Abstract
Several dengue (DEN) virus vaccines are in development; however, the lack of a reliable small animal model in which to test them is a major obstacle. Because evidence suggests that interferon (IFN) is involved in the human anti-DEN virus response, we tested mice deficient in their IFN functions as potential models. Intraperitoneally administered mouse-adapted DEN 2 virus was uniformly lethal in AG129 mice (which lack alpha/beta IFN and gamma IFN receptor genes), regardless of age. Immunized mice were protected from virus challenge, and survival times increased following passive transfer of anti-DEN polyclonal antibody. These results demonstrate that AG129 mice are a promising small animal model for DEN virus vaccine trials.
After malaria, dengue (DEN) is the most important emerging tropical infectious disease. Worldwide it is estimated that 100 million cases of DEN fever occur each year (8). The severe form of the disease, DEN hemorrhagic fever-dengue shock syndrome (DHF/DSS), is one of the most important causes of hospitalization and death among children in Asia. The average case-fatality rate for DHF is 5% when appropriate supportive treatment is given. DEN viruses (serotypes 1 through 4) are mosquito borne and are the causative agents of these diseases. Since the description of DEN etiology in 1944 (27), considerable effort has been put into vaccine development (5, 23); however, significant difficulties have been encountered. Because antibody-dependent enhancement has been associated with DHF/DSS, a tetravalent vaccine is preferable. DEN virus is not normally pathogenic in mice; therefore, an appropriate and useful small animal model has been lacking, despite numerous attempts at development (24). Evidence exists that alpha and beta interferons (IFN-α/β) and gamma IFN (IFN-γ) might be involved in human DEN infections (16, 18). In addition, exogenously administered IFN appears to protect mice from DEN virus challenge (2). This information suggested to us that mice defective in their IFN response might provide a suitable model for DEN virus infection.
Mice with a 129Sv(ev) background that had deficiencies in IFN responses were developed previously (28). When these mice were used, IFN-α/β was found to be important in the regulation of vesicular stomatitis virus and Semliki Forest virus replication (22). IFN-γ-deficient mice had increased susceptibility to tuberculosis (3), whereas the functions of IFN-α/β and IFN-γ in combination appeared to be critical for antiviral defense against Theiler’s (6), vaccinia, and lymphocytic choriomeningitis viruses (28). To determine if any of the IFN transgenic mouse strains were susceptible to DEN virus infection, mice deficient for IFN-α/β and -γ receptors in combination (AG129), as well as mice lacking IFN-α/β receptors only (A129) and their wild-type counterparts (WT129), were obtained from B & K Universal, Hull, United Kingdom. Mice without the ability to synthesize IFN-γ (GKO) (3) and BALB/c controls were also acquired, as a gift. Initially, groups of five AG129 and five WT129 mice aged 4, 6, 8, and 12 weeks old were inoculated intraperitoneally (i.p.) with 106 PFU of a mouse-adapted DEN 2 virus strain, New Guinea C. AG129 mice of all age groups began to exhibit neurological abnormalities, including hind-leg paralysis and blindness, around day 7 after inoculation, and mice were dead by day 12. In contrast, none of the WT129 mice showed symptoms. To ascertain whether a smaller challenge dose was appropriate for use in adult mice, 10-fold dilutions of virus were introduced i.p. into 8-week-old AG129 mice. Survival times increased as the viral load decreased and ranged from 10 days (106 PFU) to 28 days (103 PFU). For experimental convenience and maximum stringency, a challenge dose of 106 PFU was used in subsequent studies.
To determine which aspect of the IFN response was critical in protecting these mice from DEN virus infection, animals individually deficient in either IFN-α/β (A129) or IFN-γ (GKO) functions as well as BALB/c controls were subjected to a similar DEN virus challenge. None of these mice exhibited any overt symptoms of illness, indicating that for DEN virus infection, IFN-α, -β, and -γ abnormalities in combination were necessary for the mouse-adapted virus to be lethal when the i.p. challenge route was used. The remote possibility exists that IFN-γ-deficient mice with the 129Sv(ev) genetic background differ from the GKO mice used in this study with respect to their susceptibility to DEN 2 virus. Tissue culture-passaged DEN 2 strain 16681 failed to kill 8-week-old AG129 mice (data not shown), suggesting that mouse adaptation was necessary for DEN virus to be lethal.
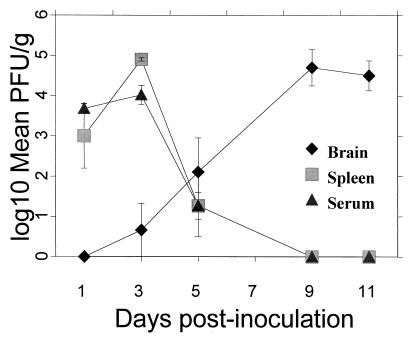
Viremia was monitored in groups of five DEN virus-infected 6-week-old AG129 mice each and in groups of two WT129 mice each. Plaque titrations of virus recovered from the brain, spleen, and serum were performed (Fig. 1) following inoculation with 106 PFU of mouse-adapted DEN virus. Samples were taken at days 1, 3, 5, and 9 and at days 10 to 12 after inoculation and homogenized with bovine albumin–phosphate-buffered saline (PBS) medium and sterile sand by using a Bellco grinder. Homogenates were centrifuged at 1,500 × g for 10 min, decanted, and adjusted to make 10% suspensions. Processed tissues were serially diluted and adsorbed for 45 min onto Vero cell monolayers in six-well plates. Infected cells were overlaid with a mixture containing 1% noble agar, 1× medium 199, 1% fetal bovine serum, and 0.75% DEAE dextran. Plates were incubated at 37°C for 7 days, after which 2.5 ml of a second overlay, containing the ingredients of the first overlay with neutral red added to a final concentration of 0.4%, was added. Plaque formation was monitored for the next 5 days. Virus levels in the serum and spleen of the AG129 mice peaked at day 3 and dropped sharply thereafter. Low levels of virus persisted until day 9 in the serum and until day 12 in the spleen. Viral titers in the brain rose logarithmically throughout the period of infection until the mice died, at days 10 to 12. Virus was not found in any of these three tissue types for the WT129 control mice. Spleen and serum virus titers within the groups of AG129 mice were fairly consistent. Levels of virus in the brain exhibited greater variability within the groups, fluctuating as much as 2 log units for any time point. However, by days 10 to 12, a demonstrable amount of virus was isolated from the brain tissue of all animals. The weights in grams of the spleens and brains were recorded, and while the brains retained consistent weights in both AG129 and WT129 mice, the spleens in the AG129 mice were considerably enlarged on days 3 and 5 after inoculation (data not shown). A retrospective virus titration was also performed on day 3 serum samples taken from groups of AG129 mice that were similarly infected with 106 PFU of DEN 2 16681 or its vaccine derivative, PDK-53 (13). Day 3 mean counts were 3 × 103 PFU/g (PFU/ml) and 1 × 102 PFU/g for these viruses, respectively, and the standard errors were calculated (data not shown). Therefore, while DEN 2 viruses 16681 and PDK-53 were nonlethal, they were shown to replicate in the AG129 mice. Evidence exists that a breakdown in the blood-brain barrier occurs in DEN 2 virus-inoculated adult Swiss albino mice (1); however, the study showed that virus could be detected only in the brains of intracranially (i.c.)-inoculated animals. Severe combined immunodeficient (SCID) mice that were reconstituted with human peripheral blood lymphocytes have also been evaluated as an animal model for DEN virus infection (29). Even in SCID mice, DEN 1 virus could be detected very rarely in the brain. Hotta et al. (9) showed that DEN 1 virus (strain Mochizuki) was present in the tissues of nu/nu and nu/+ BALB/c mice following peripheral inoculation. Nevertheless, viremias were absent, and mortality rates were only 40 to 60%. The investigation described here is the first instance in which DEN virus has been both 100% lethal in mice and consistently present in the sera and tissues following i.p. introduction into mice.
FIG. 1.
Analysis of mouse-adapted DEN 2 virus infection in AG129 mice. Groups of five AG129 mice each and groups of two WT129 mice each for each sample day were i.p. inoculated with 106 PFU of a mouse-adapted DEN 2 virus strain, New Guinea C. Mice were bled and sacrificed at days 1, 3, 5, and 9 and at days 10 to 12 (represented by day 11) postinfection. The log10 geometric mean number of plaques for the AG129 mice only were plotted. For sera, calculations were performed on the basis of 1 ml being equivalent to 1 g. Each error bar represents 1 standard error above and below the mean. No virus was detected in the tissues of WT129 mice during the experiment.
To confirm whether the AG129 mice could function as a model for DEN vaccine testing, we intraperitoneally inoculated groups of 8-week-old animals at 2-week intervals with either one or two doses of 105 PFU DEN 2 16681 or the vaccine derivative PDK-53. After a period of 14 days after the final immunizing dose, mice were bled and then were challenged with 106 PFU of mouse-adapted DEN virus. Survivors were bled 28 days after challenge. Control groups of WT129 mice were similarly immunized. In addition, a control group consisting of AG129 mice was inoculated with two doses of PBS prior to virus challenge. Antibody production was evaluated by using a plaque-reduction neutralization test (PRNT) (4) and an enzyme-linked immunosorbent assay (ELISA) (25) (Table 1). AG129 mice immunized with DEN 2 16681 produced significantly higher neutralizing antibody and ELISA titers than mice immunized with PDK-53, and the difference was especially noticeable after a single inoculation. This was consistent with the data indicating that the 16681 strain replicates more efficiently than the vaccine derivative in these mice. The 90% PRNT values revealed an appreciable increase in titer in response to both immunizing viruses after a second dose. The WT129 mice responded minimally to immunization. Group survival rates were 100% with the exceptions of the AG129 mice that received a single dose of PDK-53 and the PBS-inoculated (control) group. When PRNT data acquired before and after challenge were compared, slight increases in the titers of the AG129 mice immunized with DEN 2 16681 were observed. By contrast, the challenge virus induced significantly elevated PRNT titers in the PDK-53-immunized AG129 mice. Greater than fourfold increases in titer were observed at the 50% PRNT level after one immunizing dose and at the 90% PRNT level after one or two doses. No significant differences were noted between pre- and postchallenge ELISA titers in any group. Levels of neutralizing antibody increased substantially in the survivors after two inoculations, indicating the greater priming efficiency of the two-dose schedule. The mouse-adapted DEN virus elicited an immune response in all the WT129 mice, even though it was neither neurovirulent nor lethal.
TABLE 1.
Log geometric mean immunization and challenge titers of AG129 and WT129 mice with DEN 2 strains
| Immunogen | Strain of mouse | No. of inoculations | Before challenge
|
No. of survivors/n | After challenge
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean PRNT titer
|
Mean ELISA titer (SE) | Mean PRNT titer
|
Mean ELISA titer (SE) | ||||||
| 50% (SE) | 90% (SE) | 50% (SE) | 90% (SE) | ||||||
| DEN2 | AG129 | 1 | 2.32 (0.07) | 1.54 (0.14) | 3.93 (0.07) | 5/5 | 2.51 (0.09) | 2.08 (0.07) | 3.69 (0.07) |
| 16681 | 2 | 2.39 (0.07) | 2.02 (0.12) | 3.87 (0.11) | 5/5 | 3.05 (0.06) | 2.32 (0.07) | 3.63 (0.07) | |
| WT129 | 1 | <0.70 (0) | <0.70 (0) | <2.00 (0) | 2/2 | 2.51 (0) | 1.60 (0) | 2.90 (0) | |
| 2 | <0.70 (0) | <0.70 (0) | 2.45 (0.15) | 2/2 | 2.51 (0) | 1.15 (0.15) | 2.90 (0) | ||
| DEN 2 | AG129 | 1 | 1.68 (0.33) | 0.82 (0.12) | 2.90 (0.25) | 4/5 | 2.35 (0.15) | 1.90 (0) | 3.20 (0.17) |
| PDK-53 | 2 | 2.32 (0.12) | 1.42 (0.18) | 3.08 (0.12) | 5/5 | 2.87 (0.25) | 2.38 (0.07) | 3.39 (0.07) | |
| WT129 | 1 | <0.70 (0) | <0.70 (0) | <2.00 (0) | 2/2 | 2.20 (0.30) | 1.30 (0.30) | 2.75 (0.15) | |
| 2 | <0.70 (0) | <0.70 (0) | 2.15 (0.09) | 2/2 | 2.20 (0.30) | 1.15 (0.45) | 2.90 (0) | ||
| PBS (control) | AG129 | 2 | <0.70 (0) | <0.70 (0) | <2.00 (0) | 0/5 | |||
Conflicting evidence regarding the ability of IFN-γ knockout or receptor-deficient mice to produce a normal humoral response has been reported (7, 10). This suggests that the role of IFN-γ in an antiviral immune response might be virus dependent. Our results indicate that in AG129 mice the humoral response to DEN virus is apparently normal; however, we did not determine whether the distribution of antibody subclasses was unusual in any way. IFN-γ appears to be necessary for the production of immunoglobulin G (IgG) 2a antibodies (10), and ranges for antiviral IgG levels of virus-infected AG129 mice have been reported to be within normal limits (28) but with a heavy bias toward IgG1. In our experiments, neutralizing titers in response to DEN 2 16681 were not as high as those produced following immunization of ICR mice with DEN 2 purified inactivated virus (23). The lower titers in AG129 mice reported here are most likely due to the much smaller immunizing dose and the lack of adjuvant use rather than abnormalities of the IFN response.
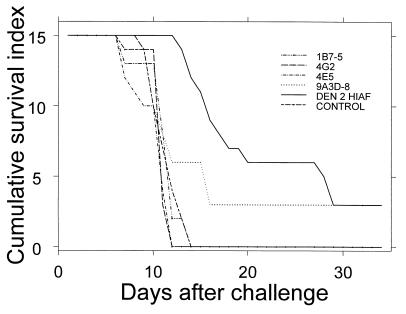
To determine if the AG129 mice could be protected via adoptive transfer of anti-DEN virus antibody, groups of five mice were given 50-μg or equivalent inoculations of monoclonal antibodies (MAbs) or PBS, intracardially, or polyclonal antibodies, i.p. At 22 h after transfer, mice were bled, and they were then challenged 2 h later with 106 PFU of mouse-adapted DEN 2 virus. Mice were monitored on a daily basis for signs of illness. Prechallenge ELISA titers for all groups ranged from 1:400 to 1:6,400 with the exception of the group inoculated with MAb 4E5 (which had a marginal average titer of 1:100) and the PBS-inoculated control group. The mice immunized with the MAbs died in the same time frame as the nonimmune controls with the single exception of one mouse in the 9A3D-8-inoculated group. The MAbs used were of isotype IgG2a and were previously shown to neutralize virus (26) or to protect mice from virus challenge (12). The amount of antibody administered here was similar to doses previously shown to protect mice from challenge with other flaviviruses (11, 12, 20) and hence should have been sufficient for protection. The failure of passively transferred MAbs to protect mice from virus challenge might indicate that other classes or isotypes of antibody are required for protection in these mice. Alternatively, multiple antibody classes or isotypes may be necessary. While only one mouse was protected, the survival time for the animals increased following passive transfer of polyclonal DEN 2 mouse hyperimmune ascitic fluid (DEN 2 HIAF) (Fig. 2). This result is similar to our observations for low challenge doses of DEN 2 virus, which also resulted in extended survival time. The DEN 2 HIAF caused an initial decrease in the effective challenge dose, requiring an extended period of viral replication before death occurred.
FIG. 2.
Survival curves of AG129 mice passively immunized with anti-DEN antibodies. MAbs (50 μg) were transferred via intracardial inoculation to groups of five mice each. Equivalent doses of polyclonal antibodies were administered to five mice via i.p. inoculation, and a control group consisting of five mice intracardially inoculated with PBS was included. Animals were challenged 24 h later with 106 PFU of the mouse-adapted DEN 2 virus strain, New Guinea C. Mice were monitored on a daily basis for signs of illness, and each mouse was assigned a score as follows: 3 for healthy, 2 for sick (losing weight, ruffled coat, hunched, and/or moving slowly), 1 for paralyzed (dragging rear legs and/or blind), and 0 for mortally ill (euthanized) or dead. Scores were summed for the mice in each group; hence, the maximum score (for a healthy group of mice) was 15.
Cytotoxic T-lymphocyte (CTL) responses have been shown previously to be fully functional in AG129 mice; however, the presence of the IFN-α/β component was important to avoid overwhelming virus replication and subsequent rapid exhaustion of CTL precursors resulting in CTL anergy (28). Though the helper T-cell responses were found to be normal in IFN-α/β and IFN-γ receptor-deficient mice (22), it is unknown whether this phenotype is additive in AG129 mice. The priming IgG responses observed in AG129 mice that were immunized with PDK-53 and were subsequently challenged with neurovirulent DEN 2 (strain New Guinea C) suggest that a helper T-cell response occurs. Whether the T-cell response in DEN virus-infected AG129 mice is normal still needs to be conclusively determined.
IFN-α/β and IFN-γ are documented as being produced in response to DEN virus infections both in vitro and in vivo. They probably play an important role in controlling both primary (14, 15, 17, 21) and secondary (30) infections. It has also been suggested that IFN-γ plays a role in modulating DEN virus-specific CD8+ CTL functions (19). Children with DEN fever and DHF produce detectable levels of IFN-α. In the individuals with DHF these levels persist even after the fever subsides (18). Given this evidence, it was not surprising to find that the near-complete loss of an IFN response in AG129 mice rendered them susceptible to a lethal peripheral DEN 2 virus infection.
The consistent susceptibility of adult AG129 mice to DEN virus via a peripheral route of inoculation is unique. The virus-immune system interactions that would be expected following the bite of an infected mosquito are able to take place. Currently, the majority of preliminary vaccine trials are performed in BALB/c mice that are no older than 6 weeks of age, using an i.c. injection of 100 to 500 times the 50% lethal dose of the virus (5). The resulting mortality rates are variable for nonimmune control groups, thus requiring that signs of morbidity, such as weight loss, be monitored closely. The use of BALB/c mice as a challenge model is paradoxical in light of the clinical symptoms induced by i.c. inoculation of mouse-adapted DEN virus into mice with various haplotypes (24), where the least susceptible strain was BALB/c. Only nude mice exhibited 100% mortality following i.c. challenge with DEN virus. In comparison to that of nude mice, husbandry of AG129 mice is more convenient, requiring only a standard specific-pathogen-free environment. The AG129 mice also have the advantage of possessing a more-normal immune response than nude mice.
Of pressing interest are whether this model can be extended to include the other DEN virus serotypes and whether it can be used to mimic DHF/DSS. This is especially important for vaccine trials. The availability of the individual IFN-α/β receptor-deficient and IFN-γ receptor-deficient mice may also aid in answering some of the questions related to IFN involvement in DHF/DSS. The AG129 mice may also be useful as models for other flaviviruses and may be helpful in development of antiviral agents against DEN virus. Clearly, there are some aspects of the DEN AG129 mouse model that need further investigation. These include identifying the effect that the lack of IFN receptors has on other aspects of the immune response to DEN virus and determining the relationship of this mouse model to primate or human infection with respect to accurate prediction of vaccine efficacy. Nevertheless, these results indicate that AG129 mice are the most promising small animal model for DEN virus infection that has been investigated to date. The use of the IFN receptor-deficient mouse model in laboratory trials of vaccines for this globally important disease should afford a significant advantage to DEN vaccine research and fill a critical experimental gap.
Acknowledgments
We acknowledge Michel Aguet for granting us permission to use the A129 and AG129 mice in this study; Andrea Cooper for supplying breeding pairs of the GKO mice; Genentech Inc., South San Francisco, Calif., for granting us permission to use the GKO mice; K. Eckels for the gift of the mouse-adapted DEN 2 virus that was used in the challenge experiments; and Richard Kinney for supplying the DEN virus strains 16681 and PDK-53. We also thank Brad Biggerstaff and Rebecca Deavours for expert assistance in the preparation of the manuscript.
REFERENCES
- 1.Chaturvedi U C, Dhawan R, Khanna M, Mathur A. Breakdown of the blood brain barrier during dengue virus infection of mice. J Gen Virol. 1991;72:859–866. doi: 10.1099/0022-1317-72-4-859. [DOI] [PubMed] [Google Scholar]
- 2.Cole G A, Wisseman C L., Jr Pathogenesis of type 1 dengue virus infection in suckling, weanling and adult mice. 1. The relation of virus replication to interferon and antibody formation. Am J Epidemiol. 1969;89:669–680. doi: 10.1093/oxfordjournals.aje.a120981. [DOI] [PubMed] [Google Scholar]
- 3.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deubel V, Kinney R M, Esposito J J, Cropp C B, Vorndam A V, Monath T P, Trent D W. Dengue 2 virus envelope protein expressed by a recombinant vaccinia virus fails to protect monkeys against dengue. J Gen Virol. 1988;69:1921–1929. doi: 10.1099/0022-1317-69-8-1921. [DOI] [PubMed] [Google Scholar]
- 5.Falgout B, Bray M, Schlesinger J J, Lai C-J. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol. 1990;64:4356–4363. doi: 10.1128/jvi.64.9.4356-4363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiette L, Aubert C, Müller U, Huang S, Aguet M, Brahic M, Bureau J-F. Theiler’s virus infection of 129Sv mice that lack the interferon α/β or interferon γ receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham M B, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon γ gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubler D J, Clark G G. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infect Dis. 1995;1:55–57. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotta H, Murakami I, Miyasaki K, Takeda Y, Shirane H, Hotta S. Inoculation of dengue virus into nude mice. J Gen Virol. 1981;52:71–76. doi: 10.1099/0022-1317-52-1-71. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilček J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 11.Hunt A R, Short W A, Johnson A J, Bolin R A, Roehrig J T. Synthetic peptides of the E2 glycoprotein of Venezuelan equine encephalitis virus. II. Antibody to the amino terminus protects animals by limiting viral replication. Virology. 1991;185:281–290. doi: 10.1016/0042-6822(91)90775-7. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman B M, Summers P L, Dubois D R, Eckels K H. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1987;36:427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- 13.Kinney R M, Butrapet S, Chang G-J J, Tsuchiya K R, Roehrig J T, Bhamarapravati N, Gubler D J. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 14.Kurane I, Meager A, Ennis F A. Induction of interferon alpha and gamma from human lymphocytes by dengue virus-infected cells. J Gen Virol. 1986;67:1653–1661. doi: 10.1099/0022-1317-67-8-1653. [DOI] [PubMed] [Google Scholar]
- 15.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Rothman A L, Livingston P G, Janus J, Ennis F A. Human immune responses to dengue viruses. Southeast Asian J Trop Med Public Health. 1990;21:658–662. [PubMed] [Google Scholar]
- 16.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Meager A, Janus J, Ennis F A. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in the sera of children with dengue. J Clin Investig. 1991;88:1473–1480. doi: 10.1172/JCI115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurane I, Janus J, Ennis F A. Dengue virus infection of human skin fibroblasts: in vitro production of IFN-beta, IL-6 and GM-CSF. Arch Virol. 1992;124:21–30. doi: 10.1007/BF01314622. [DOI] [PubMed] [Google Scholar]
- 18.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Meager A, Ennis F A. High levels of interferon alpha in the sera of children with dengue virus infection. Am J Trop Med Hyg. 1993;48:222–229. doi: 10.4269/ajtmh.1993.48.222. [DOI] [PubMed] [Google Scholar]
- 19.Livingston P G, Toomey S, Kurane I, Janus J, Ennis F A. Modulation of the functions of dengue virus-specific human CD8+ cytotoxic T cell clone by IL-2, IL-7 and IFN gamma. Immunol Investig. 1995;24:619–629. doi: 10.3109/08820139509066862. [DOI] [PubMed] [Google Scholar]
- 20.Mathews J H, Roehrig J T. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J Immunol. 1984;132:1533–1537. [PubMed] [Google Scholar]
- 21.Mori M, Kurane I, Janus J, Ennis F A. Cytokine production by dengue virus antigen-responsive human T lymphocytes in vitro examined using a double immunocytochemical technique. J Leukoc Biol. 1997;61:338–345. doi: 10.1002/jlb.61.3.338. [DOI] [PubMed] [Google Scholar]
- 22.Müller U, Steinhoff U, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 23.Putnak R, Barvir D A, Burrous J M, Dubois D R, D’Andrea V M, Hoke C H, Sadoff J C, Eckels K H. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996;174:1176–1184. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 24.Raut C G, Deolankar R P, Kolhapure R M, Goverdhan M K. Susceptibility of laboratory-bred rodents to the experimental infection with dengue virus type 2. Acta Virol. 1996;40:143–146. [PubMed] [Google Scholar]
- 25.Roehrig J T, Corser J A, Schlesinger M J. Isolation and characterization of hybrid cell lines producing monoclonal antibodies directed against the structural proteins of Sindbis virus. Virology. 1980;101:41–49. doi: 10.1016/0042-6822(80)90481-x. [DOI] [PubMed] [Google Scholar]
- 26.Roehrig J T, Bolin R A, Kelly R G. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 27.Sabin A B. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 28.van den Broek M F, Müller U, Huang S, Aguet M, Zinkernagel R M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S-J L, Hayes C G, Dubois D R, Windheuser M G, Kang Y-H, Watts D M, Sieckmann D G. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am J Trop Med Hyg. 1995;52:468–476. doi: 10.4269/ajtmh.1995.52.468. [DOI] [PubMed] [Google Scholar]
- 30.Yang K D, Lee C S, Hwang K P, Chu M L, Shaio M F. A model to study cytokine profiles in primary and heterologously secondary dengue-2 virus infections. Acta Virol. 1995;39:19–21. [PubMed] [Google Scholar]