The results of this multidatabase study suggest that users of conjugated estrogens/bazedoxifene might experience slightly higher rates of endometrial cancer and endometrial hyperplasia and a lower rate of breast cancer than estrogen/progestin users.
Key Words: Bazedoxifene, Breast cancer, Endometrial cancer, Estrogen, Hormone therapy, Multidatabase study
Abstract
Objective
To assess the risk of select safety outcomes including endometrial cancer, endometrial hyperplasia, and breast cancer among women using conjugated estrogens/bazedoxifene (CE/BZA) as compared with estrogen/progestin combination hormone therapy (EP).
Methods
We conducted a new-user cohort study in five US healthcare claims databases representing more than 92 million women. We included CE/BZA or EP new users from May 1, 2014, to August 30, 2019. EP users were propensity score (PS) matched to users of CE/BZA. Incidence of endometrial cancer, endometrial hyperplasia, breast cancer, and eight additional cancer and cardiovascular outcomes were ascertained using claims-based algorithms. Rate ratios (RR) and differences pooled across databases were estimated using random-effects models.
Results
The study population included 10,596 CE/BZA and 33,818 PS-matched EP new users. Rates of endometrial cancer and endometrial hyperplasia were slightly higher among CE/BZA users (1.6 and 0.4 additional cases per 10,000 person-years), although precision was limited because of small numbers of cases (endometrial cancer: RR, 1.50 [95% confidence interval {CI}, 0.79-2.88]; endometrial hyperplasia: RR, 1.69 [95% CI, 0.51-5.61]). Breast cancer incidence was lower in CE/BZA users (9.1 fewer cases per 10,000 person-years; RR, 0.79; 95% CI, 0.58-1.05). Rates of other outcomes were slightly higher among CE/BZA users, but with confidence intervals compatible with a wider range of possible associations.
Conclusions
CE/BZA users might experience slightly higher rates of endometrial cancer and endometrial hyperplasia, and a lower rate of breast cancer, than EP users in the first years of use.
Although hormone therapy is frequently used to treat vasomotor symptoms of menopause, it is not without other potential implications to public health.1 Among women with a uterus, the use of estrogen by itself increases the incidence of endometrial cancer.2 This increase in risk is typically mitigated by the addition of progestin to “oppose” the estrogen, but the addition of progestin seems to increase the risk of breast cancer relative to placebo or taking estrogen therapy.3-5 Other molecular entities could be considered for the purpose of estrogen opposition that may not have this undesired effect. For example, it is possible that using a selective estrogen receptor modulator (SERM) instead of progestin to oppose estrogen could mitigate the increased risk of endometrial cancer associated with estrogen therapy without increasing the risk of breast cancer.6-8
Bazedoxifene (BZA) is a SERM that was developed as an estrogen antagonist in breast and endometrial tissue.6 Combined with conjugated estrogens (CE/BZA), it is the first approved hormone therapy to pair estrogen with a SERM for the treatment of the vasomotor symptoms associated with menopause. In 2013, oral CE/BZA (Duavee) was approved in the United States for the treatment of moderate-to-severe vasomotor symptoms associated with menopause and prevention of postmenopausal osteoporosis9 in women with a uterus.10 In 2014, oral CE/BZA (Duavive) was authorized throughout the European Union for the treatment of menopause-associated symptoms in women with a uterus.11 Uptake of CE/BZA in Europe has been low,12 with most women exposed to CE/BZA located in the United States.
Evidence regarding the safety of CE/BZA is limited to preclinical studies and clinical trials, principally five clinical trials known as the Selective estrogens, Menopause, And Response to Therapy (SMART) trials.13-16 Because of their small sizes, the SMART trials were, however, not informative regarding endometrial outcomes or breast cancer risk. Preliminary evidence suggests, however, a potential for CE/BZA to reduce breast cancer risk factors such as breast density and biomarkers including insulin-like growth factor and serum progesterone.16,17
No studies have compared the rates of endometrial outcomes and breast cancer between CE/BZA and estrogen-progestin therapy (EP) users. Although a randomized controlled trial is the ideal approach to assess causal relationships, these outcomes could not be readily assessed using a trial design because of their infrequency. To assess these and other safety outcomes, the European Medicines Agency designated a noninterventional, Post-Authorization Safety Study to examine the real-world safety of CE/BZA.18 To fulfill this Post-Authorization Safety Study commitment, we used data from five, large, US healthcare claims databases containing more than 92 million women between 2014 and 2019. Because uptake of this new drug combination was low in Europe,12 these US databases likely contain a majority of the women exposed to this new treatment.
METHODS
Study design
We performed a propensity score (PS)–matched, new-user, active comparator cohort study in five US claims databases and integrated results using meta-analyses. Approval of the study protocol (Protocol Number: B2311060) was granted by the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency. The study protocol is publicly accessible through the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance's European Union electronic Register of Post-Authorisation Studies (Register Number: EUPAS11599).18
Data sources
The study included five US health insurance claims databases: research portion of Aetna's Sentinel Common Data Model (maintained by CVS Health Clinical Trial Services), HealthCore Integrated Research Database (HIRD), MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases, MarketScan Medicaid, and Optum Research Database (ORD). Each database captures the healthcare experience of persons from across the United States who are covered by commercial or public health insurance (Table 1). These data include individuals' health insurance claims and enrollment information.
TABLE 1.
Characteristics of the five study databases
| Database | Payor or servicer | Includes Medicare plans? | Data availability | Females with enrollment in study perioda |
|---|---|---|---|---|
| CVSH CTS/Aetna's SCDMb | Private (Aetna) | Advantage | 01/2008-08/2019 | 5,841,695 |
| HIRD | Private (Anthem) | Advantage | 01/2006-08/2019 | 19,045,398 |
| MarketScan CCAE-MDCR | Private (mixed, ES) | Supplemental | 01/2013-07/2019 | 41,224,183 |
| MarketScan Medicaid | Public (Medicaid) | Yes | 01/2013-12/2018 | 10,859,907 |
| ORD | Privatec | No | 05/2011-08/2019 | 15,451,242 |
CCAE-MDCR, MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases; CVSH CTS, CVS Health Clinical Trial Services; ES, employer-sponsored; HIRD, HealthCore Integrated Research Database; ORD, Optum Research Database; SCDM, Sentinel Common Data Model.
aThe study period began May 1, 2014, and ended on the final date of data availability for the respective database.
bMaintained by CVSH CTS.
cA large US health plan affiliated with Optum.
Study population
The study population included female health plan enrollees of any age with medical and pharmacy coverage and at least 12 months of continuous health plan enrollment before their first (index) dispensing of CE/BZA or EP in the database. Women were included if they initiated CE/BZA or EP during the study period from May 1, 2014, to August 30, 2019 (or end of data availability, if earlier). We excluded women with a history of hysterectomy; cancer diagnoses (other than nonmelanoma skin cancer); previous dispensings of CE/BZA, EP, or unopposed estrogen; or simultaneous dispensings of both CE/BZA and EP on the index date (Supplemental Digital Content [SDC] Tables 1 and 2, http://links.lww.com/MENO/B148).
For the analyses of endometrial hyperplasia, women diagnosed with endometrial hyperplasia in the 12 months before initiating CE/BZA or EP were excluded. For analyses of cardiovascular outcomes, women with any cardiovascular diagnosis (SDC Table 3, http://links.lww.com/MENO/B148) in the 6 months before initiating CE/BZA or EP were excluded. For the analyses of ovarian cancer, women with a history of bilateral oophorectomy were excluded.
Exposures
Women were considered exposed to CE/BZA if they had at least one pharmacy claim with a National Drug Code for CE/BZA (SDC Table 1, http://links.lww.com/MENO/B148). Women were considered exposed to EP if they had at least one pharmacy claim for oral, topical, or transdermal EP (SDC Table 1, http://links.lww.com/MENO/B148). Eligible EP drugs included CE/medroxyprogesterone acetate, estradiol and norethindrone acetate, norethindrone acetate-ethinyl estradiol, estradiol/drospirenone, estradiol/levonorgestrel, and estradiol/norgestimate (SDC Table 1, http://links.lww.com/MENO/B148). The index date for each woman was their first (index) exposure (CE/BZA or EP) recorded in the database during the intake period.
Outcomes
Endometrial cancer and endometrial hyperplasia cases were identified using algorithms developed and validated in EP users in the HIRD.19,20 Endometrial cancer was identified by the presence of at least one inpatient hospitalization with a principal diagnosis of endometrial cancer or more than two outpatient or emergency department visits on different dates with an endometrial cancer diagnosis in any position. The endometrial cancer algorithm had a positive predictive value of 91% (95% confidence interval [CI], 87%-94%).20 Endometrial hyperplasia was identified using a predictive model algorithm with a positive predictive value of 80% (95% CI, 77%-88%) in the HIRD.18
Venous thromboembolism (VTE) cases were identified by the presence of at least one inpatient hospitalization with a principal diagnosis of VTE (SDC Table 4, http://links.lww.com/MENO/B148). Myocardial infarction and stroke/transient ischemic attack (TIA) cases were identified by the presence of either (a) at least one inpatient hospitalization with a principal diagnosis for the condition with a length of stay of at least 3 days, (b) at least one inpatient hospitalization with a principal diagnosis for the condition and a discharge status of death, or (c) at least one emergency department visit with a principal diagnosis code for the condition (SDC Table 4, http://links.lww.com/MENO/B148).
Other cancer outcomes required either (a) at least one inpatient hospitalization with a principal diagnosis of the cancer under study (for breast cancer, could be a secondary diagnosis) or (b) at least two outpatient or emergency department visits on different dates with the relevant diagnosis in any position (SDC Table 4, http://links.lww.com/MENO/B148).
Covariates
Covariates were identified on or before the index date and included age, sex, geographic region, total time enrolled in health plan, calendar year of index date, cardiovascular, cerebrovascular, or coronary heart disease (in the 183 d before and including index date), prespecified comorbidities and medications (in the 365 d before and including the index date; SDC Tables 5 and 6, http://links.lww.com/MENO/B148), and the 25 most common comorbidities and procedures within each database's prematched study population. For the 25 most common comorbidities and procedures, categories were created using the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project's Clinical Classification Software.21 The Clinical Classification Software groups International Classification of Diseases, Ninth and Tenth Revisions, Current Procedural Terminology (CPT), and Healthcare Common Procedure Coding System (HCPCS) codes into broader clinically meaningful categories.21
Endometrial hyperplasia during the baseline (preindex) period was treated as a covariate in all analyses, except analyses of endometrial hyperplasia for which women with endometrial hyperplasia in the 365 days before and including the index date were excluded. When treated as a covariate or exclusion criterion (as opposed to an outcome), endometrial hyperplasia was defined by a diagnosis code for endometrial hyperplasia on any claim in any position.
The ORD included the following additional covariates on or before the index date: any emergency department visit (yes/no), time from the start of study period (May 1, 2014) to index date, number of three-digit diagnosis codes, number of inpatient stays, number of procedures, number of unique procedures, number of drugs dispensed, number of physician visits, and total healthcare costs.
Follow-up
Follow-up for each woman began on the day after the index date and continued until the earliest of death, disenrollment, the end of the available data period (Table 1), hysterectomy, treatment switch (defined as EP to CE/BZA or CE/BZA to EP, while switching among EP drugs was permitted), or occurrence of the outcome. For noncancer outcomes (ie, endometrial hyperplasia, VTE, myocardial infarction, and stroke/TIA), follow-up was also censored by any cancer diagnosis or end of current treatment episode, and women could contribute more than one treatment episode. Treatment episodes for noncancer outcomes were constructed by concatenating consecutive dispensing days' supplies, that is, fill date + days' supply, allowing for treatment gaps of 30 days (ORD allowed for up to 32 d). If a woman had a dispensing of the same study drug type (ie, CE/BZA or EP) that occurred before the end of a treatment episode, the treatment episode was extended by the number of overlapping days (ie, stockpiling was assumed).
Statistical analysis
PS (ie, the probability of receiving CE/BZA vs EP) was estimated using logistic regression models including all covariates (see Covariates). Positivity22 was assessed by comparing distributions of PS in each treatment group to visually assess the extent of nonoverlap. Women exposed to EP were matched without replacement by PS to women exposed to CE/BZA. Matching was tailored to the needs of each database per the number and characteristics of women in each treatment group. Absolute standardized differences were computed to assess covariate balance.23,24
Incidence rates (IR) for each outcome were calculated for each treatment group by database. Incidence rate ratios (RR) for each outcome were calculated with EP users as the referent.
The DerSimonian and Laird random-effects model25 was used to pool the estimates for IR and RR across databases. The Cochran Q test and I2 values were used to assess heterogeneity of results between databases. P value functions were constructed for the pooled endometrial and breast cancer RRs. These functions simultaneously convey the precision of effect estimates and the consistency of the data with various magnitudes of hypothetical effect.26
To account for endometrial and breast cancers that may have had onset before initiating CE/BZA or EP exposure, and to account for potential latency between exposure and risk period, we implemented a sensitivity analysis in three databases (HIRD, MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases, and MarketScan Medicaid) in which follow-up began 6 months after first exposure to CE/BZA or EP. Effect estimates were then pooled across these three databases.
Data management and analysis took place in 2019 to 2020 using Statistical Analysis System (SAS) Enterprise Guide version 7.15 (SAS Institute, Cary, NC) and SAS 9.4 (SAS Institute Inc., Cary, NC) and were completed by HealthCore (HIRD and MarketScan databases), Pfizer (data management for MarketScan databases only, with data extraction code authored by HealthCore), Optum (ORD), and CVS Health Clinical Trial Services (as Healthagen). Pooled analyses were completed by HealthCore using the metafor package in R.27,28
RESULTS
Participant characteristics
There were 92,422,425 women with health plan enrollment across the five databases during the study period. We identified 10,603 CE/BZA and 94,531 EP new users meeting the study entry criteria. Of these, 10,596 CE/BZA and 33,818 EP new users were included after PS matching (Table 2). In each database, demographic and clinical covariates were balanced between CE/BZA and EP users in the matched population (SDC Table 7, http://links.lww.com/MENO/B148). Treatment duration by treatment group and study database is shown in SDC Table 8, http://links.lww.com/MENO/B148, and was slightly longer in the CE/BZA group in most databases. Average follow-up time for cancer outcomes was approximately 22 months, and average follow-up time for endometrial hyperplasia and cardiovascular outcomes was 10 to 11 months (SDC Table 9, http://links.lww.com/MENO/B148).
TABLE 2.
US Food and Drug Administration–approved combination hormone therapy products available by prescription in the United States, by estrogen and progestin type, and distribution of index medication in propensity score–matched new user population across five databases
| Commercial name | Estrogen type | Progestin type | Route | Initial US approvala |
n (%)b |
|---|---|---|---|---|---|
| Duavee | CE | N/Ac | Pill | 2013 | 10,596 (24) |
| All EP | — | — | — | — | 33,818 (76) |
| Prempro & Premphase | CE | MPA | Pill | 1995 | 12,403 (28) |
| Activella | Estradiol | Norethindrone acetate | Pill | 1998 | 10,757 (24) |
| Combipatch | Estradiol | Norethindrone acetate | Patch | 1998 | 5,030 (11) |
| Femhrt | Ethinyl estradiol | Norethindrone acetate | Pill | 1999 | 3,239 (7) |
| Angeliq | Estradiol | Drospirenone | Pill | 2005 | 333 (1) |
| Climara Pro | Estradiol | Levonorgestrel | Patch | 2003 | 2,022 (5) |
| Prefest | Estradiol | Norgestimate | Pill | 1999 | 40 (0) |
CE, conjugated estrogens; EP, estrogen-progestin hormone therapy; MPA, medroxyprogesterone acetate; n, number; N/A, not applicable.
aSource: https://www.accessdata.fda.gov/scripts/cder/daf/.
bParticipants in the EP group could have more than one EP type as their index medication. This was the case for n = 6 participants (0.01%).
cBazedoxifene instead of progestin.
Pooled comparative findings
Pooled comparative findings are presented in Figure 1 and Table 3. For all pooled RR values, the P value for the Cochrane Q test was greater than 0.1, and I2 was 0%, with the exception of endometrial hyperplasia, which showed greater heterogeneity than other outcomes (Table 3).
FIG. 1.
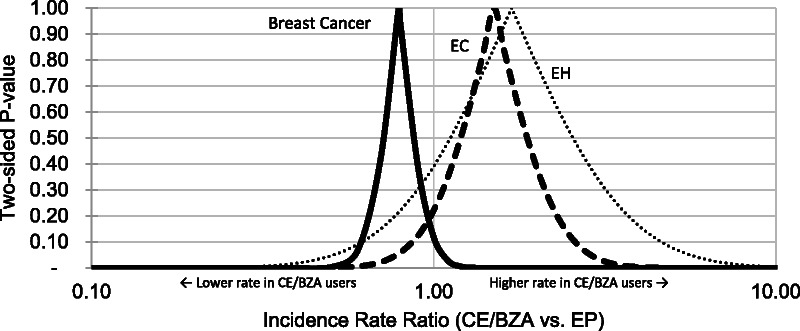
P value functions of RRs for EC, EH, and breast cancer. This plot displays all P values for a range of possible RRs. The probabilities shown are for the observed data given each hypothesized RR. The axis values of this plot can be used to infer confidence limits at varying levels of confidence. For example, x axis values at the 0.05 level of the y axis represent the 95% confidence interval limits. Similarly, the x axis values at the 0.10 level of the y axis represent the 90% confidence interval limits. The peak of each curve represents the study point estimate (rate ratio). The two-sided null hypothesis P value (familiar for its common use in statistical significance testing) is the y axis value at which the x axis value is 1 (eg, P ~ 0.10 for breast cancer). CE/BZA, conjugated estrogens/bazedoxifene; EC, endometrial cancer; EH, endometrial hyperplasia; EP, estrogen-progestin hormone therapy; RR, incidence rate ratio.
TABLE 3.
Pooled random-effects IR and RR for cancer and cardiovascular outcomes in new users of CE/BZA and new users of EP
| CE/BZA (n = 10,596) | EP (n = 33,818) | CE/BZA vs EP (ref) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | PY | IRp (95% CI)a | Q | I 2b | Cases | PY | IRp (95% CI)a | Q | I 2b | RRp (95% CI) | Q | I 2b | |
| Endometrial outcomes | |||||||||||||
| Endometrial cancer | 12 | 19,704 | 5.2 (2.0-8.4) | 3.3 | 0 | 27 | 62,754 | 3.6 (1.1-6.1) | 8.5 | 52.6 | 1.5 (0.8-2.9) | 0.6 | 0 |
| Endometrial hyperplasiad | 14 | 9,689 | 11.0 (1.8-20.2) | 5.9 | 31.8 | 34 | 29,081 | 10.6 (6.1-15.1) | 4.8 | 15.7 | 1.7 (0.5-5.6) | 8.2 | 51.3 |
| Extrauterine outcomes | |||||||||||||
| Any cancerc | 140 | 19,487 | 71.6 (59.7-83.5) | 1 | 0 | 478 | 62,066 | 76.7 (69.8-83.6) | 2.1 | 0 | 0.9 (0.8-1.1) | 0.5 | 0 |
| Breast cancer | 55 | 19,625 | 27.2 (19.9-34.5) | 2.1 | 0 | 231 | 62,422 | 36.3 (30.4-42.2) | 5 | 20.5 | 0.8 (0.6-1.1) | 3.5 | 0 |
| Ovarian cancer | 5 | 19,693 | 2.1 (0.1-4.1) | 1.8 | 0 | 10 | 62,741 | 1.6 (0.6-2.6) | 1.1 | 0 | 1.9 (0.7-5.5) | 2.5 | 0 |
| Thyroid cancer | 13 | 19,677 | 5.9 (2.5-9.2) | 2.5 | 0 | 30 | 62,717 | 4.7 (3.0-6.3) | 1.8 | 0 | 1.5 (0.8-2.9) | 1.9 | 0 |
| Renal cancer or adenoma | 1 | 19,707 | 0.7 (0.0-1.9) | 1.6 | 0 | 8 | 62,756 | 0.9 (0.2-1.7) | 2.7 | 0 | 1.1 (0.3-4.0) | 0.9 | 0 |
| GI cancer | 11 | 19,702 | 4.9 (1.8-8.1) | 1.8 | 0 | 48 | 62,712 | 7.5 (5.4-9.7) | 1.2 | 0 | 0.8 (0.4-1.5) | 1.9 | 0 |
| VTEd | 4 | 9,725 | 3.3 (0.0-6.8) | 2.3 | 0 | 13 | 29,188 | 3.6 (0.7-6.5) | 5.4 | 26.2 | 1.3 (0.5-3.5) | 1.4 | 0 |
| Myocardial Infarctiond | 3 | 9,353 | 3.9 (0.0-7.9) | 0.8 | 0 | 11 | 27,966 | 3.3 (1.2-5.4) | 2.8 | 0 | 1.2 (0.4-3.8) | 1.6 | 0 |
| Stroke/TIAd | 15 | 9,348 | 14.0 (1.0-27.1) | 7.7 | 48.2 | 41 | 27,954 | 13.4 (7.1-19.6) | 6.2 | 34.9 | 1.2 (0.7-2.1) | 1.6 | 0 |
CE/BZA, conjugated estrogens/bazedoxifene; CI, confidence interval; EP, estrogen-progestin hormone therapy; GI, gastrointestinal; IR, incidence rate; IRp, pooled incidence rate; n, number; PY, person-years; RR, incidence rate ratio; RRp, pooled incidence rate ratio; ref, referent; TIA, transient ischemic attack; VTE, venous thromboembolism.
aPer 10,000 PY. IR values were pooled independently from RR values; as a result, the CE/BZA IRp divided by the EP IRp may not equate to the RRp.
bDisplayed as %.
cAny cancer except nonmelanoma skin cancer.
dNoncancer outcomes were censored by the end of current treatment episode.
A total of 39 endometrial cancer cases occurred in 82,458 person-years of follow-up. The IR of endometrial cancer was slightly higher (1.6 additional cases per 10,000 person-years) in the CE/BZA users than in EP users (5.2 vs 3.6 per 10,000 person-years; RR, 1.50; 95% CI, 0.79-2.88; Fig. 1; Table 3). A total of 48 endometrial hyperplasia cases occurred in 38,770 person-years of follow-up. The IR of endometrial hyperplasia was similar, although also slightly higher (0.4 additional cases per 10,000 person-years) in the CE/BZA users than in EP users (11 vs 10.6 per 10,000 person-years; RR, 1.69; 95% CI, 0.51-5.61), although with greater uncertainty due to variation among data sources.
Rates and RRs for additional outcomes are shown in Table 3. The most precise result for a specific outcome was the effect estimate for breast cancer. A total of 286 breast cancer cases occurred in 82,047 person-years of follow-up. The breast cancer IR was lower (9.1 fewer cases per 10,000 person-years) in the CE/BZA users than in EP users (27.2 vs 36.3 per 10,000 person-years; RR, 0.79; 95% CI, 0.58-1.05; Fig. 1; Table 3).
The P value functions show the range of estimates compatible with the data (Fig. 1), indicating greater precision for the breast cancer RR. The P value functions for endometrial cancer and hyperplasia are shifted to the right and wider than the P value function for breast cancer, which is shifted to the left of the null.
After applying a 6-month lag period for endometrial and breast cancers, pooled RRs were 1.88 (95% CI, 0.90-3.91; Q = 0.6; I2 = 0%) for endometrial cancer and 0.65 (95% CI, 0.44-0.97; Q = 0.8; I2 = 0%) for breast cancer in three databases.
Effect estimates for extrauterine outcomes with similar precision to endometrial cancer were thyroid cancer (RR, 1.5; 95% CI, 0.8-2.9), gastrointestinal cancer (RR, 0.8; 95% CI, 0.4-1.5), and stroke (RR, 1.2; 95% CI, 0.7-2.1). The remaining outcomes showed slightly higher rates for CE/BZA but were less precise.
DISCUSSION
We assessed safety outcomes among women initiating CE/BZA for hormone therapy across five large US healthcare databases with a collective 92 million women. Rates of endometrial hyperplasia and endometrial cancer were slightly higher in women who used CE/BZA than in women who used EP, although precision was limited because of small numbers of cases. Breast cancer rates were lower in women who used CE/BZA than in women who used EP, suggesting that the use of BZA for estrogen opposition does not increase breast cancer risk as progestin may be known for.3-5 Among other outcomes, rates of thyroid cancer, ovarian cancer, and stroke/TIA were higher (albeit imprecisely so) in CE/BZA users, although these results are not strongly supported by other literature, and further study would be valuable. Similarly, the results for the remaining outcomes are less precise and are consistent with both potentially important increased and decreased rates comparing CE/BZA with EP.
Previous trials suggest a potential for CE/BZA to reduce risk factors of breast cancer, such as breast density, insulin-like growth factor, and serum progesterone.16,17 The results reported here for breast cancer incidence are consistent with results for surrogate endpoints in the aforementioned clinical trials as well as the protective effects of two other SERMS (raloxifene and tamoxifen) approved in the United States for the prevention of breast cancer in high-risk women.6
Although a randomized controlled trial is the ideal approach to assess causal relationships, these outcomes could not be readily assessed using a trial design because of their infrequency. Even observational studies in large databases are challenged to assemble large populations to study rare outcomes among users of uncommon medications. We used data from 92 million women across five US health insurance claims databases to examine the rates of safety outcomes among new CE/BZA and EP users, likely including most of the women exposed to CE/BZA to date. Despite these efforts, effects are estimated with less precision than we would like. To assess random error, we present P value functions that show the compatibility of the results across a wide range of hypothetical effect sizes for endometrial outcomes and breast cancer (Fig. 1). This strategy avoids dichotomizing continuous P values or confidence intervals into regions of “statistically significant” and “not statistically significant” that has resulted in researchers overlooking potentially important public health effects that were not “statistically significant.”29 Instead, the degree of support for possible effect sizes is considered quantitatively and in light of additional, contextualizing evidence. The data used represent the largest study available at this time, included most of the relevant population, and demonstrated a plausible and potentially important effect on breast cancer, the most common cancer among women worldwide.30
There are several important limitations to this study. Because the safety outcomes of interest and the exposure are uncommon, we pooled findings from five large US health insurance claims databases representing 10,596 new users of CE/BZA and 33,818 new users of EP. Doing so enabled us to include most study-eligible CE/BZA users in the United States during the study period.12 Despite pooling results from these large databases, interpretation is challenging because of few outcome cases and the limited precision of our estimates, and the variation among databases for some estimates.
Because average durations of use tended to be brief and follow-up times were 2 years or less for all outcomes, these data do not address possible long-term effects of CE/BZA versus EP. Important effects may still occur in the short term because of the promotion of growth in undetected existing tumor cells.31,32 After taking possible latency into account and removing cases that were diagnosed in the first 6 months of follow-up, the effect estimates for breast cancer and endometrial cancer both increased in absolute magnitude; the estimate for breast cancer was more strongly protective, whereas the effect estimate for endometrial cancer increased.
This study did not estimate effects according to duration of use, EP formulation, dose, route of administration, or time since menopause, which may affect the incidence of the studied outcomes.33,34 The study compares the effects of substituting BZA for progestin in commonly used hormone therapy and cannot assess individual contributions of each drug or how CE/BZA would compare with CE without progestin. Sample size was insufficient to compare CE/BZA with any particular estrogen dose or formulation.
Results from administrative claims databases can be affected by outcome misclassification. We used validated algorithms for the endometrial cancer and endometrial hyperplasia outcomes,19,20 but claims-based algorithms do not guarantee accurate case ascertainment. In terms of potential bias, the main concern is the extent to which misclassification errors might differ between treatment groups.35 The comparison groups in this study were, however, treated for a common indication, and users were propensity score matched to produce balance between the two groups for potential confounding factors including age, geographic region, medications filled, and comorbidities. Furthermore, screenings for endometrial cancer and breast cancer are typically performed as part of routine preventive care in this population, and baseline mammogram frequency was balanced between the treatment groups after propensity score matching.
As a claims database analysis, this real-world study reflects actual practice and could not conduct baseline imaging or biopsies to ensure that no cancer or hyperplasia existed before treatment initiation. In lieu of this, we examined healthcare claims to identify existing diagnosed malignancy or hyperplasia before treatment initiation and excluded those women from the corresponding analyses as described earlier.
Drug exposure was based on insurance claims for drug dispensings; medication use from drug samples or self-paid fills was not included. In addition, length of treatment episode was based on the days' supply accompanying each medication dispensing, and it is possible that some women took the drug differently from prescribed or not at all.
CONCLUSIONS
In summary, the results of this multidatabase study suggest that users of CE/BZA might experience slightly higher rates of endometrial cancer and endometrial hyperplasia and a lower rate of breast cancer than estrogen/progestin users, providing another option for women considering hormone therapy for vasomotor symptoms of menopause.
Supplementary Material
Footnotes
The views expressed are those of the authors and not necessarily those of Pfizer, Anthem, CVS Health Clinical Trial Services (formerly Healthagen), or Optum.
Findings from this study were presented as a podium presentation at the International Conference on Pharmacoepidemiology & Therapeutic Risk Management in August 2021, with an associated abstract published in Pharmacoepidemiology and Drug Safety (PDS).
Funding/support: This research was supported by Pfizer. This study was conducted to fulfill a postauthorization commitment to the European Medicines Agency. The sponsor of the study (Pfizer Inc.) contributed to the study design, interpretation of findings, coauthorship of the report, and decision to submit for publication. The corresponding author had final responsibility for the interpretation of the findings as submitted for publication.
Financial disclosure/conflicts of interest: Authors V.F., X.Z., R.S., and R.K.G. were employees of Pfizer during the course of the study. All other authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Contributor Information
Samuel Governor, Email: Samuel.bisilki@gmail.com.
Kimberly Daniels, Email: kimberly.daniels@carelon.com.
Ryan M. Seals, Email: ryan.m.seals@optum.com.
Najat J. Ziyadeh, Email: Najat.Ziyadeh@optum.com.
Florence T. Wang, Email: Florence.Wang@optum.com.
Dingwei Dai, Email: Dingwei.Dai@cvshealth.com.
Cheryl N. Mcmahill-Walraven, Email: Cheryl.Walraven@cvshealth.com.
Patty Shuminski, Email: Patty.Shuminski@cvshealth.com.
Vera Frajzyngier, Email: vera.frajzyngier@aetion.com.
Xiaofeng Zhou, Email: Xiaofeng.Zhou@pfizer.com.
Rongjun Shen, Email: Rongjun.Shen@pfizer.com.
Renu K. Garg, Email: Renu.Garg@pfizer.com.
Nicole Fournakis, Email: nicole.fournakis@carelon.com.
Stephan Lanes, Email: stephan.lanes@carelon.com.
Daniel C. Beachler, Email: daniel.beachler@carelon.com.
REFERENCES
- 1.Zhang GQ Chen JL Luo Y, et al. Menopausal hormone therapy and women's health: an umbrella review. PLoS Med 2021;18:e1003731. doi: 10.1371/journal.pmed.1003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beral V Bull D Reeves G, Million Women Study Collaborators . Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005;365:1543–1551. doi: 10.1016/S0140-6736(05)66455-0 [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Menopausal Hormone Therapy and Cancer . Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/mht-fact-sheet. Published 2018. Accessed October 27, 2020.
- 4.Chlebowski RT Anderson G Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med 2008;168:370–377; quiz 345. doi: 10.1001/archinternmed.2007.123 [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT Kuller LH Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 2009;360:573–587. doi: 10.1056/NEJMoa0807684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan VC. A(nother) scientific strategy to prevent breast cancer in postmenopausal women by enhancing estrogen-induced apoptosis? Menopause 2014;21:1160–1164. doi: 10.1097/GME.0000000000000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CL, Santen RJ, Komm B, Mirkin S. Breast-related effects of selective estrogen receptor modulators and tissue-selective estrogen complexes. Breast Cancer Res 2014;16:212. doi: 10.1186/bcr3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores VA, Taylor HS. The effect of menopausal hormone therapies on breast cancer: avoiding the risk. Endocrinol Metab Clin North Am 2015;44:587–602. doi: 10.1016/j.ecl.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food & Drug Administration . Drug Approval Package: Duavee (Conjugated Estrogens and Bazedoxifene). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/022247_duavee_toc.cfm. Published 2013. Accessed October 27, 2020.
- 10.Wyeth Pharmaceuticals Inc. (a Subsidiary of Pfizer) . Duavee Package Insert. Philadelphia, PA: Pfizer; 2013. [Google Scholar]
- 11.European Medicines Agency . Duavive Product Information. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/duavive#authorisation-details-section. Accessed November 9, 2022.
- 12.Shlaen M, von Bredow D. Drug utilization study of conjugated oestrogens/bazedoxifene (CE/BZA) in the European Union (EU): non-interventional study report. Available at: https://www.encepp.eu/encepp/openAttachment/studyResult/34704. Published 2020. Accessed November 9, 2022.
- 13.Pinkerton JV Harvey JA Lindsay R, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab 2014;99:E189–E198. doi: 10.1210/jc.2013-1707 [DOI] [PubMed] [Google Scholar]
- 14.Komm BS, Thompson JR, Mirkin S. Cardiovascular safety of conjugated estrogens plus bazedoxifene: meta-analysis of the SMART trials. Climacteric 2015;18:503–511. doi: 10.3109/13697137.2014.992011 [DOI] [PubMed] [Google Scholar]
- 15.Mirkin S Pinkerton JV Kagan R, et al. Gynecologic safety of conjugated estrogens plus bazedoxifene: pooled analysis of five phase 3 trials. J Womens Health (Larchmt) 2016;25:431–442. doi: 10.1089/jwh.2015.5351 [DOI] [PubMed] [Google Scholar]
- 16.Pinkerton JV Harvey JA Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013;121:959–968. doi: 10.1097/AOG.0b013e31828c5974 [DOI] [PubMed] [Google Scholar]
- 17.Fabian CJ Nye L Powers KR, et al. Effect of bazedoxifene and conjugated estrogen (Duavee) on breast cancer risk biomarkers in high-risk women: a pilot study. Cancer Prev Res (Phila) 2019;12:711–720. doi: 10.1158/1940-6207.CAPR-19-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP)'s European Union electronic Register of Post-Authorisation Studies (EU PAS) . Post-Authorization Safety Study (PASS) of conjugated estrogens/bazedoxifene (CE/BZA) in the United States (EUPAS11599). Available at: http://www.encepp.eu/encepp/viewResource.htm?id=26516. Published 2021. Accessed May 12, 2021.
- 19.Esposito D Banerjee G Yin R, et al. Development and validation of an algorithm to identify endometrial hyperplasia in US administrative claims data. Pharmacoepidemiol Drug Saf 2016;25:3–679. doi: 10.1002/pds.4070 [DOI] [Google Scholar]
- 20.Esposito DB Banerjee G Yin R, et al. Development and validation of an algorithm to identify endometrial adenocarcinoma in US administrative claims data. J Cancer Epidemiol 2019;2019:1938952. doi: 10.1155/2019/1938952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HCUP CCS . Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality. Available at: https://hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Published 2017. Accessed 2020. [PubMed]
- 22.Hernán M, Robins J. Causal Inference: What If. Boca Raton, FL: Chapman & Hall/CRC; 2020. [Google Scholar]
- 23.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannas M, Arpino B. A comparison of machine learning algorithms and covariate balance measures for propensity score matching and weighting. Biom J 2019;61:1049–1072. doi: 10.1002/bimj.201800132 [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 26.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed,. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 28.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amrhein V, Greenland S, McShane B. Retire statistical significance. Nature 2019;567:305–307. doi: 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
- 30.International Agency for Research on Cancer (IARC) Global Cancer Observatory . Estimated age-standardized incidence and mortality rates (World) in 2020, females, all ages (GLOBOCAN 2020). Available at: https://gco.iarc.fr/today/home. Published 2021. Accessed May 10, 2021.
- 31.Dietel M, Lewis MA, Shapiro S. Hormone replacement therapy: pathobiological aspects of hormone-sensitive cancers in women relevant to epidemiological studies on HRT: a mini-review. Hum Reprod 2005;20:2052–2060. doi: 10.1093/humrep/dei043 [DOI] [PubMed] [Google Scholar]
- 32.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ 2020;371:m3873. doi: 10.1136/bmj.m3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith T, Sahni S, Thacker HL. Postmenopausal hormone therapy—local and systemic: a pharmacologic perspective. J Clin Pharmacol 2020;60(Suppl 2):S74–S85. doi: 10.1002/jcph.1740 [DOI] [PubMed] [Google Scholar]
- 34.The NAMS Hormone Therapy Position Statement Advisory Panel . The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24:728–753. doi: 10.1097/GME.0000000000000921 [DOI] [PubMed] [Google Scholar]
- 35.Brenner H, Gefeller O. Use of the positive predictive value to correct for disease misclassification in epidemiologic studies. Am J Epidemiol 1993;138:1007–1015. doi: 10.1093/oxfordjournals.aje.a116805 [DOI] [PubMed] [Google Scholar]


