Background:
Urology has been at the forefront of adopting laparoscopic and robot-assisted techniques to improve patient outcomes. This systematic review aimed to examine the literature relating to the learning curves of major urological robotic and laparoscopic procedures.
Methods:
In accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, a systematic literature search strategy was employed across PubMed, EMBASE, and the Cochrane Library from inception to December 2021, alongside a search of the grey literature. Two independent reviewers completed the article screening and data extraction stages using the Newcastle–Ottawa Scale as a quality assessment tool. The review was reported in accordance with AMSTAR (A MeaSurement Tool to Assess systematic Reviews) guidelines.
Results:
Of 3702 records identified, 97 eligible studies were included for narrative synthesis. Learning curves are mapped using an array of measurements including operative time (OT), estimated blood loss, complication rates as well as procedure-specific outcomes, with OT being the most commonly used metric by eligible studies. The learning curve for OT was identified as 10–250 cases for robot-assisted laparoscopic prostatectomy and 40–250 for laparoscopic radical prostatectomy. The robot-assisted partial nephrectomy learning curve for warm ischaemia time is 4–150 cases. No high-quality studies evaluating the learning curve for laparoscopic radical cystectomy and for robotic and laparoscopic retroperitoneal lymph node dissection were identified.
Conclusion:
There was considerable variation in the definitions of outcome measures and performance thresholds, with poor reporting of potential confounders. Future studies should use multiple surgeons and large sample sizes of cases to identify the currently undefined learning curves for robotic and laparoscopic urological procedures.
Keywords: laparoscopic, learning curves, review, robotic, urology
Introduction
Highlights
The robot-assisted prostatectomy learning curve ranges from 10 to 250 cases.
The robot-assisted partial nephrectomy curve ranges from 4 to 150 cases.
Retroperitoneal lymph node dissection and laparoscopic cystectomy learning curves are undefined.
Standardized reporting of outcomes is required to enable future meta-analysis.
The concept of a ‘learning curve’ was first described in the aeronautics industry in 1936 to illustrate the correlation between improvements in the production of plane components and the increasing experience of the workforce involved1. It has subsequently been adopted in the context of surgical practice, where it has been defined as the number of cases that a surgical trainee needs to undertake in order to reach the point where their inexperience no longer affects the outcomes of the procedure2. This particular stage in their acquisition of skills is represented on conventional learning curve graphs by a plateau3. Notably, although learning curves map the achievement of technical skills proficiency, surgeons must also achieve proficiency in non-technical skills in order to gain overall surgical competency in the procedure4.
Various learning curve metrics are used to plot the surgical learning curve. Operative time (OT) and estimated blood loss (EBL) are two of the most commonly reported learning curve metrics5, with reductions in these variables being associated with surgical training progression6. Urology-specific patient-outcome variables include measures of potency and continence postoperatively, the impairment of which can have a significant impact on the patient’s quality of life7. One or more of four principal methods of analysis are then used to assess change in performance as case number increases8; graphical inspection, the split-group method (dividing the data into consecutive groups and comparing group means), logistic regression and cumulative sum (CUSUM) analysis.
It is highly important to define the learning curve for surgical procedures because detrimental outcomes associated with surgeon inexperience can have a major impact on patient safety, as exemplified by findings of the Bristol Royal Infirmary enquiry9. Knowledge of the learning curve enables the safeguarding of patients by identifying trainees in the learning phase and providing them with adequate supportive measures such as close senior supervision and additional simulation-based skill practice10. Mapping the learning curve can also inform the design of surgical training programmes by illustrating the impact of educational interventions on the learning process. This is particularly relevant in the context of the laparoscopic and robotic approaches which have been widely adopted as the standard of care for urological operations such as prostatectomy and radical nephrectomy. Given their relatively recent implementation in contrast to traditional open approaches, knowledge of their learning curves is important in informing and improving training programmes for these modalities so as to effectively achieve competency across all outcome measures and ensure patient safety.
The last systematic review to include an evaluation of the learning curve for laparoscopic prostatectomy was conducted in 201411. The most recent systematic review evaluating the learning curve for robot-assisted laparoscopic prostatectomy (RALP), robot-assisted radical cystectomy (RARC), robot-assisted partial nephrectomy (RAPN) and robotic pyeloplasty, conducted database searching in February 201812, although this particular review excluded studies published prior to January 2012 as well as single-surgeon studies which constitute the bulk of the relevant learning curve literature base.
The principal aim of this review is to provide an updated insight into the learning curves for major urological robotic and laparoscopic procedures to act as a reference for expected progress. Other aims include the evaluation of the methods used to analyse the learning curves and to provide recommendations for future studies in the field in terms of their scope and methodology.
Methods
Design
A systematic review was performed utilising the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), Supplemental Digital Content 1, http://links.lww.com/JS9/A423 statement13 and was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO) database (ID number: CRD42021251186)14. The review was reported in line with the AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews) checklist15, Supplemental Digital Content 2, http://links.lww.com/JS9/A424, achieving a moderate quality score.
Eligibility criteria
The participants for which the learning curve was mapped were surgeons at any stage of training with no restriction placed on their prior surgical experience. Studies conducted in a simulated surgical setting were excluded. The interventions included were RALP, laparoscopic radical prostatectomy (LRP), RAPN, laparoscopic nephrectomy (including both conventional laparoscopic and hand-assisted techniques), RARC, laparoscopic radical cystectomy (LRC), robotic adult and paediatric pyeloplasty, laparoscopic adult and paediatric pyeloplasty, robotic retroperitoneal lymph node dissection (RPLND) and laparoscopic RPLND. Studies that reported outcomes for multiple eligible procedures but failed to provide separate data for each procedure were excluded.
Randomised controlled trials, non-randomised interventional studies, cohort studies, case series and case–control studies evaluating the learning curve were included. No language restriction was placed on studies. Review articles, conference abstracts, editorials and letters were excluded.
Search strategy
A systematic literature search strategy was employed across PubMed, EMBASE and the Cochrane Library from inception to December 2021 for eligible studies. Both free-text terms and medical subject headings (MeSH) were used in the database search strategies as listed in the Appendix, Supplemental Digital Content 3, http://links.lww.com/JS9/A425. To find relevant full-text articles in the ‘grey literature’, the Google Scholar search engine was used alongside website searching and citation chaining.
Study selection and screening
Two independent reviewers performed the initial article title and abstract screening and then screened the full text of the potentially eligible articles against the inclusion criteria. Where discrepancies were encountered, a third reviewer was consulted to resolve the disagreement.
Data extraction and risk of bias assessment
The two independent reviewers used a pre-defined, standardised form to undertake the data extraction. No specific risk of bias assessment tool exists for learning curve studies, so the two reviewers utilised the Newcastle–Ottawa scale16 as a quality assessment tool. As before, disagreements between the reviewers over the risk of bias scores for the included studies were resolved by referral to a third reviewer.
Data synthesis
It was not possible to perform a meta-analysis of the data collected due to substantial heterogeneity in the included studies, so the results were narratively synthesised in accordance with PRISMA13 guidelines, Supplemental Digital Content 1, http://links.lww.com/JS9/A423, with summary diagrams produced to provide the range of values for which the learning curve associated with a particular procedure and its outcomes were numerically defined. This provides surgeons at any stage of training with a guide of expected progress against which they can compare their own performances. Similar descriptive and diagrammatic approaches have been utilised in previous systematic reviews evaluating urological learning curves11,17, where meta-analysis was not possible.
Results
Three thousand eight hundred and seventy-eight records were identified through database searching, with an additional 25 records identified through a search of the grey literature and citation chaining. After deduplication, 2912 studies were eligible for the title and abstract screening. Two thousand two hundred and sixty-one studies were then excluded, so 651 studies underwent full-text screening. Five hundred and fifty-four studies were then excluded, with 97 studies thus included for narrative synthesis in this review. Figure 1 illustrates this study’s selection and screening process.
Figure 1.

PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram.
Characteristics of included studies
Fifty-one studies were retrospective in design whilst 46 were prospective studies. Fifty-five studies mapped the learning curve of single surgeons, 39 involved multiple surgeons (with one study18 including as many as 744 surgeons) and 3 did not report the exact number of surgeons involved. The number of procedures ranged from 2019 to 27 34818 with study durations ranging from 10 months20 to 259 months21. The earliest publication date was 200022, with the most recent included study published in April 202123.
Table 1 displays the full study characteristics of the included studies.
Table 1.
Characteristics of the included studies.
| Study | Procedure, country | Study design | Number of surgeons | Previous experience | Number of procedures | Study duration (months) |
|---|---|---|---|---|---|---|
| Adili et al. (2017)24 | RALP (transperitoneal), Canada | Prospective | 1 | 600 LRPs | 400 | 37 |
| Ahlering et al. (2003)20 | RALP (extraperitoneal), USA | Prospective | 1 | Experienced in open | 45 | 10 |
| Alemozaffar et al. (2012)25 | RALP, USA | Retrospective | 1 | 76 open RPs, 11 months’ fellowship experience of RALP (without nerve-sparing) | 400 | 20 |
| Al-Hathal and El-Hakim (2013)26 | RALP, Canada | Prospective | 1 | NR | 250 | 72 |
| Atug et al. (2006)27 | RALP, USA | Retrospective | 3 | Experienced in open and laparoscopic | 100 | 21 |
| Bravi et al. (2019)28 | RALP (transperitoneal), Italy | Retrospective | 9 | 8 with no experience in robotics1 with >50 RALP experience | 1827 | 132 |
| Chan and Pautler (2019)29 | RALP, Canada | Retrospective | 1 | Fellowship-trained | 577 | 65 |
| Chang et al. (2016)30 | RALP (transperitoneal), China | Retrospective | 3 | 1 experienced in open only1 experienced in laparoscopic only1 experienced in open and laparoscopic | 388 | 41 |
| Chen et al. (2020)31 | RALP (transperitoneal), China | Prospective | 1 | Experienced in ORP, no LRP experience | 500 | 67 |
| Davis et al. (2014)18 | RALP, USA | Retrospective | 744 | NR | 27348 | 72 |
| Dev et al. (2012)32 | RALP (extraperitoneal), UK | Prospective | 1 | Experienced in laparoscopic | 150 | NR |
| Di Pierro et al. (2015)33 | RALP (with ePLND), Italy | Prospective | 1 | Experienced in open and laparoscopic | 233 | 47 |
| Doumerc et al. (2010)34 | RALP (transperitoneal), Australia | Prospective | 1 | Experienced in open | 212 | 35 |
| Fossati et al. (2017)35 | RALP, Italy | Prospective | 4 | NR | 1477 | 96 |
| Galfano et al. (2021)36 | RALP (Retzius-sparing), 12 international centres | Retrospective | 14 | 12 experts at standard RALP2 had no first-hand RALP experience | 626 | 84 |
| Good et al. (2015)37 | RALP (transperitoneal), UK | Retrospective | 1 | Experienced in open and laparoscopic RPs | 531 | 73 |
| LRP, UK | Retrospective | 2 | 1 with 19 open RP and 5 LRP fellowship experience1 with 160 LN, 20 open RP experience | 550 | 104 | |
| Gumus et al. (2011)38 | RALP (extraperitoneal), Turkey | Prospective | 1 | No laparoscopic experience | 120 | 27 |
| Hashimoto et al. (2013)39 | RALP, Japan | Prospective | 1 | 500 open RPs, no laparoscopic experience | 200 | 60 |
| Herrell and Smith (2005)40 | RALP, USA | Prospective | 1 | Highly experienced in open | 350 | NR |
| Islamoglu et al. (2018)41 | RALP (transperitoneal), Turkey | Retrospective | 1 | Experienced in open and laparoscopic RPs | 111 | 31 |
| Ko et al. (2009)42 | RALP (transperitoneal), South Korea | Prospective | 1 | >300 open RPs and >20 laparoscopic RPs | 63 | 13 |
| Lee et al. (2010)43 | RALP (transperitoneal), South Korea | Prospective | 1 | NR | 307 | 35 |
| Monnerat Lott et al. (2018)45 | RALP (transperitoneal), Brazil | Prospective | 2 | 10 years’ experience of open RP | 119 | 37 |
| Maddox et al. (2013)44 | RALP (extraperitoneal), USA | Prospective | 3 | 1 with limited experience with laparoscopic RP1 with significant exposure to RALP1 with no robotic experience | 575 | 49 |
| Ohwaki et al. (2020)46 | RALP, Japan | Retrospective | 4 | NR | 540 | 76 |
| Ou et al. (2011)47 | RALP, Taiwan | Prospective | 1 | Open and laparoscopic experience | 200 | 49 |
| Pardalidis et al. (2008)48 | RALP (transperitoneal), Greece | Prospective | 1 | NR | 40 | 8 |
| Patel et al. (2005)49 | RALP, USA | Prospective | 1 | Experienced in open | 200 | 18 |
| Ploussard et al. (2010)50 | RALP (extraperitoneal), France | Prospective | 2 | Experienced in LRP | 206 | 89 |
| Samadi et al. (2007)51 | RALP, USA | Prospective | 1 | Experienced in laparoscopic | 70 | 22 |
| Sammon et al. (2010)52 | RALP (extraperitoneal), USA | Prospective | 3 | 2 experienced in laparoscopic, 1 not | 225 | 36 |
| Sharma et al. (2011)53 | RALP (extraperitoneal), UK | Prospective | 2 | 1 experienced in open prostatectomy1 experienced in laparoscopy | 500 | 48 |
| Sivaraman et al. (2017)54 | RALP, France | Retrospective | 9 | 250 LRP experiences each | 1701 | 180 |
| LRP, France | Retrospective | 9 | NR | 3846 | 180 | |
| Slusarenco et al. (2020)55 | RALP (transperitoneal), Russia | Retrospective | 1 | Experienced in laparoscopy | 145 | 24 |
| Song et al. (2020)56 | RALP (transperitoneal), South Korea | Retrospective | 1 | No experience of any form of RP | 480 | 59 |
| Thompson et al. (2018)57 | RALP, Australia | Prospective | 1 | >3000 ORPs | 1520 | 120 |
| van der Poel et al. (2012)58 | RALP, Netherlands | Prospective | 2 | >50 ORPs each | 440 | 60 |
| Williams et al. (2011)59 | RALP (transperitoneal), Canada | Prospective | 1 | >200 open and laparoscopic RPs | 158 | 53 |
| Wu et al. (2008)60 | RALP (extraperitoneal), Taiwan | Prospective | 1 | Experienced in laparoscopic (limited RP experience) | 24 | 25 |
| Baumert et al. (2004)61 | LRP (transperitoneal), France | Retrospective | 1 | Performed >30 various laparoscopic procedures, assisted 20 LRPs | 100 | 16 |
| Di Gioia et al. (2013)62 | LRP (transperitoneal), Brazil | Retrospective | 1 | Experienced in open prostatectomy and upper tract laparoscopy | 240 | 84 |
| Dias et al. (2017)63 | LRP (transperitoneal), Brazil | Prospective | 2 | RALP experienced, trained in LRP | 110 | 20 |
| Eden et al. (2009)64 | LRP (transperitoneal initially, then extraperitoneal), UK | Prospective | 1 | Experienced in open RP and reconstructive laparoscopy | 1000 | 93 |
| Eden et al. (2013)65 | LRP (transperitoneal, ePLND) | Retrospective | 1 | Open RP, RALP, performed or supervised 868 LRPs (8 ePLNDs) | 500 | 48 |
| Handmer et al. (2018)66 | LRP, Australia | Retrospective | 9 | Fellowship-trained in LRP | 2943 | 132 |
| Hruza et al. (2010)67 | LRP (transperitoneal initially, then extraperitoneal), Germany | Prospective | 5 | 1 first-gen surgeon (only open exp.)2 second-gen surgeons (open exp., trained by first-gen)2 third-gen surgeons (limited open exp., trained by first-gen or second-gen) | 2200 | 96 |
| Mason et al. (2016)68 | LRP (extraperitoneal), UK | Prospective | 2 | Experienced in laparoscopic | 500 | 102 |
| Mitre et al. (2013)69 | LRP (extraperitoneal), Brazil | Prospective | 1 | Experienced in other laparoscopic procedures | 165 | 94 |
| Poulakis et al. (2005)70 | LRP (transperitoneal initially, then extraperitoneal), Germany | Prospective | 1 | >100 open RPs, no laparoscopic experience | 50 | 17 |
| Rodriguez et al. (2010)71 | LRP (extraperitoneal), USA | Retrospective | 1 | NR | 400 | 30 |
| Secin et al. (2010)72 | LRP, international | Retrospective | 51 | 1 with 285 LRP experience50 with open RP experience | 8544 | 102 |
| So et al. (2011)73 | LRP (mostly transperitoneal, last 11 were extraperitoneal), South Korea | Retrospective | 1 | Fellowship-trained in laparoscopy | 100 | 31 |
| Vickers et al. (2009)74 | LRP, European and North American institutions | Retrospective | 29 | Only 1 with LRP experience | 5328 | 113 |
| Bajalia et al. (2020)75 | RAPN, USA | Retrospective | 1 | Fellowship-trained | 418 | 135 |
| Castilho et al. (2020)76 | RAPN, Brazil | Retrospective | 1 | No laparoscopic experience | 101 | 48 |
| Dias et al. (2018)77 | RAPN, India | Prospective | 1 | Experienced in laparoscopy | 108 | 59 |
| Hanzly et al. (2015)78 | RAPN, USA | Retrospective | 1 | RALP experience, no LPN experience | 116 | NR |
| LPN, USA | Retrospective | 2 | Fellowship experience | 116 | NR | |
| Haseebuddin et al. (2010)79 | RAPN, USA | Prospective | 1 | >200 LPNs | 38 | 12 |
| Larcher et al. (2019)80 | RAPN, 2 European centres | Prospective | Multiple | NR | 457 | 132 |
| Lavery et al. (2011)81 | RAPN, USA | Retrospective | 1 | >100 LPNs, 100 RALPs, 15 robotic pyeloplasties | 20 | 21 |
| Motoyama et al. (2020)82 | RAPN, Japan | Retrospective | 1 | 300 RALPs, >100 open PNs, 15 LPNs | 65 | 20 |
| Mottrie et al. (2010)83 | RAPN, Belgium | Prospective | 1 | 15 LPN, experienced in robotic | 62 | 39 |
| Omidele et al. (2018)84 | RAPN, USA | Retrospective | 1 | Fellowship-trained in robotics and laparoscopy | 131 | 91 |
| Pierorazio et al. (2011)85 | RAPN, USA | Retrospective | 1 | Experienced in minimally invasive surgery | 48 | NR |
| LPN, USA | Retrospective | 1 | Experienced in minimally invasive surgery | 102 | NR | |
| Tobis et al. (2012)86 | RAPN, USA | Retrospective | 3 | Experienced in minimally invasive surgery | 100 | 30 |
| Xie et al. (2016)87 | RAPN, China | Retrospective | 1 | >1000 LPNs | 144 | 12 |
| Zeuschner et al. (2021)88 | RAPN, Germany | Retrospective | 7 | NR | 500 | 132 |
| Azawi et al. (2014)89 | HALPN (with early arterial clamp removal), Denmark | Prospective | 3 | NR | 60 | 21 |
| Bhayani (2008)90 | LPN, USA | Retrospective | 1 | Fellowship-trained in LPN | 50 | 30 |
| Gaston et al. (2004)19 | HALN, USA | Prospective | 6 | Residents each with experience of ≥15 open nephrectomies | 30 | 22 |
| Gill et al. (2001)91 | LRN, USA | Prospective | 1 | NR | 100 | 35 |
| Gozen et al. (2017)21 | LRN, Germany | Prospective | 6 | 1st gen surgeons: experienced in open only.2nd gen surgeons: experienced in open, trained by 1st gen.3rd gen surgeons: no open experience, trained by 1st or 2nd gen | 330 | 259 |
| Jeon et al. (2009)92 | LRN, South Korea | Retrospective | 3 | No laparoscopic experience | 150 | 59 |
| Kanno et al. (2006)93 | LN, Japan | Retrospective | 6 | NR | 78 | 47 |
| Kawauchi et al. (2005)94 | HALN, Japan | Retrospective | 18 | Each performed ≥20 open RNs or nephroureterectomies | 166 | NR |
| Link et al. (2005)95 | LPN, USA | Retrospective | 1 | Experienced in laparoscopic | 178 | 63 |
| Masoud et al. (2020)96 | LN, Egypt | Prospective | 1 | Trainee urologist, access to laparoscopic simulation training | 40 | 28 |
| Porpiglia et al. (2013)97 | LPN, Italy | Prospective | 1 | NR | 302 | 132 |
| Collins et al. (2014)98 | RARC (with intracorporeal neobladder), Sweden | Prospective | 2 | NR | 67 | 106 |
| Dell’Oglio et al. (2020)99 | RARC (with intracorporeal urinary diversion), Belgium | Prospective | 2 | Limited robotic experience (<20 cases) | 164 | 156 |
| Guru et al. (2009)100 | RARC (with ePLND), USA | Retrospective | 1 | Experienced in RALP, formal robotic fellowship | 100 | 34 |
| Hayn et al. (2011)101 | RARC, USA | Prospective | 1 | Experienced in robotics | 164 | 45 |
| Hellenthal et al. (2010)102 | RARC, 15 international centres | Prospective | 22 | NR | 513 | 72 |
| Honore et al. (2019)103 | RARC, Australia | Retrospective | 1 | Experienced robotic pelvic surgeon | 100 | 72 |
| Pruthi et al. (2008)104 | RARC (with extracorporeal urinary diversion, USA | Retrospective | 1 | Experienced in open cystectomy and RALP | 50 | 23 |
| Wang et al. (2019)105 | RARC (with intracorporeal urinary diversion), USA | Retrospective | 2 | Fellowship-trained | 56 | 39 |
| Aboumarzouk et al. (2012)106 | Laparoscopic radical cystectomy (with PLND and urinary diversion), Poland | Prospective | 1 | NR | 65 | 61 |
| Chammas et al. (2019)107 | Robotic pyeloplasty (adult), Brazil | Prospective | 1 | Experienced in open | 100 | 62 |
| Cundy et al. (2015)108 | Robotic pyeloplasty (paediatric), UK | Prospective | 1 | Experienced in laparoscopy | 90 | 92 |
| Dothan et al. (2021)109 | Robotic pyeloplasty (paediatric), Israel | Retrospective | 1 | Experienced in open and laparoscopic pyeloplasty | 33 | 192 |
| Kassite et al. (2018)110 | Robotic pyeloplasty (paediatric), France | Retrospective | 2 | Experienced in laparoscopy (but no laparoscopic pyeloplasty experience) | 42 | 121 |
| Sorensen et al. (2011)111 | Robotic pyeloplasty (paediatric), USA | Retrospective | 2 | Experienced in laparoscopy (but minimal laparoscopic pyeloplasty experience) | 33 | 34 |
| Tasian et al. (2013)112 | Robotic pyeloplasty (paediatric), USA | Prospective | 5 | 4 fellows with adult robotic experience1 attending with paediatric robotic experience | 100 | 48 |
| Calvert et al. (2008)113 | Laparoscopic pyeloplasty (adult), UK | Retrospective | NR | NR | 49 | 60 |
| Panek et al. (2020)114 | Laparoscopic pyeloplasty (paediatric), Poland | Retrospective | 1 | NR | 95 | 120 |
| Janetschek et al. (2000)22 | Laparoscopic RPLND, Austria | Retrospective | NR | NR | 64 | 76 |
| Schermerhorn et al. (2021)23 | Robotic RPLND, USA | Retrospective | 4 | Trained in robotics and laparoscopy | 121 | 120 |
ePLND, extended pelvic lymph node dissection; gen., generation; HALN, hand-assisted laparoscopic nephrectomy; HALPN, hand-assisted laparoscopic partial nephrectomy; LN, lymph nodes; LPN, laparoscopic partial nephrectomy; LRN, laparoscopic radical nephrectomy; LRP, laparoscopic radical prostatectomy; NR, not reported; ORP, open radical prostatectomy; PLND, pelvic lymph node dissection; PN, partial nephrectomy; RALP, robot-assisted laparoscopic prostatectomy; RAPN, robot-assisted partial nephrectomy; RARC, robot-assisted radical cystectomy; RN, radical nephrectomy; RP, radical prostatectomy; RPLND, retroperitoneal lymph node dissection.
Risk of bias assessment
Included studies scored either 5 or 6 on the Newcastle–Ottawa scale, scoring lowest in the ‘Selection’ domain due to the potential for selection bias and the lack of control groups.
Findings
The main findings of the included studies are listed in Table 2. Where studies defined the learning curve for ‘overall performance’, this referred to the number of cases required to achieve competency across the range of outcomes they measured.
Table 2.
Results of the included studies.
| Study | Procedure, country | Outcome measures | Learning Curve Model, statistical analysis | Thresholds in surgeon performance | Learning curve, outcome measure: number of cases |
|---|---|---|---|---|---|
| Adili et al. (2017)24 | RALP (transperitoneal), Canada | PSM, OT, EBL, LOS | Split-group (quartiles); logistic regression model, Student’s t-test | Performance in most recent quartile used as reference standard | PSM: no statistically significant learning curve, OT, EBL: reductions from first quartile compared to last quartile |
| Ahlering et al. (2003)20 | RALP (extraperitoneal), USA | OT, individual step time, EBL, LOS, PSM, C, TR, post-op Hb decrease, continence, potency | Graphical inspection, split-group; no tests for statistical significance reported | Number of cases to achieve 4 h proficiency | OT: 12 |
| Alemozaffar et al. (2012)25 | RALP, USA | PSM, EPIC sexual function (at 5 and 12 months), potency (at 5 and 12 months) | Split-group (octiles); linear regression models, Cochran–Armitage trend test | Number of cases to reach plateau | EPIC sexual function: 250–300 |
| Al-Hathal and El-Hakim (2013)26 | RALP, Canada | OT, EBL, C, TR, CR, LOS, PSM, continence, potency | Split-group (quintiles); no tests for statistical significance reported | Number of cases to reach plateau | OT: 50, PSM (pT2): 50 |
| Atug et al. (2006)27 | RALP, USA | PSM | Split-group (thirds); χ 2 test, ANOVA | Reduction in PSM rates | PSM: ≈30 |
| Bravi et al. (2019)28 | RALP (transperitoneal), Italy | PSM, BCR | Multivariable logistic regression model; Wilcoxon rank-sum test, χ 2 test, multivariable Cox regression model | Number of cases to reach plateau | PSM: 200 |
| Chan and Pautler (2019)29 | RALP, Canada | C, continence, LOS, BCR | CUSUM method; no tests for statistical significance reported | Number of cases to reach plateau | C: ≈500 |
| Chang et al. (2016)30 | RALP (transperitoneal), China | OT, LOS, EBL, PSM, continence, BCR | Graphical inspection, split-group (groups of 20); χ 2 test, Student–Newman–Keuls test, ANOVA, Kruskal–Wallis test | Number of cases to reach plateau | For open exp surgeon: OT and EBL plateau after 20No plateau for lap-exp surgeonFor surgeon exp. in both open and lap: OT plateaus after 40, no plateau for EBL |
| Chen et al. (2020)31 | RALP (transperitoneal), China | OT, EBL, LOS, PSM | Split-group (quintiles); ANOVA, Kruskal–Wallis test | Number of cases to reach plateau | OT, EBL, LOS: 200 |
| PSM: no statistically significant learning curve | |||||
| Davis et al. (2014)18 | RALP, USA | OT, LOS, C, CR | Split-group; Student’s t-test, χ 2 test, Jonckheere–Terpstra test | NR | OT, LOS and C all improve in first 100 with additional improvements in next 100 |
| Dev et al. (2012)32 | RALP (extraperitoneal), UK | OT, individual step time, EBL, LOS, PSM | Split-group; linear regression model, Wald test | NR | Individual step times all significantly decreased between the first 25 and last 50 procedures, except for the Rocco stitch step |
| Di Pierro et al. (2015)33 | RALP (with ePLND), Italy | OT, time for ePLND, C, resected lymph node yield, positive lymph nodes, PSM | Split-group (quartiles); χ 2 test, Kruskal–Wallis test, logistic regression models, mixed linear regression models | Number of cases to reach plateau | Lymph node yield: 60 |
| Doumerc et al. (2010)34 | RALP (transperitoneal), Australia | OT, EBL, TR, C, LOS, PSM, continence | Join point regression model; Monte Carlo permutation method, χ 2 test, ANOVA | Number of cases to reach 3 h proficiencyNumber of cases to reach plateauNumber of cases to match open performances | OT: 110 (3 h proficiency)PSM (pT2): plateau at 140PSM (pT3): plateau at 170200 for early continence (6 weeks) |
| Fossati et al. (2017)35 | RALP, Italy | Continence | Graphical inspection; multivariable Cox regression analysis | NR | Continence: no plateau reached after >200>400 to increase continence recovery rate at 1 year from 60% to ≈90% |
| Galfano et al. (2021)36 | RALP (Retzius-sparing), 12 international centres | OT, console time, EBL, TR, C, LOS, continence, potency, PSM, BCR | Split-group (halves), graphical inspection with LOWESS; Mann–Whitney U-test, χ 2 test, Kaplan–Meier method | Number of cases to reach plateau | OT, console time, continence, C: all show continued improvement over first 50 with no plateauPSM: no improvement over first 50 |
| Good et al. (2015)37 | RALP (transperitoneal), UK | OT, EBL, C, PSM, continence (at 3 months) | Split-group, LOWESS; Mann–Whitney U test, Pearson χ 2 test | Number of cases to reach plateau | OT: 250EBL: 250PSM (overall): 300PSM (pT2): 300PSM (pT3): No plateau reachedC: 250Continence: 100 |
| Gumus et al. (2011)38 | RALP (extraperitoneal), Turkey | OT, EBL, TR, PSM, BCR, LOS, continence, potency | Split-group (thirds); χ 2 test, ANOVA, Kruskal–Wallis test | Number of cases to achieve comparable outcomes to the published results of high-volume centres | 80–120 |
| Hashimoto et al. (2013)39 | RALP, Japan | OT, EBL, PSM, C, continence | Split-group; χ 2 test, Spearman’s rank correlation test, Mann–Whitney U-test | Number of cases to achieve ‘acceptable’ outcomes | OT: 25PSM, C: 50Continence: 100 |
| Herrell and Smith (2005)40 | RALP, USA | EBL, haematocrit decrease, TR, LOS, post-op pain, QoL, PSM, continence, potency | NR | Number of cases to achieve outcomes comparable to open approach | >150 |
| Islamoglu et al. (2018)41 | RALP (transperitoneal), Turkey | OT, LOS, haematocrit decrease, PSM, BCR | Moving average method, split-group; Fisher’s exact test, Pearson’s χ 2 test, Student’s t-test, Mann–Whitney U-test | Number of cases to reach plateau | OT: 50 |
| Ko et al. (2009)42 | RALP (transperitoneal), South Korea | OT, setup time, console time, EBL, LOS, C, TR, PSM, urine leakage on cystogram at 14 days, continence, potency | Split-group; Spearman’s rank correlation test, χ 2 test, Mann–Whitney U-test | Number of cases for TR and urine leakage to reach zero | TR: >15Urine leakage on cystogram at 14 days: >20 |
| Lee et al. (2010)43 | RALP (transperitoneal), South Korea | OT, EBL, PSM, C, LOS | Split-group; ANOVA, χ 2 test, Spearman’s rank correlation test | Number of cases to reach plateau | OT, EBL: >24PSM: continuous decrease with no plateau |
| Monnerat Lott et al. (2018)45 | RALP (transperitoneal), Brazil | OT, console time, EBL, PSM, C, continence, potency | Split-group; Fisher’s exact test, χ 2 test, ANOVA, Tukey test | Number of cases to reach plateau | Continence, potency, PSM: plateau not reached in first 100 |
| Maddox et al. (2013)44 | RALP (extraperitoneal), USA | OT, console time, C, PSM, TR, CR | Split-group (quintiles); ANOVA | Number of cases to reach plateau | Minor C: stable throughoutMajor C: drop after 100 |
| Ohwaki et al. (2020)46 | RALP, Japan | OT, EBL, PSM | CUSUM method; χ 2 test, Kruskal–Wallis test | Number of cases to achieve competency | PSM: 45 |
| Ou et al. (2011)47 | RALP, Taiwan | OT, console time, EBL, C, TR, PSM | Split-group (quartiles); Mann–Whitney U-test, Fisher’s exact test | Number of cases to significantly reduce complications | C, TR: 150 |
| Pardalidis et al. (2008)48 | RALP (transperitoneal), Greece | OT, EBL, C, LOS, pain, continence, potency, PSM | Split-group; no tests for statistical significance reported | NR | 10–12 |
| Patel et al. (2005)49 | RALP, USA | OT, EBL, C, LOS, PSM, continence | Split-group (quartiles); no tests for statistical significance reported | Number of cases to achieve outcomes comparable to open approach | 20–25 |
| Ploussard et al. (2010)50 | RALP (extraperitoneal), France | OT, EBL, TR, C, CR, LOS, PSM continence, potency | Split-group (quintiles); χ 2 test, Fisher’s exact test, Mann–Whitney U-test | Number of cases to achieve competency | OT: 10 (for 3 h proficiency)EBL, C, TR, LOS: ≈30PSM (pT2): ≈60 |
| Samadi et al. (2007)51 | RALP, USA | OT, EBL, LOS, duration of catheterisation, continence | Split-group (quartiles); tests used for statistical significance were not reported | Number of cases to reach plateau | Plateau not reachedGreatest improvements occurred in first 20 |
| Sammon et al. (2010)52 | RALP (extraperitoneal), USA | OT, EBL, LOS, BNC, PSM | Graphical inspection, non-linear regression model; t-tests, Fisher’s exact test | Number of cases to reach plateau | OT: 25 |
| Sharma et al. (2011)53 | RALP (extraperitoneal), UK | OT, EBL, LOS, C, CR, PSM, continence, potency | Moving average, split-group; multivariable logistic regression, χ 2 test | Number of cases to reach plateau | OT, EBL: continuous improvement, no plateau reached in 330 |
| Sivaraman et al. (2017)54 | RALP, France | PSM, BCR | CUSUM method, logistic regression; Kaplan–Meier curves, Cox regression | Number of cases to reach ‘transition point’ (plateau) in the learning phase | PSM, BCR: 100 |
| Slusarenco et al. (2020)55 | RALP (transperitoneal), Russia | OT, EBL, C, CR, TR, PSM, duration of catheterisation, continence, potency | Split-group; tests used for statistical significance were not reported | Number of cases to reach 3 h proficiencyNumber of cases to achieve median blood loss (150 ml) | OT: >80EBL: 50 |
| Song et al. (2020)56 | RALP (transperitoneal), South Korea | OT, EBL, C, PSM, continence, potency | Graphical inspection with LOWESS; independent t-test, Pearson’s χ 2 test | Number of cases to reach lowest point for each outcome | OT: 200EBL: 230 |
| Thompson et al. (2018)57 | RALP, Australia | PSM, QoL, BCR | Modelled as natural log function; logistic regression, Cox regression | Number of cases to reach plateau | PSM (pT2): 477PSM (pT3/pT4): 360BCR: 226 |
| van der Poel et al. (2012)58 | RALP, Netherlands | OT, time for lymph node dissection, number of removed lymph nodes, % patients with nodal metastases, C | Split-group: univariate and multivariate logistic regression models | Number of cases to reach plateau | OT: 150Lymph node yield: 250Node positivity rate: 300C: 400 |
| Williams et al. (2011)59 | RALP (transperitoneal), Canada | OT, EBL, PSM | CUSUM method; Fisher’s exact test, Kruskal–Wallis test | Number of cases to reach plateau | PSM (pT2): ≈110 (no PSMs in last 50) |
| Wu et al. (2008)60 | RALP (extraperitoneal), Taiwan | OT, console time, EBL, TR, C, PSM, continence | Graphical inspection, split-group; no tests for statistical significance reported | Number of cases to reach plateau | 18 |
| Baumert et al. (2004)61 | LRP (transperitoneal), France | PSM | Split-group (quartiles); χ 2 test, Fisher’s exact test, Student’s t-test, ANOVA | NR | PSM length and rate significantly lower in last 50 cases |
| Di Gioia et al. (2013)62 | LRP (transperitoneal), Brazil | OT, anastomosis time, EBL, LOS, C, continence, potency, PSM | Split-group (thirds); Kruskal–Wallis test, χ 2 test, Fisher’s exact test | Number of cases to reach potency | OT, anastomosis time: 80 |
| Dias et al. (2017)63 | LRP (transperitoneal), Brazil | OT, EBL, LOS, C, continence, PSM, CR, TR | Split-group (quartiles); Fisher’s exact test, Kruskal–Wallis test, ANOVA, Tukey test, logistic regression | Number of cases to reach plateau | OT: 40 (plateaued at 150 min)Continence: 70 (plateaued at 95%) |
| Eden et al. (2009)64 | LRP (transperitoneal initially, then extraperitoneal), UK | OT, EBL, TR, CR, LOS, C, PSM, continence, potency | Logistic regression model; independent samples t-test, Fisher’s exact test | Number of cases to reach plateau | OT, EBL: 100–150C, continence: 150–200Potency: 700 |
| Eden et al. (2013)65 | LRP (transperitoneal, ePLND) | OT, EBL, TR, LOS, C (generic and PLND-specific), lymph node yield | CUSUM method; independent samples t-test, Fisher’s exact test | Number of cases to reach plateauNumber of cases to achieve acceptable failure rate (FR) | OT: 130 (plateau)C (generic): 136 (10% FR), 346 (5% FR)C (PLND-specific): 40 (5% FR)Lymph node yield: 150 (plateau) |
| Good et al. (2015)37 | LRP, UK | OT, EBL, C, PSM, continence (at 3 months) | Split-group, LOWESS; Mann–Whitney U-test, Pearson χ 2 test | Number of cases to reach plateau | OT: 250EBL: 250PSM (overall): 200PSM (pT2): 250PSM (pT3): 200C: 250Early continence: 350 |
| Handmer et al. (2018)66 | LRP, Australia | OT, EBL, TR, CR, PSM | Split-group (first 100 vs. second 100); Pearson’s χ 2 test | NR | Improvements noted in second 100 compared to first 100, no significant improvement in PSM rates between the two groups |
| Hruza et al. (2010)67 | LRP (transperitoneal initially, then extraperitoneal), Germany | OT, EBL, LOS, PSM, NSM, lymph node status, C | Split-group; Pearson χ 2 test, Fisher’s exact test, logistic regression model | Number of cases to reach plateau | C: 700 (1st gen.), 250 (3rd gen.) |
| Mason et al. (2016)68 | LRP (extraperitoneal), UK | OT, EBL, LOS, C, PSM | Split-group; multivariable regression models | Number of cases to reach plateau | LOS: 100PSM (pT2): 150 |
| Mitre et al. (2013)69 | LRP (extraperitoneal), Brazil | OT, EBL, TR, C, CR, PSM, BCR | Split-group (thirds); χ 2 test, ANOVA, Bonferroni test | Number of cases for significant decreaseNumber of cases to reach plateau | C, CR: significant decrease after first 51OT, EBL, PSM: 110 |
| Poulakis et al. (2005)70 | LRP (transperitoneal initially, then extraperitoneal), Germany | OT, EBL, LOS, PSM, BCR, difficulty scores (relative to open RP), duration of catheterisation | Multivariate regression; t-test, Fisher’s exact test, linear regression | Number of cases to achieve rapid decrease in outcome | OT: 21 |
| Rodriguez et al. (2010)71 | LRP (extraperitoneal), USA | OT, EBL, PSM, LOS, TR | Split-group (quartiles); Kruskal–Wallis test, Fisher’s exact test, χ 2 test, Wilcoxon rank-sum test | Numbers of cases for significant decrease | OT: 100PSM (pT2): 200 |
| Secin et al. (2010)72 | LRP, international | PSM | Split-group; multivariable regression models | Number of cases to reach plateau | PSM: 200–250 |
| Sivaraman et al. (2017)54 | LRP, France | PSM, BCR | CUSUM method, logistic regression; Kaplan–Meier curves, Cox regression | Number of cases to reach ‘transition point’ (plateau) in the learning phase | PSM, BCR: 350 |
| So et al. (2011)73 | LRP (mostly transperitoneal, last 11 were extraperitoneal), South Korea | OT, EBL, PSM, continence, potency | Split-group; Student’s t-test, χ 2 test, Fisher’s exact test, Mann–Whitney U-test, Kruskal–Wallis test, Spearman’s rank correlation test, logistic and linear regression models | Number of cases to reach plateau | OT: 40 |
| Vickers et al. (2009)74 | LRP, European and North American institutions | BCR | Linear and logistic regression models; multivariable, parametric survival-time regression model | NR | No plateau, absolute risk difference of ~10% for BCR at 5 years between the most and least experienced surgeons |
| Bajalia et al. (2020)75 | RAPN, USA | OT, Trifecta (NSM, WIT, C), functional volume loss | Multivariable logistic and linear regression models; 2-sided statistical tests | Number of cases to reach plateauNumber of cases to maximise outcomes | Trifecta: ≈77OT: 77 |
| Castilho et al. (2020)76 | RAPN, Brazil | OT, EBL, Trifecta (NSM, WIT, C) | Split-group (halves); Mann–Whitney U-test, Friedman paired test, χ 2 test, Fisher’s exact test, logistic regression | Number of cases to achieve >60% Trifecta rate | Trifecta: 50 |
| Dias et al. (2018)77 | RAPN, India | OT, console time, LOS, EBL, Trifecta (NSM, WIT, C) | Split-group; ANOVA, Pearson’s correlation test, Spearman’s rank correlation test | Number of cases to achieve OT <120 min, WIT <20 min, EBL <100 ml | OT: 44WIT: 44EBL: 54 |
| Hanzly et al. (2015)78 | RAPN, USA | OT, WIT, EBL, post-op eGFR, C | Split-group (quartiles); Wilcoxon rank-sum test, Fisher’s exact test | Number of cases to achieve mastery | OT: gradual declineC: undefined learning curveWIT: learning curve reached between cases 29 and 58 |
| Haseebuddin et al. (2010)79 | RAPN, USA | OT, WIT | Polynomial regression; Student’s t-test | Numbers of cases to reach plateau | OT: 16WIT: 26 |
| Larcher et al. (2019)80 | RAPN, 2 European centres | WIT, C, PSM | Multivariable logistic and linear regression models; 2-sided statistical tests | Number of cases to reach plateau | WIT: 150C: no plateau reached in first 300 |
| Lavery et al. (2011)81 | RAPN, USA | OT, WIT, EBL, LOS, C | Graphical inspection; χ 2 test, Student’s t-test | Number of cases to match or better the average OT and WIT of the last 18 LPNs performed by the surgeon (179.7 and 24.7 min, respectively) | OT: 5WIT: 5 |
| Motoyama et al. (2020)82 | RAPN, Japan | OT, console time, EBL, Trifecta (NSM, WIT, C), LOS, TR | Split-group (quintiles); ANOVA, logistic regression | Number of cases to achieve console time ≤150 min, WIT ≤20 min | Console time: 6WIT: 4 |
| Mottrie et al. (2010)83 | RAPN, Belgium | Console time, WIT, EBL, pelvicalyceal repair, C, PSM | Split-group; ANOVA, Kruskal–Wallis test, Pearson χ 2 test, paired-samples t-test | Number of cases to achieve console time <100 min, WIT <20 min | Console time: 20WIT: 30 |
| Omidele et al. (2018)84 | RAPN, USA | OT, EBL, LOS, Trifecta (NSM, WIT, C), decrease in post-op eGFR | Split-group (quartiles); paired-samples t-test | Number of cases to achieve:- 0 C- NSM- WIT ≤25 min- Decrease in post-op eGFR ≤15% | >61–90 |
| Pierorazio et al. (2011)85 | RAPN, USA | OT, WIT, EBL | Split-group; t-test, χ 2 test, ANOVA | Number of cases to reach plateau | No significant learning curve identified |
| Tobis et al. (2012)86 | RAPN, USA | OT, EBL, WIT, CR, LOS, PSM | Split-group (halves); Mann–Whitney U-test, Fisher’s exact test, multivariable linear regression models | Number of cases to reach plateau | No plateaus reachedOT decreased as surgeon experience increased |
| Xie et al. (2016)87 | RAPN, China | OT, EBL, MIC (NSM, WIT, C) | Split-group (quartiles); Mann–Whitney U-test, ANOVA, Kruskal–Wallis test, Pearson’s χ 2 test | Number of cases to achieve MIC rate >80% | MIC: ≈75 |
| Zeuschner et al. (2021)88 | RAPN, Germany | OT, EBL, Trifecta (NSM, WIT, C), MIC, CR, LOS | Logistic and linear regression analysis; Spearman’s rank correlation test, Fisher’s exact test, Mann–Whitney U-test, ROC analysis | Number of cases to become ‘experienced’ (have a 70% probability of achieving MIC or Trifecta) | >35 |
| Azawi et al. (2014)89 | HALPN (with early arterial clamp removal), Denmark | OT, MIC (NSM, WIT, C), LOS, LKF | Split-group (thirds); Paired t-test, Kruskal–Wallis test, multiple regression analysis | Number of cases to achieve 95% MIC rateNumber of cases to achieve WIT ≤5 min | MIC: 40WIT: 40 |
| Bhayani (2008)90 | LPN, USA | OT, EBL, WIT, C, CR, pelvicalyceal system repair, LOS | Split-group (halves); t-test | NR | No significant differences between groups except for LOS |
| Gaston et al. (2004)19 | HALN, USA | OT, EBL, LOS, difficulty scores | Graphical inspection, split-group; ANOVA | NR | OT, difficulty scores; significantly decreased by case 4EBL, LOS: stable throughout |
| Gill et al. (2001)91 | LRN, USA | OT, EBL, C, CR, LOS, PSM | Split-group (halves); Spearman’s rank correlation test, Wilcoxon rank-sum test, χ 2 test, multivariate regression | NR | OT: significant decrease from first 50 to second 50 |
| Gozen et al. (2017)21 | LRN, Germany | OT, EBL, C, TR, PSM, NSM | Split-group; Pearson’s χ 2 test | Number of cases to reach plateau | C: 40 (1st gen), 25 (3rd gen) |
| Hanzly et al. (2015)78 | LPN, USA | OT, WIT, EBL, post-op eGFR, C | Split-group (quartiles); Wilcoxon rank-sum test, Fisher’s exact test | Number of cases to achieve mastery | OT, C: undefined learning curveWIT: learning curve reached between cases 58 and 87 |
| Jeon et al. (2009)92 | LRN, South Korea | EBL, C, TR | Split-group; independent t-test, Pearson’s χ 2 test, Fisher’s exact test, ANOVA, Dunnett t-tests | Number of cases to achieve competence | 15 |
| Kanno et al. (2006)93 | LN, Japan | OT, C | Split-group; Student’s t-test, χ 2 test | Number of cases to achieve acceptable outcomes comparable to the published literature | OT, C: 50 |
| Kawauchi et al. (2005)94 | HALN, Japan | OT, EBL, C, CR | Split-group; Student’s t-test | Number of cases to gain ‘average’ operating skills | OT: 5–10 |
| Link et al. (2005)95 | LPN, USA | OT, WIT | Stepwise linear regression model | Association between surgeon experience and outcome | OT: significant association with surgeon experience |
| Masoud et al. (2020)96 | LN, Egypt | OT, EBL, TR, CR, LOS, C | Split-group (halves); Fisher’s exact test, Student’s t-test, Mann–Whitney U-test | Number of cases to reach plateau | OT:22 |
| Pierorazio et al. (2011)85 | LPN, USA | OT, WIT, EBL | Split-group; t-test, χ 2 test, ANOVA | Number of cases to reach plateau | 25 |
| Porpiglia et al. (2013)97 | LPN, Italy | MIC (NSM, WIT, C) | Split-group (quartiles); Mann–Whitney U-test, Kruskal–Wallis test, ANOVA | Number of cases to achieve acceptable MIC rate (80%) | 150 |
| Collins et al. (2014)98 | RARC (with intracorporeal neobladder), Sweden | OT, EBL, C, CR, lymph node yield, PSM, LOS | Split-group; Jonckheere–Terpstra test, ANOVA, Mann–Whitney U-test, Fisher’s exact test, Brown–Forsythe test | Decrease in outcomes with increasing surgeon experience | OT, C, CR, LOS: decreases noted with increasing experienceEBL, PSM, LOS: stable throughout |
| Dell’Oglio et al. (2020)99 | RARC (with intracorporeal urinary diversion), Belgium | OT, lymph node yield, PSM, C, 18-month recurrence | Multivariable linear and logistic regression models, LOWESS; tests used for statistical significance were not reported | Number of cases to reach plateau | OT: 50 |
| Guru et al. (2009)100 | RARC (with ePLND), USA | OT, cystectomy time, PLND time, EBL, PSM, C, LOS, lymph node yield | Graphical inspection, split-group (quartiles); logistic regression model | Number of cases to reach plateau (where a <1% change occurred in the outcome) | OT: 16EBL: 11LOS:12Lymph node yield: 30 |
| Hayn et al. (2011)101 | RARC, USA | OT, EBL, C, PSM, lymph node yield | Split-group (thirds); χ 2 test, Kaplan–Meier survival analyses | Association between sequential case number and outcome | OT, lymph node yield: significant association with surgeon experienceC, EBL, PSM: no significant association |
| Hellenthal et al. (2010)102 | RARC, 15 international centres | PSM | Split-group; logistic regression model | Association between sequential case number and outcome | PSM: no significant association |
| Honore et al. (2019)103 | RARC, Australia | OT, EBL, LOS, C, PSM, lymph node yield | Split-group (halves); Student’s t-test, Mann–Whitney U-test | Decrease in outcomes with increasing surgeon experience | OT: significant reduction from first 50 to second 50 |
| Pruthi et al. (2008)104 | RARC (with extracorporeal urinary diversion, USA | OT, EBL, C, PSM, lymph node yield, bladder entry, time to flatus, time to bowel movement, LOS | Graphical inspection, split-group (quintiles); multiple paired regression models | Number of cases to reach plateau | OT, EBL: 20 |
| Wang et al. (2019)105 | RARC (with intracorporeal urinary diversion), USA | OT, lymph node yield, ureteral stricture rate, PSM | CUSUM method; no tests for statistical significance reported | Number of cases to reach plateau | OT: 10–11Lymph node yield, ureteral stricture rate, PSM: no learning curve noted |
| Aboumarzouk et al. (2012)106 | Laparoscopic radical cystectomy (with PLND and urinary diversion), Poland | OT, EBL, lymph node yield, LOS, morphine requirement, C | Split-group; Mantel–Haenszel χ 2 test, inverse variance analysis | NR | OT: significantly decreased with increasing surgeon experience |
| Chammas et al. (2019)107 | Robotic pyeloplasty (adult), Brazil | OT, suturing time, LOS, EBL, C, CR | Split-group (quartiles); Kruskal–Wallis test, ANOVA | Number of cases to achieve significant decrease in outcomes | OT, LOS: 25 |
| Cundy et al. (2015)108 | Robotic pyeloplasty (paediatric), UK | OT, individual step time, C, CR | CUSUM method; Student’s t-test, ANOVA, Kruskal–Wallis test | Number of cases of cases to transition beyond learning phase | OT: 57Setup time: 10Docking time: 15Console time: 42 |
| Dothan et al. (2021)109 | Robotic pyeloplasty (paediatric), Israel | OT, LOS, C | Split-group (early vs. late phase); Student’s t-test, Mann–Whitney U-test, χ 2 test, Fisher’s exact test | Significant decrease in outcome between early and late phase | No significant decrease in any outcome with increasing surgeon experience; short learning curve |
| Kassite et al. (2018)110 | Robotic pyeloplasty (paediatric), France | OT, adjusted OT (AOT), composite outcome | CUSUM method; Student’s t-test, Mann–Whitney U-test | Number of cases to achieve proficiency | OT: 23AOT: 19Composite outcome: 22 |
| Sorensen et al. (2011)111 | Robotic pyeloplasty (paediatric), USA | OT, post-op pain, LOS, EBL, C | Logistic regression model; χ 2 test, Student’s t-test | Number of cases to achieve ‘rudimentary’ proficiency (achieve comparable outcomes to those of open surgery) | 15–20 |
| Tasian et al. (2013)112 | Robotic pyeloplasty (paediatric), USA | Console time | Linear regression model; Fisher’s exact test, Kruskal–Wallis test, Mann–Whitney U-test | Number of cases for fellows to achieve comparable outcomes to those of the attending | Console time: 37 (projected) |
| Calvert et al. (2008)113 | Laparoscopic pyeloplasty (adult), UK | OT, LOS, time to normal diet, Hb drop, C, CR | Split-group; Student’s t-test, χ 2 test | Number of cases for significant decrease | C, CR: ≈30 |
| Panek et al. (2020)114 | Laparoscopic pyeloplasty (paediatric), Poland | OT, LOS, failure rate (failure being any surgical reintervention at the ureteropelvic junction) | Split-group; Fisher’s exact test | NR | Failure rate: no change, comparable to previous studies |
| Janetschek et al. (2000)22 | Laparoscopic RPLND, Austria | OT, C | Split-group; no tests for statistical significance reported | Number of cases to achieve outcomes comparable to open surgery | OT: continued reduction over first 64 |
| Schermerhorn et al. (2021)23 | Robotic RPLND, USA | OT, setup time, lymph node count, C | Linear and logistic regression models; tests used for statistical significance not reported | Predicted number of cases to decrease OT by 1 h | OT: 44 (predicted) |
ANOVA, analysis of variance; BCR, biochemical recurrence; BNC, bladder neck contractures; C, complications; CR, conversion rate; CUSUM, cumulative sum; EBL, estimated blood loss; eGFR, estimated glomerular filtration rate; EPIC, Expanded Prostate Cancer Index Composite; ePLND, extended pelvic lymph node dissection; exp., experienced; gen., generation; HALN, hand-assisted laparoscopic nephrectomy; HALPN, hand-assisted laparoscopic partial nephrectomy; Hb, haemoglobin; LKF, loss of kidney function; LN, lymph nodes; LOS, length of stay; LOWESS, Locally Weighted Scatterplot Smoothing; LPN, laparoscopic partial nephrectomy; LRN, laparoscopic radical nephrectomy; LRP, laparoscopic radical prostatectomy; MIC, ‘Margins, Ischaemia, and Complications’ score; NR, not reported; NSM, negative surgical margins; OT, operative time; PLND, pelvic lymph node dissection; PSM, positive surgical margins; QoL, quality of life; RALP, robot-assisted laparoscopic prostatectomy; RAPN, robot-assisted partial nephrectomy; RARC, robot-assisted radical cystectomy; ROC, receiver operating characteristic; RP, radical prostatectomy; RPLND, retroperitoneal lymph node dissection; TR, transfusion rate; WIT, warm ischaemia time.
Robot-assisted laparoscopic prostatectomy (RALP)
Thirty-nine studies18,20,24–60 evaluated the learning curve of RALP. While the majority of RALP studies focussed on defining the learning curve for OT and positive surgical margins (PSMs), Alemozaffar et al.’s study25 was unique in defining the learning curve for potency, demonstrating that surgeon experience correlated with improved sexual function at 5 months (P=0.007) and 12 months (P=0.061) up to a plateau phase of 250–300 nerve-sparing RALP cases. Fossatti et al.35 reported urinary continence recovery increasing from 60% initially to 90% after 400 procedures, with surgeon experience being a significant predictor of continence recovery (P<0.001).
Samadi et al. 51 evaluated a single surgeon’s first 70 RALPs, noting a sustained downward trend in OT (P<0.0001), length of stay (P=0.003) and EBL (P<0.00001). Gumus et al. 38 also observed a continued decrease in OT, with it decreasing from 182 min in the first 40 patients to 168 min in the second 40 patients and then down to 139 min in the third 40 patients.
Bravi et al. 28 found that the risk of PSMs decreased from 15.3% for a surgeon with 10 prior RALPs experience to 6.7% for a surgeon with experience of 250 RALPs. Williams et al. 59 used the CUSUM method to define the PSM learning curve in RALP, setting acceptable and unacceptable positive margin rates at 10 and 15%, respectively. They concluded that around 110 cases are required to overcome the learning curve for pT2 PSMs.
Van der Poel et al. 58 reported an increase in lymph node yield and in the node positivity rate, which significantly increased from 4 to 23.1% from the first 50 cases to their 351st–400th cases. A decrease in Clavien–Dindo grade I and II complications were also noted as surgeon experience increased, but this downward trend was not observed for grade III and IV complications.
The four studies20,48,49,60 reporting the lowest number of cases to overcome the learning curve notably used carefully selected patients so as to ease the transition for open surgeons to the robotic interface. For example, Pardalidis et al. 48 initially only included patients with a prostate volume less than 50 cm3, Gleason score ≤7, BMI <30 and with no previous major pelvic surgery.
Laparoscopic radical prostatectomy (LRP)
Sixteen studies37,54,61–74 analysed the learning curve of LRP. Handmer et al. 66 retrospectively reviewed data from nine Australian surgeons, reporting lower rates of mean blood loss (413 vs. 378 ml), blood transfusions (2.4 vs. 0.8%) and decreased length of stay (2.7 vs. 2.4 days) in the surgeons’ first 100 combined cases compared to the second 100. Di Gioia et al. 62, examining the first 240 LRPs performed by a surgeon with open and laparoscopic experience, reported a significant decrease in the mean anastomosis time (P<0.001) as case number increased, concluding that up to 80 cases were required to achieve a plateau in time-related outcomes.
Mitre et al.69 noted a significant decrease in intraoperative complications after the first 51 cases (P<0.05) alongside a significant decrease in the PSM rates from 29.1 to 21.8 to 5.5% for a single surgeon’s first, second and third groups of 55 patients, respectively. Vickers et al. 74 reported that surgeons who experienced an open radical prostatectomy achieved significantly poorer results for biochemical recurrence than those naïve to the open procedure (risk difference of 12.3%; 95% CI: 8.8–15.7%). A similar trend was observed with regard to complications by Hruza et al. 67 , who concluded that 700 cases were required for surgeons experienced in the open approach to overcome the learning curve compared to 250 cases for surgeons with minimal open surgical experience.
Figure 2 summarises the RALP and LRP learning curves.
Figure 2.
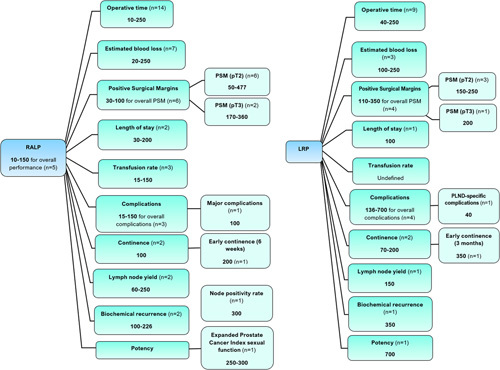
The number of cases required to overcome the learning curve for RALP and LRP. Values are given as a range, with ‘n’ denoting the number of studies which numerically defined the learning curve for the outcome. LRP, laparoscopic radical prostatectomy; PLND, pelvic lymph node dissection; PSM, positive surgical margins; RALP, robot-assisted laparoscopic prostatectomy. Where studies defined learning curve for ‘overall performance’, this referred to the number of cases required to achieve competency across the range of outcomes they measured.
Robot-assisted partial nephrectomy (RAPN)
Fourteen studies75–88 analysed the learning curve for RAPN. Mottrie et al.83 reported a short RAPN learning curve for an experienced robotic surgeon (prior experience of 100 RALPs) with only 20 cases required to achieve a console time of under 100 min. Motoyama et al.82 reported similar findings with 6 and 4 cases required for a surgeon with prior experience of over 300 RALPs to achieve a console time of under 150 min and a warm ischaemia time (WIT) of under 20 min, respectively.
Bajalia et al.75 evaluated 418 consecutive RAPNs, concluding that OT decreased by 2.5% per 50% increase in the case number (P<0.001) up to the plateau phase at 77 cases. Larcher et al.80 adjusted for case mix through the use of multivariable regression analyses, reporting that surgeon experience was significantly associated with decreased WIT (P<0.0001) and increased probability of a postoperative course without a Clavien–Dindo grade II or higher complication (P=0.001). Haseebuddin et al.79 concluded that the RAPN learning curve for WIT is short at just 26 cases for a surgeon with substantial experience in laparoscopic partial nephrectomy.
Laparoscopic partial nephrectomy
Five studies78,85,90,95,97 evaluated the learning curve for laparoscopic partial nephrectomy. Bhayani et al.90 evaluated the first 50 laparoscopic partial nephrectomy cases of a single surgeon, noting that only the length of hospital stay significantly decreased from 3.1 days in the first 25 patients down to 2.5 days in the last 25 patients (P=0.01). Porpiglia et al.97 assessed the laparoscopic partial nephrectomy learning curve using the ‘margin, ischaemia and complication’ (MIC) scoring system, reporting that MIC rates increased from 29.4% in the first 51 patients up to 84.9% in the 150th–206th cases.
Other forms of laparoscopic nephrectomy
Three studies21,91,92 evaluated the learning curve for laparoscopic radical nephrectomy, while two studies93,95 grouped multiple techniques together to map an overall learning curve under the umbrella term of ‘laparoscopic nephrectomy’. Gill et al.91 analysed a single surgeon’s initial 100 laparoscopic radical nephrectomies, with the only significant decrease between the first 50 and second 50 cases being shorter OT (P=0.02).
Three studies19,89,94 assessed the learning curve for hand-assisted laparoscopic nephrectomy. Azawi et al.89 reported achievement of 5 min or less WIT after 40 procedures due to the safe and easily learned procedural step of early arterial clamp removal.
Figure 3 provides a summary of the RAPN and laparoscopic nephrectomy learning curves.
Figure 3.

The number of cases required to overcome the learning curve for robot-assisted and laparoscopic nephrectomies. MIC, ‘Margins, Ischaemia and Complications’, score; RAPN, robot-assisted partial nephrectomy. Where studies defined learning curve for ‘overall performance’, this referred to the number of cases required to achieve competency across the range of outcomes they measured.
Robot-assisted radical cystectomy
Eight studies98–105 analysed the learning curve for RARC. Dell’Oglio et al.99 used multivariable regression models to adjust for case mix and found that surgeon experience correlated with shorter OT (P=0.003), lower 18-month recurrence rates (P=0.002) and decreased likelihood of Clavien–Dindo grade II or higher complications 30 days postoperatively (P=0.01). Hellenthal et al.102 found no significant association between sequential case number and PSMs, whereas Guru et al.100 reported increased lymph node yield from 14 to 23 and decreased PSMs from 4 to 0 between the first 25 cases and 76th–100th cases.
Figure 4 illustrates a summary of the RARC learning curve.
Figure 4.

The number of cases required to overcome the learning curve for robot-assisted robotic cystectomy and robotic pyeloplasty. RARC, robot-assisted radical cystectomy. Where studies defined learning curve for ‘overall performance’, this referred to the number of cases required to achieve competency across the range of outcomes they measured.
Laparoscopic radical cystectomy
One study106 evaluated the learning curve for LRC. Aboumarzouk et al.106 split a single surgeon’s first 65 LRCs into halves, reporting a significant decrease only in OT (303±28 vs. 285±22.93 min, P=0.002), with a nonsignificant decrease in EBL. The learning curve for LRC and the number of cases required to achieve the plateau phase still remain numerically undefined.
Robotic pyeloplasty
Six studies107–112 evaluated the learning curve for robotic pyeloplasty, one of which involved an adult patient population107 whilst the other five involved paediatric patient populations. Chammas et al.107 analysed 100 consecutive adult robotic pyeloplasty procedures, reporting significant decreases in OT (144.6 vs. 94.6 min, P<0.001) and length of stay (7.08 vs. 4.20 days, P<0.001) from the first 25 cases to the last 25. Sorensen et al.111 concluded that only 15–20 paediatric robotic pyeloplasty cases were required to attain an OT with no significant difference to that of open pyeloplasty (P=0.23). Cundy et al.108 identified a classically shaped learning curve for the console time CUSUM chart, but the CUSUM charts for setup and docking times unexpectedly displayed second transition points, which reflected a relocation of the surgical service to a different institution and a change of staff.
The learning curve for robotic pyeloplasty is summarised in Figure 4.
Laparoscopic pyeloplasty
Two studies113,114 evaluated the learning curve for laparoscopic pyeloplasty.
Calvert et al.113 retrospectively evaluated the adult laparoscopic pyeloplasty learning curve over 49 procedures. A nonsignificant decrease in conversion rate to open was observed from 18% in the first third of cases to 6% in the last third of cases (P=0.53). The authors reported around 30 cases are required to overcome the learning curves associated with complications and conversion rate.
Panek et al.114 examined the learning curve of paediatric laparoscopic pyeloplasty by splitting a single surgeon’s 95 cases into a group of the first 37 and a group of the remaining 58. A statistically nonsignificant decrease in failure rate (rate of surgical reintervention at the ureteropelvic junction) between the two groups was observed from 16.2 to 5.1% (P=0.147).
Retroperitoneal lymph node dissection
One study22 assessed the learning curve for laparoscopic RPLND, while another23 studied the learning curve for robotic RPLND. Janetschek et al.22 studied 64 laparoscopic RPLND procedures, noting a trend for decreased mean OT from 480 min in the first 14 patients to 222 min in the last 19, thus suggesting the presence of a learning curve. Schermerhorn et al.23 used linear and logistic regression models to define the learning curve for robotic RPLND. As case number increased, OT and overall complications decreased (P=0.001 and P=0.001, respectively), with OT predicted to decrease by 1 h after 44 cases. However, an insufficient number of cases were studied for a plateau phase to be reached. Therefore, the learning curves for both laparoscopic and robotic RPLND remain undefined.
Comparative studies
The four studies37,54,78,85 examining the learning curves of both laparoscopic and robotic procedures also undertook comparative analyses of these. Good et al.37 identified similar, lengthy learning curves for RALP and LRP but noted that RALP carries the advantages of comparatively lower PSM rates and improved early continence rates. With regard to PSM and biochemical recurrence rates, Sivaraman et al.54 identified a shorter learning curve of 100 cases for RALP compared to 350 cases for LRP.
Hanzly et al.78 reported that RAPN has a shorter learning curve for OT than LPN and more effectively preserves the estimated glomerular filtration rate (eGFR) postoperatively. Furthermore, the authors identified that the learning curve for WIT was reached between cases 29 and 58 for RAPN and between cases 58 and 87 for LPN. Pierororazio et al.85 similarly reported superior outcomes in OT, WIT and EBL for the RAPN cohort compared to the LPN cohort.
Discussion
Summary
This systematic review evaluated the existing evidence base for the learning curves of major robotic and laparoscopic urological procedures. For all procedures, the learning curve values varied substantially depending on the outcome measure used to define it, differing learning curve definitions and also on the surgeons’ prior surgical experience. Prior surgical experience was not consistently reported and was poorly quantified with general labels of ‘experienced in open and/or laparoscopic surgery’20,27,34, providing no precise indication of the number of procedures performed. Multiple studies report that previous laparoscopic experience does reduce the robotic surgery learning curve in the clinical setting, particularly with regard to OT115,116, so it is important for authors to report it in order to contextualise the learning curve value.
OT was the metric most commonly used by the included studies to define the procedural learning curves. However, no uniform definition of OT was used, with one study defining it as the time between the carbon dioxide gas going on and off73, whereas another described it as the time between ‘knife-to-skin’ and wound closure108. pT3-specific PSM learning curves were defined by several studies34,37,57; this is a much more tumour-dependent outcome than surgeon-dependent (whereas the reverse is true for pT2 PSM), so it has limited validity in assessing surgeon performance on the basis of oncological parameters68.
Complications and LOS were amongst the patient-outcome variables used to define learning curves, but these also have limitations. LOS is not necessarily representative of the patient’s condition leaving the hospital64, given it can be affected by patient wishes and even the day of the week that the procedure is performed due to the varied distribution of hospital resources and ancillary support across the week117.
Complications also are not always completely reflective of the surgeon’s performance as they correlate with the quality of complication reporting which thus introduces potential bias to the results118. Urinary incontinence is one of the most important patient-outcome variables for RALP and LRP, given its nature as the most disruptive side-effect post-prostatectomy119, but its learning curve was only defined by 6 of the 53 included prostatectomy studies.
Learning curve metrics are also subject to the effects of other confounders. In the context of urological robotic surgery, the format of training undergone by the surgeon influences the learning process and hence the rate at which safe surgical outcomes are achieved120. The skills and expertise of the whole operative team have also been reported to affect surgical outcomes in urological procedures. Using an expert bedside assistant has been shown to decrease EBL and PSM early in the learning curve for RALP121, with the benefits of a skilled bedside assistant including their ability to handle potential emergencies and thereby decrease the risk of complications, while the console surgeon is seated unscrubbed away from the patient81. Gumus et al. 38 proposed a possible learning curve for anaesthetists on the basis that transfusion rates were disproportionately high relative to the EBL for the initial 80 RALP cases and as the decision to transfuse was made by an anaesthetist naïve to robotics and laparoscopy, their inexperience explained this discrepancy. Furthermore, the presence of mentors and the extent of mentorship received by trainees were poorly reported despite it being demonstrated to affect urological learning curves122.
The split-group method of analysing the learning curve lacks the sensitivity to define the exact number of cases for which learning curve transitions occur5, yet it was the most commonly employed method. Regression techniques were also used by studies, but these may also obscure the identification of key learning curve characteristics such as rate and plateau, given the forced match of the best-fit lines on the data collected8.
The conventional view of the surgical learning curve having just one ascent and one plateau phase was challenged in this review by reports of a multiphasic learning curve arising from the tendency of surgeons to take on increasingly complex cases as their confidence improved with experience94,108. The case mix effect is well-documented in the literature as a key factor in shaping the learning curve3,123, particularly in the post-plateau phase124.
Strengths and limitations
All of the included studies were observational in design, which can introduce confounding and selection bias to results125, but measurement of learning curves necessitates such a design as they are based on the observation of changes in variables as surgeon experience increases. Fifty-five studies were single-surgeon in design which limits the generalisability and external validity of their reported learning curves given the vast interpersonal variation between surgeons in terms of technical attributes and prior experience126. The lack of adjusting for confounders and variation in outcome measures across the included studies in this review is consistent with the findings of reviews assessing the heterogeneity of the surgical learning curve literature bases127,128. Another limitation at the study level was the lack of cost-effectiveness analyses to investigate the association between the length of a surgeon’s learning curve and the economic impact on their institution in terms of training costs.
At review-level, the exclusion of conference abstracts has been identified as a source of publication bias129, but the justification for doing so is that the data presented in such abstracts frequently involves preliminary results which do not necessarily provide an accurate representation of the eventual findings upon study completion130. Another limitation is that no sensitivity analysis was performed to investigate the effect of including studies at high risk of bias on the conclusions formed.
No date restriction was imposed on studies, so the results of older studies may be confounded by the procedural ‘discovery’ curve in which the surgeon is standardising novel techniques as opposed to learning a standard procedure12. However, date restriction would not have been an effective way of eliminating bias from technical changes, given that individual institutions appear to undergo their own procedural discovery curves according to their own local experiences of procedures and outcomes, irrespective of study date. One such example is Sharma et al.’s53 RALP learning curve study in which visual port placement was altered from the paraumbilical space to the umbilical space in order to reduce an initially high rate of port-site hernias, thereby decreasing complications independent of increasing surgeon experience.
Key strengths of this review include its comprehensive search strategy of multiple databases and the grey literature in order to elicit eligible full-text articles and adherence to PRISMA guidelines), Supplemental Digital Content 1, http://links.lww.com/JS9/A423. This review updates the urological learning curve literature base and is the first to examine the literature pertaining to the learning curves of laparoscopic nephrectomy, LRC, laparoscopic pyeloplasty and both robotic and laparoscopic RPLND.
Implications for research and clinical practice
As is consistent with the findings of previous reviews5,11,12,17,126, standardised reporting of outcomes and performance measures is needed in order to reduce heterogeneity and thereby enable a meta-analysis to be performed to combine learning curve values across studies. Given that surgeons’ background expertise affects their learning curve131, surgeons’ baseline characteristics must be reported so as to categorise learning curves by prior experience.
There are ethical concerns raised over surgeons learning on real patients given that more experienced surgeons often attain better outcomes28, so increasing surgical experience through simulation-based training is a safer method for reducing the learning curve associated with procedures132. Mentorship is another educational intervention that has been demonstrated to reduce the surgical learning curve133, with the ‘Leipzig Model’ of supervision in LRP being one such example134. In this example, the trainee performs all the steps they are competent at with a mentor acting as a first assistant, then the student becomes the first assistant and observes for the remaining steps to enhance the learning process. Indeed, surgical outcomes in RALP have been shown to improve if the console surgeon has first gained experience as a bedside assistant because this role improves troubleshooting ability and confidence in dealing with more challenging cases135.
Although underused in the included studies, CUSUM analysis is a well-described method for not only precisely plotting an individual’s learning curve8 but also for auditing purposes in the continuous monitoring of a trainee’s competency as has been demonstrated in the context of cataract surgery136 and gynaecology137. CUSUM charts enable assessment of a trainee’s performance relative to target values and can alert trainers to out-of-control processes, at which point trainees can be instructed to either stop and undergo further education or conduct their next procedures under observation to ensure that patient safety is not compromised while the learning process restabilises46.
The results of the comparative studies37,54,78,85 support the view that the robot-assisted approaches to radical prostatectomy and partial nephrectomy have a shorter learning curve than the conventional laparoscopic approaches. Across all outcomes, the laparoscopic modality was not found to confer any advantage over the robot-assisted approach with regard to the learning curve. Thus RALP would be recommended over LRP on the basis of accelerated attainment of competency for the outcomes of PSMs, biochemical recurrence and early continence. In a similar fashion, RAPN would be recommended over LPN owing to its shorter learning curves for OT, WIT and EBL.
Finally, the learning curves for LRC and robotic and laparoscopic RPLND remain undefined, so future studies should evaluate hundreds of consecutive cases to enable the identification of the plateau phase for these procedures and assess the learning curves of multiple surgeons to increase the external validity of their findings.
Conclusion
This systematic review outlines the range of values for the learning curves of major robotic and laparoscopic urological procedures. As has been described in previous reviews, there was substantial variation in the definitions of outcome measures and performance thresholds, with poor reporting of confounders such as prior surgical experience. Although the majority of studies used the split-group method of analysing the learning curve, the CUSUM method is recommended for more precise characterisation of the key learning curve phases as well as for monitoring the competency of surgeons over time as a form of an appraisal. The use of simulation training, mentorship, and gaining experience as a bedside assistant is recommended in order to reduce the learning curve prior to taking full control as the lead surgeon. Lastly, the results of the comparative studies demonstrate that RALP and RAPN have shorter learning curves for a range of metrics compared to their laparoscopic counterparts.
Ethical approval
Ethical approval was not required.
Sources of funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
B.C.: planned the review, performed the search strategies, extracted the data and wrote the manuscript; M.S.A.A.: acted as the second reviewer and contributed to writing the manuscript; A.A.: acted as the third reviewer, aided in planning the review, and edited and reviewed the manuscript; A.K., M.S.K., K.A. and P.D.: contributed to writing the manuscript and providing revisions.
Conflicts of interest disclosure
Baldev Chahal, Abdullatif Aydin, Mohammad S.A. Amin, Azhar Khan, Muhammad S. Khan and Kamran Ahmed have no conflicts of interest or financial ties to disclose. Prokar Dasgupta declares financial ties as Chief Medical Officer for Proximie Ltd. and Chief Scientific Officer for MysteryVibe Ltd. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique identifying number or registration ID: CRD42021251186.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=25 1186.
Guarantor
Baldev Chahal and Abdullatif Aydin.
Data availability statement
Search results and extracted data are available on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 3 May 2023
Contributor Information
Baldev Chahal, Email: baldev.chahal@kcl.ac.uk.
Abdullatif Aydin, Email: abdullatif.aydin@kcl.ac.uk.
Mohammad S.A. Amin, Email: ali.amin@medsci.ox.ac.uk.
Azhar Khan, Email: azharkhan1@nhs.net.
Muhammad S. Khan, Email: shamim.khan@gstt.nhs.uk.
Kamran Ahmed, Email: kamran.ahmed@kcl.ac.uk.
Prokar Dasgupta, Email: prokar.dasgupta@kcl.ac.uk.
References
- 1. Wright TP. Factors affecting the cost of airplanes. J Aeronaut Sci 1936;3:122–128. [Google Scholar]
- 2. Eden CG, Arora A, Hutton A. Cancer control, continence, and potency after laparoscopic radical prostatectomy beyond the learning and discovery curves. J Endourol 2011;25:815–819. [DOI] [PubMed] [Google Scholar]
- 3. Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J 2007;83:777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satava RM, Gallagher AG, Pellegrini CA. Surgical competence and surgical proficiency: definitions, taxonomy, and metrics. J Am Coll Surg 2003;196:933–937. [DOI] [PubMed] [Google Scholar]
- 5. Khan N, Abboudi H, Khan MS, et al. Measuring the surgical ‘learning curve’: methods, variables and competency. BJU Int 2013;113:504–508. [DOI] [PubMed] [Google Scholar]
- 6. Sheehan H, Gray T, Farrell T. The potential value of surrogate performance markers at caesarean section for the assessment of surgical competence. Eur J Obstet Gynecol Reprod Biol 2018;231:30–34. [DOI] [PubMed] [Google Scholar]
- 7. Seth J, Pakzad M, Hamid R, et al. The assessment and management of post-prostatectomy stress urinary incontinence. Rev Méd Clín Las Condes 2018;29:193–196. [Google Scholar]
- 8. Valsamis EM, Chouari T, Dowd-Booth C, et al. Learning curves in surgery: variables, analysis and applications. Postgrad Med J 2018;94:525–530. [DOI] [PubMed] [Google Scholar]
- 9. Treasure T. The learning curve. BMJ 2004;329:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Wang J, Ferguson MK. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150–1154. [DOI] [PubMed] [Google Scholar]
- 11. Abboudi H, Khan MS, Guru KA, et al. Learning curves for urological procedures: a systematic review. BJU Int 2013;114:617–629. [DOI] [PubMed] [Google Scholar]
- 12. Soomro NA, Hashimoto DA, Porteous AJ, et al. Systematic review of learning curves in robot-assisted surgery. BJS Open 2020;4:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 14. Chahal B, Aydin A, Amin MSA, et al. The learning curves of major laparoscopic and robotic procedures in urology: a systematic review. PROSPERO 2021 CRD42021251186. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=251186 [DOI] [PMC free article] [PubMed]
- 15. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. Accessed 30 April 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 17. Quirke K, Aydin A, Brunckhorst O, et al. Learning curves in urolithiasis surgery: a systematic review. J Endourol 2018;32:1008–1020. [DOI] [PubMed] [Google Scholar]
- 18. Davis JW, Kreaden US, Gabbert J, et al. Learning curve assessment of robot-assisted radical prostatectomy compared with open-surgery controls from the premier perspective database. J Endourol 2014;28:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaston KE, Moore DT, Pruthi RS. Hand-assisted laparoscopic nephrectomy: prospective evaluation of the learning curve. J Urol 2004;171:63–67. [DOI] [PubMed] [Google Scholar]
- 20. Ahlering TE, Skarecky D, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol 2003;170:1738–1741. [DOI] [PubMed] [Google Scholar]
- 21. Gozen AS, Gherman V, Akin Y, et al. Evaluation of the complications in laparoscopic retroperitoneal radical nephrectomy; an experience of high volume centre. Arch Ital Urol Androl 2017;89:266–271. [DOI] [PubMed] [Google Scholar]
- 22. Janetschek G, Hobisch A, Peschel R, et al. Laparoscopic retroperitoneal lymph node dissection. Urology 2000;55:136–140. [DOI] [PubMed] [Google Scholar]
- 23. Schermerhorn SM, Christman M, Rocco NR, et al. Learning curve for robotic-assisted laparoscopic retroperitoneal lymph node dissection. J Endourol 2021;35:1483–1489. [DOI] [PubMed] [Google Scholar]
- 24. Adili AF, Di Giovanni J, Kolesar E, et al. Positive surgical margin rates during the robot-assisted laparoscopic radical prostatectomy learning curve of an experienced laparoscopic surgeon. Can Urol Assoc J 2017;11:E409–e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alemozaffar M, Duclos A, Hevelone ND, et al. Technical refinement and learning curve for attenuating neurapraxia during robotic-assisted radical prostatectomy to improve sexual function. Eur Urol 2012;61:1222–1228. [DOI] [PubMed] [Google Scholar]
- 26. Al-Hathal N, El-Hakim A. Perioperative, oncological and functional outcomes of the first robotic prostatectomy program in Quebec: single fellowship-trained surgeon’s experience of 250 cases. Can Urol Assoc J 2013;7:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atug F, Castle EP, Srivastav SK, et al. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol 2006;49:866–871. [DOI] [PubMed] [Google Scholar]
- 28. Bravi CA, Tin A, Vertosick E, et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: a learning curve study. J Urol 2019;202:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan G, Pautler SE. Use of cumulative summation (CUSUM) as a tool for early feedback and monitoring in robot-assisted radical prostatectomy outcomes and performance. Can Urol Assoc J 2019;13:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang Y, Qu M, Wang L, et al. Robotic-assisted laparoscopic radical prostatectomy from a single Chinese center: a learning curve analysis. Urology 2016;93:104–111. [DOI] [PubMed] [Google Scholar]
- 31. Chen H, Lian B, Dong Z, et al. Experience of one single surgeon with the first 500 robot-assisted laparoscopic prostatectomy cases in mainland China. Asian J Urol 2020;7:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dev H, Sharma NL, Dawson SN, et al. Detailed analysis of operating time learning curves in robotic prostatectomy by a novice surgeon. BJU Int 2012;109:1074–1080. [DOI] [PubMed] [Google Scholar]
- 33. Di Pierro GB, Grande P, Wirth JG, et al. Extended pelvic lymph node dissection at the time of robot-assisted radical prostatectomy: impact of surgical volume on efficacy and complications in a single-surgeon series. Can Urol Assoc J 2015;9:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doumerc N, Yuen C, Savdie R, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 2010;106:378–384. [DOI] [PubMed] [Google Scholar]
- 35. Fossati N, Di Trapani E, Gandaglia G, et al. Assessing the impact of surgeon experience on urinary continence recovery after robot-assisted radical prostatectomy: results of four high-volume surgeons. J Endourol 2017;31:872–877. [DOI] [PubMed] [Google Scholar]
- 36. Galfano A, Secco S, Dell’Oglio P, et al. Retzius-sparing robot-assisted radical prostatectomy: early learning curve experience in three continents. BJU Int 2021;127:412–417. [DOI] [PubMed] [Google Scholar]
- 37. Good DW, Stewart GD, Laird A, et al. A critical analysis of the learning curve and postlearning curve outcomes of two experience- and volume-matched surgeons for laparoscopic and robot-assisted radical prostatectomy. J Endourol 2015;29:939–947. [DOI] [PubMed] [Google Scholar]
- 38. Gumus E, Boylu U, Turan T, et al. The learning curve of robot-assisted radical prostatectomy. J Endourol 2011;25:1633–1637. [DOI] [PubMed] [Google Scholar]
- 39. Hashimoto T, Yoshioka K, Gondo T, et al. Learning curve and perioperative outcomes of robot-assisted radical prostatectomy in 200 initial Japanese cases by a single surgeon. J Endourol 2013;27:1218–1223. [DOI] [PubMed] [Google Scholar]
- 40. Herrell SD, Smith JA, Jr. Robotic-assisted laparoscopic prostatectomy: what is the learning curve. Urology 2005;66(5 suppl):105–107. [DOI] [PubMed] [Google Scholar]
- 41. Islamoglu E, Karamik K, Ozsoy C, et al. The learning curve does not affect positive surgical margin status in robot-assisted laparoscopic prostatectomy. Urol J 2018;15:333–338. [DOI] [PubMed] [Google Scholar]
- 42. Ko YH, Ban JH, Kang SH, et al. Does robot-assisted laparoscopic radical prostatectomy enable to obtain adequate oncological and functional outcomes during the learning curve? From the Korean experience. Asian J Androl 2009;11:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee JW, Jeong WJ, Park SY, et al. Learning curve for robot-assisted laparoscopic radical prostatectomy for pathologic T2 disease. Korean J Urol 2010;51:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maddox M, Lasser M, Renzulli J, II, et al. An updated report on complications following robotic prostatectomy: results of an unbiased prospective database. J Endourol. 2013;27:554-559. [DOI] [PubMed] [Google Scholar]
- 45. Monnerat Lott F, Siqueira D, Argolo H, et al. Analysis of the learning curve of surgeons without previous experience in laparoscopy to perform robot-assisted radical prostatectomy. Adv Urol 2018;2018:9073807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohwaki K, Endo F, Shimbo M, et al. The use of cumulative sum analysis to derive institutional and surgeon-specific learning curves for robot-assisted radical prostatectomy. J Endourol 2020;34:969–973. [DOI] [PubMed] [Google Scholar]
- 47. Ou YC, Yang CR, Wang J, et al. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int 2011;108:420–425. [DOI] [PubMed] [Google Scholar]
- 48. Pardalidis NP, Andriopoulos NA, Tsiga A, et al. Robotic radical prostatectomy in Greece: the learning curve and beyond. The initial 40 cases. J Robot Surg 2008;2:77–80. [DOI] [PubMed] [Google Scholar]
- 49. Patel VR, Tully AS, Holmes R, et al. Robotic radical prostatectomy in the community setting – the learning curve and beyond: initial 200 cases. J Urol 2005;174:269–272. [DOI] [PubMed] [Google Scholar]
- 50. Ploussard G, Xylinas E, Salomon L, et al. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: experience in a high-volume laparoscopy reference centre. BJU Int 2010;105:1155–1160. [DOI] [PubMed] [Google Scholar]
- 51. Samadi D, Levinson A, Hakimi A, et al. From proficiency to expert, when does the learning curve for robotic-assisted prostatectomies plateau? The Columbia University experience. World J Urol 2007;25:105–110. [DOI] [PubMed] [Google Scholar]
- 52. Sammon J, Perry A, Beaule L, et al. Robot-assisted radical prostatectomy: learning rate analysis as an objective measure of the acquisition of surgical skill. BJU Int 2010;106:855–860. [DOI] [PubMed] [Google Scholar]
- 53. Sharma NL, Papadopoulos A, Lee D, et al. First 500 cases of robotic-assisted laparoscopic radical prostatectomy from a single UK centre: learning curves of two surgeons. BJU Int 2011;108:739–747. [DOI] [PubMed] [Google Scholar]
- 54. Sivaraman A, Sanchez-Salas R, Prapotnich D, et al. Learning curve of minimally invasive radical prostatectomy: comprehensive evaluation and cumulative summation analysis of oncological outcomes. Urol Oncol 2017;35:149.e1–6. [DOI] [PubMed] [Google Scholar]
- 55. Slusarenco RI, Mikheev KV, Prostomolotov AO, et al. Analysis of learning curve in robot-assisted radical prostatectomy performed by a surgeon. Adv Urol 2020;2020:9191830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song W, Lee SW, Chung JH, et al. Relationship between robotic-assisted radical prostatectomy and retropubic radical prostatectomy in the learning curve of a single surgeon as a novice in radical prostatectomy: a retrospective cohort study. Int J Surg 2020;81:74–79. [DOI] [PubMed] [Google Scholar]
- 57. Thompson JE, Egger S, Böhm M, et al. Superior biochemical recurrence and long-term quality-of-life outcomes are achievable with robotic radical prostatectomy after a long learning curve-updated analysis of a prospective single-surgeon cohort of 2206 consecutive cases. Eur Urol 2018;73:664–671. [DOI] [PubMed] [Google Scholar]
- 58. van der Poel HG, de Blok W, Tillier C, et al. Robot-assisted laparoscopic prostatectomy: nodal dissection results during the first 440 cases by two surgeons. J Endourol 2012;26:1618–1624. [DOI] [PubMed] [Google Scholar]
- 59. Williams AK, Chalasani V, Martínez CH, et al. Cumulative summation graphs are a useful tool for monitoring positive surgical margin rates in robot-assisted radical prostatectomy. BJU Int 2011;107:1648–1652. [DOI] [PubMed] [Google Scholar]
- 60. Wu ST, Tsui KH, Tang SH, et al. Laparoscopic radical prostatectomy: initial experience of robotic surgery in Taiwan. Anticancer Res 2008;28(4A):1989–1992. [PubMed] [Google Scholar]
- 61. Baumert H, Fromont G, Adorno Rosa J, et al. Impact of learning curve in laparoscopic radical prostatectomy on margin status: prospective study of first 100 procedures performed by one surgeon. J Endourol 2004;18:173–176. [DOI] [PubMed] [Google Scholar]
- 62. Di Gioia RF, Rubinstein M, Velasque L, et al. Impact of a low-volume laparoscopic radical prostatectomy learning curve on perioperative outcomes: is it acceptable? J Laparoendosc Adv Surg Tech A 2013;23:841–848. [DOI] [PubMed] [Google Scholar]
- 63. Dias JAN, Dall’oglio MF, Colombo JR, Jr, et al. The influence of previous robotic experience in the initial learning curve of laparoscopic radical prostatectomy. Int Braz J Urol 2017;43:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eden CG, Neill MG, Louie-Johnsun MW. The first 1000 cases of laparoscopic radical prostatectomy in the UK: evidence of multiple ‘learning curves’. BJU Int 2009;103:1224–1230. [DOI] [PubMed] [Google Scholar]
- 65. Eden CG, Zacharakis E, Bott S. The learning curve for laparoscopic extended pelvic lymphadenectomy for intermediate- and high-risk prostate cancer: implications for compliance with existing guidelines. BJU Int 2013;112:346–354. [DOI] [PubMed] [Google Scholar]
- 66. Handmer M, Chabert C, Cohen R, et al. The Australian laparoscopic radical prostatectomy learning curve. ANZ J Surg 2018;88:100–103. [DOI] [PubMed] [Google Scholar]
- 67. Hruza M, Weiss HO, Pini G, et al. Complications in 2200 consecutive laparoscopic radical prostatectomies: standardised evaluation and analysis of learning curves. Eur Urol 2010;58:733–741. [DOI] [PubMed] [Google Scholar]
- 68. Mason S, Van Hemelrijck M, Chandra A, et al. Laparoscopic radical prostatectomy outcome data: how should surgeon’s performance be reported? A retrospective learning curve analysis of two surgeons. Ecancermedicalscience 2016;10:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mitre AI, Chammas MF, Jr, Rocha, et al. Laparoscopic radical prostatectomy: the learning curve of a low volume surgeon. ScientificWorldJournal 2013;2013:974276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Poulakis V, Dillenburg W, Moeckel M, et al. Laparoscopic radical prostatectomy: prospective evaluation of the learning curve. Eur Urol 2005;47:167–175. [DOI] [PubMed] [Google Scholar]
- 71. Rodriguez AR, Rachna K, Pow-Sang JM. Laparoscopic extraperitoneal radical prostatectomy: impact of the learning curve on perioperative outcomes and margin status. J Soc Laparoendosc Surg 2010;14:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Secin FP, Savage C, Abbou C, et al. The learning curve for laparoscopic radical prostatectomy: an international multicenter study. J Urol 2010;184:2291–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. So BK, Choi JD, Lee SY, et al. Experience of 100 laparoscopic radical prostatectomies performed by a single surgeon: an analysis of surgical and functional outcomes. Korean J Urol 2011;52:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vickers AJ, Savage CJ, Hruza M, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol 2009;10:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bajalia EM, Parikh KA, Haehn DA, et al. Assessment of advanced perioperative outcomes to identify the true learning curve of robotic-assisted partial nephrectomy. Urology 2020;144:136–141. [DOI] [PubMed] [Google Scholar]
- 76. Castilho TML, Lemos GC, Cha JD, et al. Transition from open partial nephrectomy directly to robotic surgery: experience of a single surgeon to achieve “TRIFECTA”. Int Braz J Urol 2020;46:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dias BH, Ali MS, Dubey S, et al. Impact of learning curve on the perioperative outcomes following robot-assisted partial nephrectomy for renal tumors. Indian J Urol 2018;34:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hanzly M, Frederick A, Creighton T, et al. Learning curves for robot-assisted and laparoscopic partial nephrectomy. J Endourol 2015;29:297–303. [DOI] [PubMed] [Google Scholar]
- 79. Haseebuddin M, Benway BM, Cabello JM, et al. Robot-assisted partial nephrectomy: evaluation of learning curve for an experienced renal surgeon. J Endourol 2010;24:57–61. [DOI] [PubMed] [Google Scholar]
- 80. Larcher A, Muttin F, Peyronnet B, et al. The learning curve for robot-assisted partial nephrectomy: impact of surgical experience on perioperative outcomes. Eur Urol 2019;75:253–256. [DOI] [PubMed] [Google Scholar]
- 81. Lavery HJ, Small AC, Samadi DB, et al. Transition from laparoscopic to robotic partial nephrectomy: the learning curve for an experienced laparoscopic surgeon. J Soc Laparoendosc Surg 2011;15:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Motoyama D, Matsushita Y, Watanabe H, et al. Initial learning curve for robot-assisted partial nephrectomy performed by a single experienced robotic surgeon. Asian J Endosc Surg 2020;13:59–64. [DOI] [PubMed] [Google Scholar]
- 83. Mottrie A, De Naeyer G, Schatteman P, et al. Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol 2010;58:127–132. [DOI] [PubMed] [Google Scholar]
- 84. Omidele OO, Davoudzadeh N, Palese M. Trifecta outcomes to assess learning curve of robotic partial nephrectomy. J Soc Laparoendosc Surg 2018;22:e2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pierorazio PM, Patel HD, Feng T, et al. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology 2011;78:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tobis S, Venigalla S, Knopf JK, et al. Robot-assisted partial nephrectomy: analysis of the first 100 cases from a single institution. J Robot Surg 2012;6:139–147. [DOI] [PubMed] [Google Scholar]
- 87. Xie Y, Ma X, Gu L, et al. Associating the learning curve and tumor anatomical complexity with the margins, ischemia, and complications rate after robot-assisted partial nephrectomy. Int J Surg 2016;36(Pt A):219–224. [DOI] [PubMed] [Google Scholar]
- 88. Zeuschner P, Meyer I, Siemer S, et al. Three different learning curves have an independent impact on perioperative outcomes after robotic partial nephrectomy: a comparative analysis. Ann Surg Oncol 2021;28:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Azawi NH, Norus TP, Wittendorff HE, et al. Hand-assisted partial nephrectomy with early arterial clamp removal: impact of the learning curve. Scand J Urol 2014;48:538–543. [DOI] [PubMed] [Google Scholar]
- 90. Bhayani SB. Laparoscopic partial nephrectomy: fifty cases. J Endourol 2008;22:313–316. [DOI] [PubMed] [Google Scholar]
- 91. Gill IS, Meraney AM, Schweizer DK, et al. Laparoscopic radical nephrectomy in 100 patients: a single center experience from the United States. Cancer 2001;92:1843–1855. [DOI] [PubMed] [Google Scholar]
- 92. Jeon SH, Han KS, Yoo KH, et al. How many cases are necessary to develop competence for laparoscopic radical nephrectomy? J Endourol 2009;23:1965–1969. [DOI] [PubMed] [Google Scholar]
- 93. Kanno T, Shichiri Y, Oida T, et al. Complications and the learning curve for a laparoscopic nephrectomy at a single institution. Int J Urol 2006;13:101–104. [DOI] [PubMed] [Google Scholar]
- 94. Kawauchi A, Fujito A, Soh J, et al. Learning curve of hand-assisted retroperitoneoscopic nephrectomy in less-experienced laparoscopic surgeons. Int J Urol 2005;12:1–6. [DOI] [PubMed] [Google Scholar]
- 95. Link RE, Bhayani SB, Allaf ME, et al. Exploring the learning curve, pathological outcomes and perioperative morbidity of laparoscopic partial nephrectomy performed for renal mass. J Urol 2005;173:1690–1694. [DOI] [PubMed] [Google Scholar]
- 96. Masoud M, Ibrahim A, Elemam A, et al. Learning curve of laparoscopic nephrectomy: a prospective pilot study. Afr J Urol 2020;26:24. [Google Scholar]
- 97. Porpiglia F, Bertolo R, Amparore D, et al. Margins, ischaemia and complications rate after laparoscopic partial nephrectomy: impact of learning curve and tumour anatomical characteristics. BJU Int 2013;112:1125–1132. [DOI] [PubMed] [Google Scholar]
- 98. Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder – what is the effect of the learning curve on outcomes? BJU Int 2014;113:100–107. [DOI] [PubMed] [Google Scholar]
- 99. Dell’Oglio P, Mazzone E, Lambert E, et al. The effect of surgical experience on perioperative and oncological outcomes after robot-assisted radical cystectomy with intracorporeal urinary diversion: evidence from a referral centre with extensive experience in robotic surgery. Eur Urol Focus 2021;7:352–358. [DOI] [PubMed] [Google Scholar]
- 100. Guru KA, Perlmutter AE, Butt ZM, et al. The learning curve for robot-assisted radical cystectomy. J Soc Laparosc Robot Surg 2009;13:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hayn MH, Hellenthal NJ, Seixas-Mikelus SA, et al. Is patient outcome compromised during the initial experience with robot-assisted radical cystectomy? Results of 164 consecutive cases. BJU Int 2011;108:882–887. [DOI] [PubMed] [Google Scholar]
- 102. Hellenthal NJ, Hussain A, Andrews PE, et al. Surgical margin status after robot assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol 2010;184:87–91. [DOI] [PubMed] [Google Scholar]
- 103. Honore M, Roberts MJ, Morton A, et al. Outcomes and learning curve for robotic-assisted radical cystectomy: an Australian experience. ANZ J Surg 2019;89:1593–1598. [DOI] [PubMed] [Google Scholar]
- 104. Pruthi RS, Smith A, Wallen EM. Evaluating the learning curve for robot-assisted laparoscopic radical cystectomy. J Endourol 2008;22:2469–2474. [DOI] [PubMed] [Google Scholar]
- 105. Wang A, Polotti CF, Wang S, et al. Characterization of a learning curve for robotic cystectomy with intracorporeal urinary diversion at two institutions using the cumulative sum (CUSUM) method. Canadian. J Urol 2019;26:10033–10038. [PubMed] [Google Scholar]
- 106. Aboumarzouk OM, Drewa T, Olejniczak P, et al. Laparoscopic radical cystectomy: a 5-year review of a single institute’s operative data and complications and a systematic review of the literature. Int Braz J Urol 2012;38:330–340. [DOI] [PubMed] [Google Scholar]
- 107. Chammas MF, Mitre AI, Arap MA, et al. Learning robotic pyeloplasty without simulators: an assessment of the learning curve in the early robotic era. Clinics (Sao Paulo, Brazil) 2019;74:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cundy TP, Gattas NE, White AD, et al. Learning curve evaluation using cumulative summation analysis - a clinical example of pediatric robot-assisted laparoscopic pyeloplasty. J Pediatr Surg 2015;50:1368–1373. [DOI] [PubMed] [Google Scholar]
- 109. Dothan D, Raisin G, Jaber J, et al. Learning curve of robotic-assisted laparoscopic pyeloplasty (RALP) in children: how to reach a level of excellence? J Robot Surg 2021;15:93–97. [DOI] [PubMed] [Google Scholar]
- 110. Kassite I, Braik K, Villemagne T, et al. The learning curve of robot-assisted laparoscopic pyeloplasty in children: a multi-outcome approach. J Pediatr Urol 2018;14:570.e1–570.e10. [DOI] [PubMed] [Google Scholar]
- 111. Sorensen MD, Delostrinos C, Johnson MH, et al. Comparison of the learning curve and outcomes of robotic assisted pediatric pyeloplasty. J Urol 2011;185(6 suppl):2517–2522. [DOI] [PubMed] [Google Scholar]
- 112. Tasian GE, Wiebe DJ, Casale P. Learning curve of robotic assisted pyeloplasty for pediatric urology fellows. J Urol 2013;190(4 suppl):1622–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Calvert RC, Morsy MM, Zelhof B, et al. Comparison of laparoscopic and open pyeloplasty in 100 patients with pelvi-ureteric junction obstruction. Surg Endosc Other Interv Tech 2008;22:411–414. [DOI] [PubMed] [Google Scholar]
- 114. Panek W, Szmer J, Kuijper CF, et al. (2020). Learning curve or experience-related outcome: what really matters in paediatric laparoscopic pyeloplasty. Wideochir Inne Tech Maloinwazyjne 2020;15:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Eddib A, Jain N, Aalto M, et al. An analysis of the impact of previous laparoscopic hysterectomy experience on the learning curve for robotic hysterectomy. J Robot Surg 2013;7:295–299. [DOI] [PubMed] [Google Scholar]
- 116. Kilic GS, Walsh TM, Borahay M, et al. Effect of residents’ previous laparoscopic surgery experience on initial robotic suturing experience. ISRN Obstet Gynecol 2012;2012:569456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gilmore DM, Curran T, Gautam S, et al. Timing is everything-colectomy performed on Monday decreases length of stay. Am J Surg 2013;206:340–345. [DOI] [PubMed] [Google Scholar]
- 118. Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol 2009;182:914–921. [DOI] [PubMed] [Google Scholar]
- 119. Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol 2005;23:2772–2780. [DOI] [PubMed] [Google Scholar]
- 120. Palagonia E, Mazzone E, De Naeyer G, et al. The safety of urologic robotic surgery depends on the skills of the surgeon. World J Urol 2020;38:1373–1383. [DOI] [PubMed] [Google Scholar]
- 121. Garbens A, Lay AH, Steinberg RL, et al. Experienced bedside-assistants improve operative outcomes for surgeons early in their learning curve for robot assisted laparoscopic radical prostatectomy. J Robot Surg 2020;15:619–626. [DOI] [PubMed] [Google Scholar]
- 122. Hay D, Khan MS, Van Poppel H, et al. Current status and effectiveness of mentorship programmes in urology: a systematic review. BJU Int 2015;116:487–494. [DOI] [PubMed] [Google Scholar]
- 123. Roman A, Ahmed K, Challacombe B. Robotic partial nephrectomy – evaluation of the impact of case mix on the procedural learning curve. Int J Surg 2016;29:132–136. [DOI] [PubMed] [Google Scholar]
- 124. Pernar LIM, Robertson FC, Tavakkoli A, et al. An appraisal of the learning curve in robotic general surgery. Surg Endosc 2017;31:4583–4596. [DOI] [PubMed] [Google Scholar]
- 125. Jepsen P, Johnsen SP, Gillman MW, et al. Interpretation of observational studies. Heart 2004;90:956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Grantcharov TP, Bardram L, Funch-Jensen P, et al. Learning curves and impact of previous operative experience on performance on a virtual reality simulator to test laparoscopic surgical skills. Am J Surg 2003;185:146–149. [DOI] [PubMed] [Google Scholar]
- 127. Kassite I, Bejan-Angoulvant T, Lardy H, et al. A systematic review of the learning curve in robotic surgery: range and heterogeneity. Surg Endosc 2019;33:353–365. [DOI] [PubMed] [Google Scholar]
- 128. Mazzon G, Sridhar A, Busuttil G, et al. Learning curves for robotic surgery: a review of the recent literature. Curr Urol Rep 2017;18:89. [DOI] [PubMed] [Google Scholar]
- 129. Dalton JE, Bolen SD, Mascha EJ. Publication bias: the elephant in the review. Anesth Analg 2016;123:812–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev 2007;2007:MR000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Castellan P, Marchioni M, Rizzoli A, et al. The surgical experience influences the safety and efficacy of photovaporization of prostate with 180-W XPS GreenLight laser: comparison between novices vs expert surgeons learning curves. J Endourol 2018;32:1071–1077. [DOI] [PubMed] [Google Scholar]
- 132. Bryant-Smith A, Rymer J, Holland T, et al. ‘Perfect practice makes perfect’: the role of laparoscopic simulation training in modern gynaecological training. Obstet Gynaecol 2019;22:69–74. [Google Scholar]
- 133. Ichikawa N, Homma S, Yoshida T, et al. Mentor tutoring: an efficient method for teaching laparoscopic colorectal surgical skills in a general hospital. Surg Laparosc Endosc Percutan Tech 2017;27:479–484. [DOI] [PubMed] [Google Scholar]
- 134. Ramírez Backhaus M, Uwe Stolzenburg J, Do M, et al. Learning laparoscopic radical prostatectomy with the Leipzig program. Analysis of the training module program. Actas Urol Esp 2009;33:290–295. [DOI] [PubMed] [Google Scholar]
- 135. Cimen HI, Atik YT, Gul D, et al. Serving as a bedside surgeon before performing robotic radical prostatectomy improves surgical outcomes. Int Braz J Urol 2019;45:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Salowi MA, Choong YF, Goh PP, et al. CUSUM: a dynamic tool for monitoring competency in cataract surgery performance. Br J Ophthalmol 2009;94:445–449. [DOI] [PubMed] [Google Scholar]
- 137. Eisenberg VH, Alcazar JL, Arbib N, et al. Applying a statistical method in transvaginal ultrasound training: lessons from the learning curve cumulative summation test (LC-CUSUM) for endometriosis mapping. Gynecol Surg 2017;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Search results and extracted data are available on request.


