Mexico (Durango) raised alarms over a sudden increase in the number of aseptic meningitis (ASM) with nine deaths and 59 confirmed cases.
In infective etiology of ASM, viral meningitis cases are seen predominantly, followed by fungi.
Noninfective ASM can be caused by systemic diseases with meningeal involvement, iatrogenic or nosocomial, drugs, etc.
Dear Editor,
On 4 November 2022, in Durango, Mexico, alarms were raised over a sudden increase in the number of aseptic meningitis (ASM) cases. Initially, 11 women were diagnosed with the disease; however, the number rapidly increased, resulting in the death of nine individuals, with a confirmed case count of 59 as of 21 November 2022. Upon investigation, it was found that these women had recently undergone surgeries at the Hospital del Parque.
Thus, there was an urgent need to understand the root cause of the outbreak, given the possible nosocomial origin of the outbreak. Reports confirmed that a batch of bupivacaine, the anesthetic agent used for the surgery, was contaminated with a deadly and aggressive fungus leading to the spread of the infection (Fig. 1). To contain the spread, authorities seized the drugs from all hospitals in that area1. As per the General Director of Epidemiology, Ministry of Health, there is permanent surveillance done by health care workers and follow-up with Mexican and international experts on the protocolization of treatment and the possible outcome of the reported cases. National institutes and medical specialists are approving the treatment2.
Figure 1.
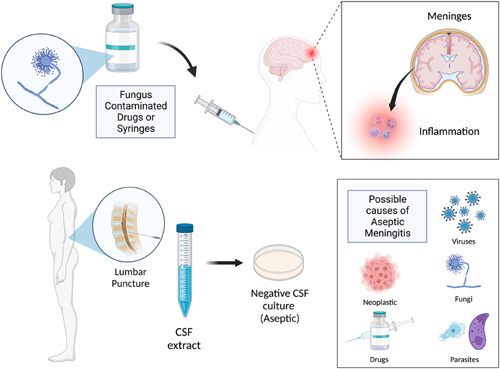
The spectrum of events leading to aseptic meningitis (created with BioRender.com; publishing license acquired). CSF, cerebrospinal fluid.
ASM refers to inflammation of the meninges with cerebrospinal fluid (CSF) pleocytosis of greater than 5 cells/mm3, which tests negatively for Gram stain and routine bacterial cultures. Although meningitis-related fatalities have reduced over the years, the incidence of meningitis has increased globally. In 2016, over 2.82 million people worldwide suffered from meningitis, of whom around 318,000 died, contributing to a disease-associated life years (DALYs) of 21.86 million3. Most cases of ASM run a benign, self-limited course. Classically, ASM presents as a triad of fever, headache, and nuchal rigidity. Mental status changes are expected, varying from lethargy to coma. Brudzinski’s and Kernig’s signs are physical examination findings that strongly suggest meningitis.
The etiology of ASM can be classified as infective and noninfective. In infective etiology, viral meningitis cases are seen predominantly, followed by fungi. The term ASM is still commonly used even though a microorganism is responsible in most cases3. Nonpolio enteroviruses are implicated the most, followed by herpes simplex virus (HSV) type 2, varicella zoster virus, and HSV type 1. Other less common viruses are HIV, arboviruses, West Nile virus, cytomegalovirus, mumps, Epstein–Barr virus, influenza virus, adenovirus, and lymphocytic choriomeningitis virus. Among the fungi, Cryptococcus is the most prevalent, followed by Coccidioides, Histoplasma, and Candida. Transmission is via inhalation of spores, and they do not spread from person to person4–6.
Noninfective ASM can be caused by systemic diseases with meningeal involvement, malignancies, autoimmune, or autoinflammatory systemic diseases such as systemic lupus erythematosus, rheumatoid arthritis, Behcet’s disease, iatrogenic or nosocomial such as postlumbar puncture complications or as adverse drug effects associated with NSAIDs, intravenous immunoglobulin, certain antimicrobials like trimethoprim–sulfamethoxazole, monoclonal antibodies like OKT3 (muromonab), anticonvulsants like lamotrigine and carbamazepine7.
Detailed history, including personal and familial, geographical location, patient contacts, history of recent travel, recent vaccination, and recent drug intake, if any, can aid in reaching a conclusive diagnosis. Clinical presentation can be similar to bacterial meningitis, but patients with bacterial meningitis clinically appear more critically ill. In addition to bacterial meningitis, other diseases, such as intracranial hemorrhage (in particular subarachnoid hemorrhage), other types of headaches (migraine), and inflammation of brain structures (brain abscess, epidural abscess) should also be taken into account for alternative differential diagnosis4.
Head computed tomography should be carried out, followed by lumbar puncture. CSF is clear in appearance with normal or elevated opening pressure. CSF laboratory tests should include total leukocyte count, glucose, protein, Gram stain and culture, viral PCR (e.g. for enterovirus, HSV-1, HSV-2, varicella zoster virus, CMV, Epstein–Barr virus, arbovirus), latex agglutination test for Cryptococcus and serum/urine antigen test for Coccidioides and Histoplasma. Findings are usually normal glucose, normal to moderately increased proteins (>50 mg/dl), and 10–1000 cells/µl (early: neutrophils; late: lymphocytes). Serum C-reactive protein and CSF lactate levels may also be increased. Rapid lateral-flow devices, loop-mediated isothermal amplification, and T2 Candida panels are some of the newer diagnostic tests used to detect Cryptococcus, Aspergillus, Histoplasma, and Candida, respectively4.
ASM associated with drug intake, called drug-induced aseptic meningitis, may be due to a direct meningeal irritation caused by the intrathecal administration of drugs or an immunologic hypersensitivity reaction to a systemic administration. Drug-induced aseptic meningitis is a diagnosis of elimination, that is, it has a rapid onset after initiation, rapid reversion after discontinuation, and recurrence after re-administration of the drug. After ruling out other causes, this diagnosis should be sought6.
Meningitis has a relatively low incidence in Mexico, making it one of the nations with the fewest documented cases. In 2016, the case fatality rate of meningitis reported in Mexico was 9.8%, contributing to a DALY of 48,608 compared to neighboring countries like the United States, with a case fatality rate of 8.4%, contributing to a DALY of 65,8051.
ASM is a much less understood subtype as compared to bacterial meningitis. In Durango, the outbreak was found to be of fungal etiology. However, the origin of the infection and the species are yet to be determined. Multiple factors can have plausible involvement leading up to this crisis. Rarely could drugs be contaminated with some microorganisms because of a breach in the process of sterilization and storage. Several species of fungi are known to thrive in drugs, specifically steroids. However, there has yet to be an explanation that describes fungal affinity to bupivacaine. The authorities should have conducted epidemiological investigations of cases, including interviews with patients. Environmental samples should have been investigated to detect the presence of fungus. Further, some pharmaceutical companies fail to meet Food and Drug Administration manufacturing standards, which compromises the quality and safety of drugs8.
A similar incident occurred in Sri Lanka in 2007 when six women who underwent cesarean section presented with ASM due to contamination of the spinal needles and syringes by Aspergillus. In North Carolina, two more epidemics of iatrogenic ASM occurred in 2002 and 2012. The fungal species implicated were Exophiala and Exserohilum, which contaminated the methylprednisolone acetate injections. At least 14,000 people were exposed in 2002. During the 2012 outbreak, steroid injections tainted with Exserohilum caused over 50 fatalities and 700 illnesses nationwide9–11.
Another similar incident was the 2012 fungal meningitis outbreak in the United States. Exserohilum was discovered as the underlying cause. A prompt public health response managed to locate, monitor, and treat infected patients. However, the lack of standard sterilization practices resulted in 664 infections and 40 fatalities. A common denominator underlying these conditions is the mode of transmission, which is the contamination of syringes administered to patients12.
The term ‘aseptic meningitis’ encompasses all types of meningitis that have negative routine bacterial cultures. The unknown nature of the etiology makes the treatment challenging, often limited to symptomatic relief. The infective agent goes unidentified in most cases. A probable reason could be the underutilization of available diagnostic tests, including serologies and PCR for viral causes. Fungal causes can be accounted for by antigen testing. ASM with an unidentified infectious etiology draws attention to drugs as a potential cause.
An unidentified etiology results in the majority of patients undergoing unnecessary cranial imaging and antibiotic therapy without specific indications, despite CSF cultures being negative. Point-of-care gram staining of CSF can distinguish bacterial meningitis from ASM, thereby significantly reducing the empirical treatment with antibiotics. Clinicians tend to immediately move to radiological assessment following a negative CSF culture. With the introduction of rapid multiplex PCR, this will likely change in the future. Pharmaceuticals must ensure proper sterilization, transport, and storage. The flow of medication from delivery to disposal must be assessed. Temperature and pH should be regulated in storage areas. Personal and hospital hygiene must be advocated.
In conclusion, although clinical outcome in ASM is primarily benign, with complete symptom resolution within the first few weeks, there is always a risk of complications in terms of intracranial hypertension, encephalitis, or cerebral vasculitis with subsequent ischemia. It is imperative to put the currently available tools to diagnostic use for the identification of the etiologic agent, along with simultaneous inspection and audits of the hospital and pharmaceutical facilities, to assess the quality and efficiency of sterilization and disinfection techniques to prevent further outbreaks. Maintaining a high index of caution and requesting the appropriate laboratory and radiological tests may be tremendously useful in diagnosing ASM.
Ethical approval
Not applicable.
Sources of funding
No funding was received.
Author contribution
V.S., R.R., V.A., M.R., K.B., and V.G. designed the original draft. P.K., A.M., B.K.P., and R.S. reviewed the literature, critically reviewed and edited the manuscript, and supervised the manuscript. All authors approved the final manuscript.
Research registration unique identifying number (UIN)
None.
Guarantor
R. Sah.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 3 March 2023
Contributor Information
Vinay Suresh, Email: dr.vinay.neuro@gmail.com.
Ranjana Rohilla, Email: ranjana86choudhary@gmail.com.
Vibhor Agrawal, Email: 2000.vibhor@gmail.com.
Malavika Rudrakumar, Email: malavika.rudrakumar@gmail.com.
Balakrishnan Kamaraj, Email: balakrishnanmmc015@gmail.com.
Victor Ghosh, Email: victor.aryaghosh@gmail.com.
Pradhyumn Kumar, Email: co.pradhyumn@gmail.com.
Aroop Mohanty, Email: aroopmohanty7785@yahoo.com.
Bijaya K. Padhi, Email: bkpadhi@gmail.com.
Ranjit Sah, Email: ranjitsah@iom.edu.np.
References
- 1. Health authorities confirm the presence of fungus in infections by aseptic meningitis in Durango Mexico Daily Post. https://mexicodailypost.com/2022/11/14/health-authorities-confirm-the-presence-of-fungus-in-infections-by-aseptic-meningitis-in-durango.
- 2. Durango reports 10th meningitis death in outbreak. Mexico Daily Post. Durango reports 10th meningitis death in outbreak. https://mexiconewsdaily.com/news/durango-10th-meningitis-death-outbreak/.
- 3. Zunt JR, Kassebaum NJ, Blake N, et al. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:1061–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leuci S, Coppola N, Cantile T, et al. Aseptic meningitis in oral medicine: exploring the key elements for a challenging diagnosis: a review of the literature and two case reports. Int J Environ Res Public Health 2022;19:3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logan SAE, MacMahon E. Viral meningitis. BMJ 2008;336:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centre for Disease control and prevention. Meningitis Home. Fungal meningitis. https://www.cdc.gov/meningitis/fungal.html.
- 7. Yelehe-Okouma M, Czmil-Garon J, Pape E, et al. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol, 32 2018:252–260. [DOI] [PubMed] [Google Scholar]
- 8. Ng TTC, Robson GD, Denning DWY. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology 1994;140:2475–9. [DOI] [PubMed] [Google Scholar]
- 9. Rodrigo N, Perera KNT, Ranwala R, et al. Aspergillus meningitis following spinal anaesthesia for caesarean section in Colombo, Sri Lanka. Int J Obstet Anesth 2007;16:256–60. [DOI] [PubMed] [Google Scholar]
- 10. Perfect JR. Iatrogenic fungal meningitis: tragedy repeated. Ann Intern Med 2012;157:825–6. [DOI] [PubMed] [Google Scholar]
- 11. Arnold C. Fungal meningitis outbreak affects over 700. Lancet Neurol 2013;12:429–30. [DOI] [PubMed] [Google Scholar]
- 12. Todd B. Fungal meningitis outbreak. Am J Nurs 2013;113:52. [DOI] [PubMed] [Google Scholar]


