539–49. doi: 10.1210/en.2006-0838. [DOI] [PubMed] [Google Scholar]
- 133.Masaki T, Chiba S, Yasuda T, Tsubone T, Kakuma T, Shimomura I, et al. Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes. 2003;52:2266–73. doi: 10.2337/diabetes.52.9.2266. [DOI] [PubMed] [Google Scholar]
- 134.Wei Q, Lee JH, Wang H, Bongmba OYN, Wu CS, Pradhan G, et al. Adiponectin is required for maintaining normal body temperature in a cold environment. BMC Physiol. 2017;17:8. doi: 10.1186/s12899-017-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013;17:901–15. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo L, Wang L, Luo Y, Romero E, Yang X, Liu M. Glucocorticoid/adiponectin Axis mediates full activation of cold-induced beige fat thermogenesis. Biomolecules. 2021;11:1573. doi: 10.3390/biom11111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qiao L, Yoo H, Bosco C, Lee B, Feng GS, Schaack J, et al. Adiponectin reduces thermogenesis by inhibiting brown adipose tissue activation in mice. Diabetologia. 2014;57:1027–36. doi: 10.1007/s00125-014-3180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saito K, Arata S, Hosono T, Sano Y, Takahashi K, Choi-Miura NH, et al. Adiponectin plays an important role in efficient energy usage under energy shortage. Biochim Biophys Acta. 2006;1761:709–16. doi: 10.1016/j.bbalip.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 139.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, et al. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–61. doi: 10.1074/jbc.c200362200. [DOI] [PubMed] [Google Scholar]
- 140.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–9. doi: 10.1056/nejm199810013391403. [DOI] [PubMed] [Google Scholar]
- 141.Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–7. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 142.Martella E, Bellotti C, Dozza B, Perrone S, Donati D, Lucarelli E. Secreted adiponectin as a marker to evaluate in vitro the adipogenic differentiation of human mesenchymal stromal cells. Cytotherapy. 2014;16:1476–85. doi: 10.1016/j.jcyt.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 143.Shan T, Liu W, Kuang S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013;27:277–87. doi: 10.1096/fj.12-211516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang W, Yang C, Luo J, Wei Y, Wang W, Zhong Y. Adiponectin promotes preadipocyte differentiation via the PPARgamma pathway. Mol Med Rep. 2018;17:428–35. doi: 10.3892/mmr.2017.7881. [DOI] [PubMed] [Google Scholar]
- 145.Abbott MJ, Roth TM, Ho L, Wang L, O’Carroll D, Nissenson RA. Negative skeletal effects of locally produced adiponectin. PLoS One. 2015;10:e0134290. doi: 10.1371/journal.pone.0134290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690–9. doi: 10.1016/j.ajpath.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Astapova O, Leff T. Adiponectin and PPARgamma: cooperative and interdependent actions of two key regulators of metabolism. Vitam Horm. 2012;90:143–62. doi: 10.1016/b978-0-12-398313-8.00006-3. [DOI] [PubMed] [Google Scholar]
- 148.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 149.Siersbaek R, Nielsen R, Mandrup S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010;584:3242–9. doi: 10.1016/j.febslet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 150.Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord. 2003;27:147–61. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 151.Azrad M, Gower BA, Hunter GR, Nagy TR. Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine. 2013;43:586–92. doi: 10.1007/s12020-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma XL, et al. Role of adipokines in cardiovascular disease. Circ J. 2017;81:920–8. doi: 10.1253/circj.cj-17-0458. [DOI] [PubMed] [Google Scholar]
- 154.Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–75. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–81. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231:R77–99. doi: 10.1530/joe-16-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Piccio L, Cantoni C, Henderson JG, Hawiger D, Ramsbottom M, Mikesell R, et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol. 2013;43:2089–100. doi: 10.1002/eji.201242836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 159.Yamamoto R, Ueki S, Moritoki Y, Kobayashi Y, Oyamada H, Konno Y, et al. Adiponectin attenuates human eosinophil adhesion and chemotaxis: implications in allergic inflammation. J Asthma. 2013;50:828–35. doi: 10.3109/02770903.2013.816725. [DOI] [PubMed] [Google Scholar]
- 160.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. doi: 10.1182/blood.v96.5.1723.h8001723_1723_1732. [DOI] [PubMed] [Google Scholar]
- 161.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–5. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 162.Luo N, Chung BH, Wang X, Klein RL, Tang CK, Garvey WT, et al. Enhanced adiponectin actions by overexpression of adiponectin receptor 1 in macrophages. Atherosclerosis. 2013;228:124–35. doi: 10.1016/j.atherosclerosis.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramos-Ramirez P, Malmhall C, Tliba O, Radinger M, Bossios A. Adiponectin/AdipoR1 Axis promotes IL-10 release by human regulatory T cells. Front Immunol. 2021;12:677550. doi: 10.3389/fimmu.2021.677550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boursereau R, Abou-Samra M, Lecompte S, Noel L, Brichard SM. New targets to alleviate skeletal muscle inflammation: role of microRNAs regulated by adiponectin. Sci Rep. 2017;7:43437. doi: 10.1038/srep43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ryu J, Hadley JT, Li Z, Dong F, Xu H, Xin X, et al. Adiponectin alleviates diet-induced inflammation in the liver by suppressing MCP-1 expression and macrophage infiltration. Diabetes. 2021;70:1303–16. doi: 10.2337/db20-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem. 2012;287:36896–904. doi: 10.1074/jbc.m112.409516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ding X, Luo Y, Zhang X, Zheng H, Yang X, Yang X, et al. IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J Endocrinol. 2016;231:35–48. doi: 10.1530/joe-16-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.cir.0000127953.98131.ed. [DOI] [PubMed] [Google Scholar]
- 169.Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007;179:5483–92. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- 170.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–62. doi: 10.1074/jbc.c200312200. [DOI] [PubMed] [Google Scholar]
- 171.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/jci200317797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Folco EJ, Rocha VZ, Lopez-Ilasaca M, Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem. 2009;284:25569–75. doi: 10.1074/jbc.m109.019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254–63. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 174.Haugen F, Drevon CA. Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology. 2007;148:5478–86. doi: 10.1210/en.2007-0370. [DOI] [PubMed] [Google Scholar]
- 175.Nyirenda MH, Fadda G, Healy LM, Mexhitaj I, Poliquin-Lasnier L, Hanwell H, et al. Pro-inflammatory adiponectin in pediatric-onset multiple sclerosis. Mult Scler. 2021;27:1948–59. doi: 10.1177/1352458521989090. [DOI] [PubMed] [Google Scholar]
- 176.Kitahara K, Kusunoki N, Kakiuchi T, Suguro T, Kawai S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2009;378:218–23. doi: 10.1016/j.bbrc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 177.Tomizawa A, Hattori Y, Kasai K. Induction of gene expression in response to globular adiponectin in vascular endothelial cells. Life Sci. 2009;85:457–61. doi: 10.1016/j.lfs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 178.Sanz-Garcia C, Nagy LE, Lasuncion MA, Fernandez M, Alemany S. Cot/tpl2 participates in the activation of macrophages by adiponectin. J Leukoc Biol. 2014;95:917–30. doi: 10.1189/jlb.0913486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wan Z, Mah D, Simtchouk S, Klegeris A, Little JP. Globular adiponectin induces a pro-inflammatory response in human astrocytic cells. Biochem Biophys Res Commun. 2014;446:37–42. doi: 10.1016/j.bbrc.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 180.Surendar J, Frohberger SJ, Karunakaran I, Schmitt V, Stamminger W, Neumann AL, et al. Adiponectin limits IFN-gamma and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front Immunol. 2019;10:2555. doi: 10.3389/fimmu.2019.02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Diniz TA, Aquino Junior JCJ, Mosele FC, Cabral-Santos C, Lima Junior EA, Teixeira AAS, et al. Exercise-induced AMPK activation and IL-6 muscle production are disturbed in adiponectin knockout mice. Cytokine. 2019;119:71–80. doi: 10.1016/j.cyto.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 182.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang K, Tai Z, Han Q, Pang Y, Li Q. Adiponectin as inducer of inflammatory and apoptosis involving in immune defense in lamprey. Fish Shellfish Immunol. 2019;90:446–55. doi: 10.1016/j.fsi.2019.04.045. [DOI] [PubMed] [Google Scholar]
- 187.Zhang R, Wu J, Liu D, Shan H, Zhang J. Anti-inflammatory effect of full-length adiponectin and proinflammatory effect of globular adiponectin in esophageal adenocarcinoma cells. Oncol Res. 2013;21:15–21. doi: 10.3727/096504013x13786659070235. [DOI] [PubMed] [Google Scholar]
- 188.Jin X, Wang Y. Mechanisms of adiponectin in regulation of proinflammatory cytokine production and migration in macrophages. J Inflamm Res. 2021;14:981–93. doi: 10.2147/jir.s292137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee KH, Jeong J, Woo J, Lee CH, Yoo CG. Globular adiponectin exerts a pro-inflammatory effect via IkappaB/NF-kappaB pathway activation and anti-inflammatory effect by IRAK-1 downregulation. Mol Cells. 2018;41:762–70. doi: 10.14348/molcells.2018.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;(1 Suppl)23:S50–3. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 191.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–9. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pun NT, Subedi A, Kim MJ, Park PH. Globular adiponectin causes tolerance to LPS-induced TNF-alpha expression via autophagy induction in RAW 264.7 macrophages: involvement of SIRT1/FoxO3A Axis. PLoS One. 2015;10:e0124636. doi: 10.1371/journal.pone.0124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 194.Ahonen T, Saltevo J, Laakso M, Kautiainen H, Kumpusalo E, Vanhala M. Gender differences relating to metabolic syndrome and proinflammation in Finnish subjects with elevated blood pressure. Mediators Inflamm. 2009;2009:959281. doi: 10.1155/2009/959281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ter Horst R, van den Munckhof ICL, Schraa K, Aguirre-Gamboa R, Jaeger M, Smeekens SP, et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol. 2020;40:1787–800. doi: 10.1161/atvbaha.120.314508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Straub LG, Scherer PE. Metabolic Messengers: adiponectin. Nat Metab. 2019;1:334–9. doi: 10.1038/s42255-019-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Adu-Gyamfi EA, Fondjo LA, Owiredu W, Czika A, Nelson W, Lamptey J, et al. The role of adiponectin in placentation and preeclampsia. Cell Biochem Funct. 2020;38:106–17. doi: 10.1002/cbf.3458. [DOI] [PubMed] [Google Scholar]
- 198.Peng YJ, Shen TL, Chen YS, Mersmann HJ, Liu BH, Ding ST. Adiponectin and adiponectin receptor 1 overexpression enhance inflammatory bowel disease. J Biomed Sci. 2018;25:24. doi: 10.1186/s12929-018-0419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Szumilas K, Szumilas P, Sluczanowska-Glabowska S, Zgutka K, Pawlik A. Role of adiponectin in the Pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2020;21:8265. doi: 10.3390/ijms21218265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Y, Peltonen M, Andersson-Assarsson JC, Svensson PA, Herder C, Rudin A, et al. Elevated adiponectin predicts the development of rheumatoid arthritis in subjects with obesity. Scand J Rheumatol. 2020;49:452–60. doi: 10.1080/03009742.2020.1753808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chan KH, Yeung SC, Yao TJ, Ip MS, Cheung AH, Chan-Yeung MM, et al. , C. S. G. o. t. H. K. T. Society Elevated plasma adiponectin levels in patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2010;14:1193–200. [PubMed] [Google Scholar]
- 202.Kirdar S, Serter M, Ceylan E, Sener AG, Kavak T, Karadag F. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest. 2009;69:219–24. doi: 10.1080/00365510802474400. [DOI] [PubMed] [Google Scholar]
- 203.Lin YH, Jiang TX, Hu SX, Shi YH. Association between serum adiponectin concentrations and chronic obstructive pulmonary disease: a meta-analysis. Biosci Rep. 2020;40:BSR20192234. doi: 10.1042/BSR20192234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miller M, Pham A, Cho JY, Rosenthal P, Broide DH. Adiponectin-deficient mice are protected against tobacco-induced inflammation and increased emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299:L834–42. doi: 10.1152/ajplung.00326.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yoon HI, Li Y, Man SFP, Tashkin D, Wise RA, Connett JE, et al. The complex relationship of serum adiponectin to COPD outcomes COPD and adiponectin. Chest. 2012;142:893–9. doi: 10.1378/chest.11-2173. [DOI] [PubMed] [Google Scholar]
- 206.Jang AY, Scherer PE, Kim JY, Lim S, Koh KK. Adiponectin and cardiometabolic trait and mortality: where do we go? Cardiovasc Res. 2021:cvab199. doi: 10.1093/cvr/cvab199. [DOI] [PubMed] [Google Scholar]
- 207.Sente T, Gevaert A, Van Berendoncks A, Vrints CJ, Hoymans VY. The evolving role of adiponectin as an additive biomarker in HFrEF. Heart Fail Rev. 2016;21:753–69. doi: 10.1007/s10741-016-9578-z. [DOI] [PubMed] [Google Scholar]
- 208.Minamino H, Katsushima M, Yoshida T, Hashimoto M, Fujita Y, Shirakashi M, et al. Increased circulating adiponectin is an independent disease activity marker in patients with rheumatoid arthritis: a cross-sectional study using the KURAMA database. PLoS One. 2020;15:e0229998. doi: 10.1371/journal.pone.0229998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang Y, Aldridge J, Vasileiadis GK, Edebo H, Ekwall AH, Lundell AC, et al. Recombinant adiponectin induces the production of pro-inflammatory Chemokines and cytokines in circulating mononuclear cells and fibroblast-like Synoviocytes from non-Inflamed subjects. Front Immunol. 2020;11:569883. doi: 10.3389/fimmu.2020.569883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang Y, Johansson L, Andersson-Assarsson J, Taube M, Peltonen M, Svensson PA, et al. Adiponectin Associates with rheumatoid arthritis risk in Overweight and obesity independently of other adipokines. J Clin Med. 2021;10:2791. doi: 10.3390/jcm10132791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Esmaili S, Xu A, George J. The multifaceted and controversial immunometabolic actions of adiponectin. Trends Endocrinol Metab. 2014;25:444–51. doi: 10.1016/j.tem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 212.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–30. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 213.Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol. 2016;8:101–9. doi: 10.1093/jmcb/mjw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hossain MM, Mukheem A, Kamarul T. The prevention and treatment of hypoadiponectinemia-associated human diseases by up-regulation of plasma adiponectin. Life Sci. 2015;135:55–67. doi: 10.1016/j.lfs.2015.03.010. [DOI] [PubMed] [Google Scholar]