Abstract
In monogamous species, pair bonding leads to striking changes in social behavior and neural circuitry. We outline the cognitive building blocks of monogamous pair bonding in prairie voles (Microtus ochrogaster), as well as opportunities afforded by the species to investigate diverse mechanisms underlying social experience-dependent plasticity and gain insights into the neurobiology of complex social behavior more generally.
Socially monogamous behavior in prairie voles
The ability and desire to form long-lasting bonds with our romantic partners is a defining feature of the human social experience – these bonds are a powerful form of attachment characterized by persistent selective and exclusive affiliation between mates. Enduring pair bonds are something of an evolutionary anomaly in the mammalian class, and their study requires neuroscientists to embrace less commonly used laboratory species to study the underlying biology. Unlike standard laboratory rodents (mice and rats), socially monogamous prairie voles (Microtus ochrogaster, Figure 1) form life-long mating-based pair bonds, engage in robust biparental care, and exhibit distress upon loss of a partner [1,2]. We highlight how the neuroethological relevance of the prairie vole pair-bonding model continues to expand our understanding of social experience-dependent plasticity in behavioral and neural phenotypes.
Figure 1. Adult prairie voles exhibit distinctive shifts in social behavior once they form a pair bond.
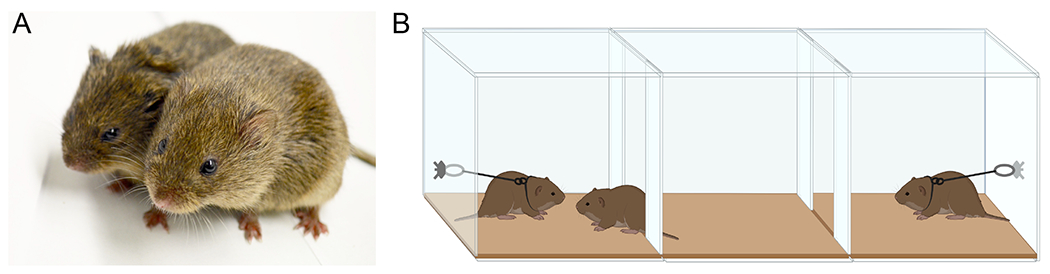
(A) When prairie voles form a pair bond, they are frequently observed together engaging in their characteristic ‘huddling’ behavior. Bonded prairie voles are also more aggressive than their sexually naive counterparts, but this heightened aggression is directed towards non-partner animals. Photo courtesy of Vanessa Gutzeit (laboratory of Z.R.D.). (B) A pair bond is operationalized by measuring the amount of time a subject spends huddling with either of two other-sex animals in a three-chamber apparatus. A pair-bonded prairie vole will demonstrate a robust preference to spend time with their partner versus a novel stimulus animal.
The first hints that prairie voles are monogamous originated with the unusual observation that two individuals were often trapped together in population monitoring studies [3]. After establishing laboratory colonies of prairie voles, early neuroendocrinologists assessed their social preferences by allowing voles to freely explore a three-chamber apparatus that held a mate at one end and a novel other-sex conspecific at the opposite end. When given the option, prairie voles exhibited a strong preference to affiliate with their mates and showed heightened aggression towards the non-partner. Leveraging this partner-preference test, neuroendocrinologists identified the hormones, oxytocin and vasopressin, in the mesolimbic reward pathway as key regulators of pair-bonding behaviors. Because they are deeply conserved across taxa, oxytocin and vasopressin are also extensively implicated in human bonding and social behavior, providing foundational insights into the potential convergent evolution of monogamous mating systems [1].
The cognitive building blocks that underpin pair bonding include traits that are present in most, if not all, mammals, and include recognition of other individuals, reward and motivation, and social aversion. However, in pair bonding, the unique confluence of these cognitive abilities enables a specificity that does not occur in promiscuous species; specifically, in pair-bonding prairie voles, a partner–reward association forms, and the subsequent preference to interact with that individual is maintained by partner-specific motivational processes and selective aggression towards (or avoidance of) non-partners.
Pair bonding as a model for social experience-dependent plasticity
Prairie vole pair bonding is a robust example of social experience-dependent plasticity in which mating leads to long-term changes in social behavior. As such, the behavioral shift from non-bonded to bonded states provides an ideal opportunity to study the mechanisms of behavioral plasticity at multiple levels of neural function.
Bonds in prairie voles strengthen over time; both males and females show more robust partner-directed affiliation the longer they are together [4]. The mechanisms underlying bond maturation are relatively understudied, but likely involve diverse physiological changes. Within the nucleus accumbens, pair-bonded voles exhibit sensitized dopamine release [5] and divergent patterns of excitatory synaptic activity following oxytocin receptor activation [6]. Calcium imaging also revealed an expansion of cells whose activity predicts partner approach [7]. Underlying these neural changes, pair bonding promotes stable shifts in gene expression that subsequently erode following partner loss, suggesting that bond status may be maintained at the level of sustained gene regulatory patterning [8]. Thus, the experience of bonding appears to fundamentally reorganize neural systems at almost every level of biology examined – from gene regulation to physiology, synaptic activity, and circuit modulation.
Not all pair bonds are the same, providing an opportunity to identify important variation in the processes driving experience-dependent plasticity. There are well-characterized sex differences, as well as individual differences in behavior; some voles exhibit an overwhelming desire to be with their mating partners whereas others show comparatively weaker preferences. Both genetic and environmental factors contribute to this behavioral diversity. Specifically, early life environments and pharmacological manipulations produce sex-specific effects on bonding behaviors [9], and males and females exhibit divergent changes in accumbal gene expression following bonding [10]. Regarding individual differences, genetic variants in key neuromodulatory genes have been linked to differences in wild and captive behavior [11]. Capitalizing upon the natural behavioral variation of prairie voles will continue to shed light on gene–environment interactions that shape behavioral diversity and plasticity.
Cross-species comparisons in vole neuroethology have also offered insight into the evolution of behavioral variation and complex sociocognitive processes. Pronounced differences in oxytocin and vasopressin receptor patterns of closely related vole species suggest that monogamous and non-monogamous phenotypes are driven by species-specific neuromodulatory signaling systems [1]. Expanding these comparative approaches may help to identify evolutionary adaptations underlying monogamous mating systems and determine whether mechanisms of socially induced plasticity are shared across pair-bonding species, and thus represent a generalized biological principle.
Pair bonding uniquely engages mating processes; only monogamous species form a mating-based reward association with a specific individual. As a result, pair bond-induced changes in prosocial behavior are highly selective and directed towards the mating partner. This differs, for instance, from socially induced plasticity in the transition to parenthood, where social behavior may be dramatically altered but is not necessarily specific to the identity of an interaction partner (i.e., in the case of rodents, general heightened aggression towards others and a generalized motivation to care for pups). As such, comparison of different types of relationships provides an opportunity to identify the unique and shared features of prosocial behavior.
Potential translational value of fundamental research in voles
The value of work on prairie voles extends well beyond fundamental research – the prairie vole model has powerful translational potential for understanding the etiology of social neurodiversity. Social behaviors are altered in several disorders including, but not limited to, major depression, anxiety disorders, and autism spectrum disorder (ASD). Investigation of oxytocin and vasopressin function in prairie voles has revealed neurogenetic features that are associated with variation in social behavior. Such studies can broaden our understanding of the spectrum of neurodiversity and the associated vulnerabilities experienced by neurodiverse individuals. In addition, the prairie vole system is being leveraged to model social influences on substance use, cardiac and autonomic functioning, and the health consequences of social isolation or grief [2,8]. Existing rodent models (rats and mice) are simply not suitable for these research applications because these species do not exhibit pair bonds.
Concluding remarks
The potential of prairie voles as a neuroscience model organism remains to be fully realized. Prairie voles offer unique opportunities to address the mechanisms of social neurobehavioral plasticity with unprecedented sophistication and resolution [12]. Virus-mediated techniques for real-time imaging, and optical control of molecularly defined cells and projections, are now possible through implementation of optogenetics, fiber photometry, and microendoscope imaging using a growing suite of biosensors. Molecular cell typing and (epi)genetic regulatory/editing techniques are allowing researchers to identify and interrogate the genetic basis of complex behavior. Robust supervised and unsupervised machine-learning approaches for analyzing complex and lengthy behavioral recordings are rapidly advancing the ability to characterize fine-grained behavioral repertoires during multi-animal social interactions. These gains will be particularly useful for expanding the scope of analysis to incorporate the dynamic behaviors and biology of multiple individuals within an interaction. As investigations are scaled up across individuals and timescales, a better appreciation of the complex processes that shape social experience, and are shaped by it, is likely to emerge.
Acknowledgments
This work was supported by National Institutes of Health (NIH) awards DP2OD026143, R01MH125423, and U01NS122124, National Science Foundation (NSF) award IOS-2045348, and the Dana Foundation (to Z.R.D.), and DP5OD033437 and a National Science Foundation (NSF) postdoctoral research fellowship in biology 2010817 (to L.C.H.).
Footnotes
Declarations of interests
The authors declare no competing interests.
References
- 1.Young LJ and Wang ZX (2004) The neurobiology of pair bonding. Nat. Neurosci 7, 1048–1054 [DOI] [PubMed] [Google Scholar]
- 2.McGraw LA and Young LJ (2010) The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 33, 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter CS and Getz LL (1993) Monogamy and the prairie vole. Sci. Am 268,100–106 [DOI] [PubMed] [Google Scholar]
- 4.Brusman LE et al. (2022) Emergent intra-pair sex differences and organized behavior in pair bonded prairie voles (Microtus ochrogaster). Genes Brain Behav. 21, e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resendez SL et al. (2016) Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. Elife 5, e15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borie AM et al. (2022) Social experience alters oxytocinergic modulation in the nucleus accumbens of female prairie voles. Curr. Biol 32, 1026–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scribner JL et al. (2020) A neuronal signature for monogamous reunion. Proc. Natl. Acad. Sci. U. S. A 117, 11076–11084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadino JM et al. (2021) Prolonged partner separation erodes nucleus accumbens transcriptional signatures of pair bonding in male prairie voles. BioRxiv Published online May 20, 2022. 10.1101/2021.07.14.452355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bales KL and Carter SC (2003) Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Horm. Behav 44, 178–184 [DOI] [PubMed] [Google Scholar]
- 10.Duclot F. et al. (2022) Transcriptomic regulations underlying pair-bond formation and maintenance in the socially monogamous male and female prairie vole. Biol. Psychiatry 91, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadino JM and Donaldson ZR (2018) Prairie voles as a model for understanding the genetic and epigenetic regulation of attachment behaviors. ACS Chem. Neurosci 9,1939–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenkel WM et al. (2021) A neuroscientist’s guide to the vole. Curr. Protoc 1, e175. [DOI] [PMC free article] [PubMed] [Google Scholar]


