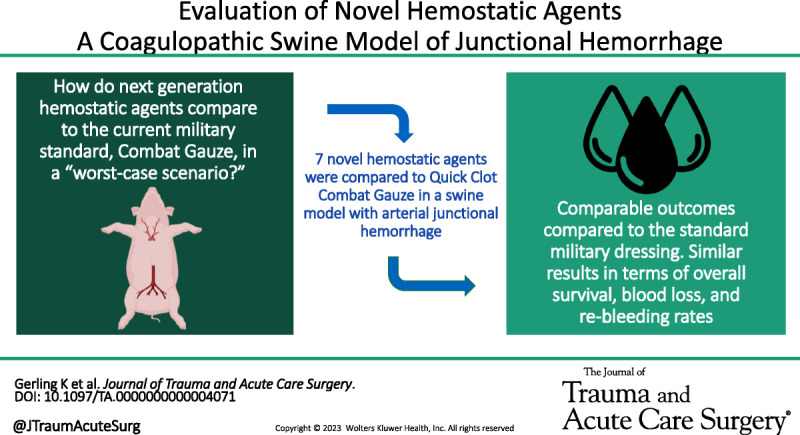
Most novel hemostatic agents demonstrated comparable efficacy compared to the currently military standard hemostatic dressing, Combat Gauze, in a hemorrhagic, coagulopathic swine model.
KEY WORDS: Hemostasis, coagulopathy, vascular injury
BACKGROUND
Hemostatic dressings are used extensively in both military and civilian trauma to control lethal noncompressible hemorrhage. The ideal topical hemostatic agent would provide reliable hemostasis in patients with profound acidosis, coagulopathy, and shock. This study aimed to compare next-generation hemostatic agents against the current military standard in a translational swine model of vascular injury and coagulopathy.
METHODS
Female Yorkshire swine were randomized to eight groups (total n = 63; control n = 14, per group n = 7) of hemostatic agents and included: QuikClot Combat Gauze (Teleflex, Morrisville, NC), which served as the control; BloodSTOP IX (LifeScience Plus, Mountain View, CA); Celox Rapid (Medtrade Product, Crewe, United Kingdom); ChitoSAM 100 (Sam Medical, Tualatin, OR); EVARREST Fibrin Sealant Patch (Ethicon, Raritan, NJ); TAC Wrapping Gauze (H&H Medical, Williamsburg, VA); ChitoGauze XR Pro (Tricol Biomedical, Portland, OR); and X-Stat 30 (RevMedX, Wilsonville, OR). Hemodilution via exchange transfusion of 6% hetastarch was performed to induce acidosis and coagulopathy. An arteriotomy was created, allowing 30 seconds of free bleeding followed by application of the hemostatic agent and compression via an external compression device. A total of three applications were allowed for continued/recurrent bleeding. All blood loss was collected, and hemostatic agents were weighed to calculate blood volume loss. Following a 180-minute observation period, angiography was completed to evaluate for technical complication and distal perfusion of the limb. Finally, the limb was ranged five times to assess for rebleeding and clot stability.
RESULTS
All swine were confirmed coagulopathic with rotational thromboelastography and acidotic (pH 7.2 ± 0.02). BloodSTOP IX allowed a significant increase in blood loss and number of applications required to obtain hemostasis compared with all other groups. BloodSTOP IX demonstrated a decreased survival rate (29%, p = 0.02). All mortalities were directly attributed to exsanguination as a result of device failure. In surviving animals, there was no difference in extravasation. BloodSTOP IX had an increased rebleeding rate after ranging compared with QuikClot Combat Gauze (p = 0.007).
CONCLUSION
Most novel hemostatic agents demonstrated comparable efficacy compared with the currently military standard hemostatic dressing, CG.
The majority of potentially preventable deaths after combat-related injury occur in the prehospital environment because of hemorrhage.1–3 There are numerous hemostatic agents and specialized tourniquets that have been created to address this problem, particularly in the setting of junctional hemorrhage, where standard tourniquet application is frequently not adequate. Given the difficult challenge of managing a junctional hemorrhage injury pattern, there has been a rapid increase in the development of novel hemostatic agents. The ideal agent would provide reliable hemostasis even in the setting of arterial hemorrhage that is difficult to access in the prehospital setting and not amenable to tourniquet applications. We also expect an ideal hemostatic dressing to have sustained performance in the setting of profound acidosis, coagulopathy, and hypothermia during transport.
The most widely used hemostatic agent in the military setting has been QuikClot Combat Gauze (CG; Teleflex, Morrisville, NC) and has been shown in swine models to effectively achieve hemostasis, reduce blood loss, and improve survival.4–6 It is also the recommended dressing in the Tactical Combat Casualty Care guidelines published by the military serving as the standard for treatment for all medical personnel in combat.7 Despite these guidelines, there are is a paucity of data comparing some of the novel agents currently on the market to the current military standard (i.e., CG) in a “worst case” physiologic scenario. This study aimed to evaluate the efficacy of novel, commercially available hemostatic agents in controlling junctional, arterial hemorrhage with a swine model under coagulopathic conditions compared with the current military standard—CG.
PATIENTS AND METHODS
Overview and Study Design
This study was approved by our Institutional Animal Care and Use Committee. All animal care and use were in strict compliance with the Guide for the Care and Use of Laboratory Animals and the National Institutes of Health guide for the care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. An Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0 author's checklist was completed and is available in the supplemental material (Supplemental Digital Content, Supplementary Data 1, http://links.lww.com/TA/D64).
Healthy adult, nonpregnant female Yorkshire-cross swine (Sus scrofa), obtained from Animal Biotech Industries Inc. (Doylestown, Pennsylvania), were acclimated for a minimum of 3 days while housed at the animal vivarium. Female swine were used to mitigate and control for the hormonal impact and influence on the physiologic response to severe hemorrhage. At the time of experimentation, animals weighed between 65 and 90 kg.
The study design was a prospective translational swine model. The swine were allocated to eight groups including CG (control group) and seven novel hemostatic agents (total, n = 63; CG control, n = 14; per group, n = 7; Fig. 1). The control group consisted of the current military standard, which is CG (Teleflex, Morrisville, NC). The novel hemostatic agents included BloodSTOP IX (BL-IX; LifeScience Plus, Mountain View, CA), Celox Rapid (CR; Medtrade Product, United Kingdom), ChitoSAM 100 (Sam Medical, Tualatin, OR), EVARREST Fibrin Sealant Patch (Ethicon, Raritan, NJ), TAC Wrapping Gauze (TAC; H&H Medical, Williamsburg, VA), ChitoGauze XR Pro (Tricol Biomedical, Portland, OR), and X-Stat 30 (XS; RevMedX, Wilsonville, OR). As described hereinafter, all animals were subjected to the same preparation and instrumentation, baseline evaluation, hemodilution, vascular injury, device implantation, and critical care phases. Arterial laboratory samples were collected during the preinjury baseline phase, at the completion of hemodilution, end of intervention phase, and time of death. Samples included blood gas, electrolytes, chemistry analysis, and rotational thromboelastography (ROTEM) (ABL 800 FLEX; 201 Radiometer America, Brea, CA).
Figure 1.

Characteristics of each hemostatic agent including dimension, packaging characteristics, and images.
Animal Preparation
Healthy adult, nonpregnant female Yorkshire-cross swine (S. scrofa) were obtained from Animal Biotech Industries Inc. (Doylestown, PA). They were allowed to acclimate for at least 3 days while housed with ad librium access to water while on a 12-hour diurnal light cycle. Animals were induced with 6 mg/kg of tiletamine/zolazepam (Telazol; Fort Dodge Animal Health, Fort Dodge, IA) intramuscularly. They were subsequently intubated and maintained under anesthesia with 1% to 5% isoflurane. All animals were mechanically ventilated with tidal volumes of 7 to 10 mL/kg and a respiratory rate of 10 to 15 breaths per minute sufficient to maintain end-tidal carbon dioxide (CO2) at 40 ± 5 mm Hg. Bilateral peripheral intravenous access was established on each ear. Intravenous fluids initiated at a rate of 5 to 10 mL/kg/h. The swine were placed on a warming blanket to maintain temperature >36.7°C to 39.2°C. A Lidocaine infusion (20 μg/kg/h) was initiated to prevent tachyarrhythmias.
Injury and Intervention
After anesthetic induction, all swine were prepared with bilateral carotid artery cannulation via percutaneous or open cutdown techniques. A 7-Fr sheath was placed in the right common carotid and 5-Fr sheath placed in the left common carotid artery. The 7-Fr sheath allowed for placement of a Solid-State Pressure Catheter (Transonic Systems Inc., Ithaca, NY) to measure arterial blood pressure and allow for blood draws throughout the experiment. A limited, lower midline laparotomy was performed for open cystostomy, and abdominal domain was restored using clamps. Next, a surgical cutdown was performed to access the femoral artery circumferentially for a length of approximately 2 to 3 cm.8 Proximal and distal control was obtained. A temperature probe was placed into the wound at the site of the planned arteriotomy. Baseline physiologic data were obtained including mean arterial pressure, heart rate, and temperature. Animals were excluded from analysis if they were unable to maintain a mean arterial pressure greater than 50 mm Hg without pharmacologic intervention before the hemorrhage phase of the experiment.
Following baseline measurements, a 50% to 60% exchange transfusion with 6% hetastarch in lactated electrolyte solution was performed to induce acidosis and coagulopathy. Target hematocrit was 15%, and ROTEM was obtained to assess and ensure adequate coagulopathy. Repeat laboratory values were obtained following the exchange transfusion. A 4.5-mm arteriotomy was created using an aortic punch with 30 seconds of free bleeding to simulate a large arterial injury in a prehospital setting that would require immediate intervention when surgical capabilities are not immediately available. The hemostatic agent was then applied, and compression was maintained via an external compression device (Femostop; St. Jude Medical, Plymouth, MN) to a pressure of 25 mm Hg for a total of 5 minutes to standardize extrinsic pressure applied across all experiments. Complete occlusion of the vessel and distal perfusion were not evaluated or confirmed at the time of compression. Failure of gauze was defined as pooling of blood around the gauze and compression device following application. A total of three applications of the hemostatic agent were allowed for continued or recurrent bleeding within this 5 minutes after which no further external compression was permitted. The number of applications was limited to simulate replicate prehospital, military casualty scenarios where resources are limited. All blood loss was collected during the intervention and critical care phase. In addition, the hemostatic agents were weighed at the completion of the experiment to calculate total blood volume lost.
Critical Care Phase
After hemostasis was achieved (or all three applications were completed), the animals were resuscitated with a 500 mL 6% hetastarch bolus and monitored for 180 minutes. During the critical care phase, maintenance intravenous fluids were continued at 5 to 10 mL/kg; however, no further fluid boluses or vasopressors were administered. All blood loss during this phase was collected for measurement of total blood loss.
Evaluation and Assessment
Following the 180-minute critical care phase, angiographic assessment was performed via the carotid sheath to evaluate for technical complication including extravasation, arterial thrombosis, and distal perfusion of the limb. Any distal perfusion was counted even if there appeared to be stenosis or spasm of the artery. Complete occlusion was noted if there was no evidence of contrast runoff into the distal extremity. There was no evaluation in size of clot or quantification of distal perfusion performed. Next, a full range of motion (full extension to full flexion) of the affected limb was performed up to five times to assess for rebleeding and clot stability. Any bleeding from the wound observed during this cycle was counted as a failure. At the completion of the experiment, the animals were humanely euthanized using an overdose of pentobarbital-based euthanasia solution (100 mg/kg) consistent with the 2020 American Veterinary Medical Association Guidelines for the Euthanasia of Animals6. Immediately following euthanasia, the exposed section of the femoral artery and surrounding hind limb was harvested and sent for pathologic evaluation. The hind limb muscle was analyzed with hematoxylin and eosin, and Masson trichrome stains. A board-certified histopathologist evaluated muscle samples for myocyte degeneration and necrosis in a blinded fashion. Arterial samples were analyzed with hematoxylin and eosin, and Movat's pentachrome stain (which identifies fibrin deposition as purplish/red) by a board certified veterinary pathologist, who evaluated tissues for fibrosis in a blinded fashion.
Data Collection and Statistical Analysis
Hemodynamic and physiologic parameters were continuously measured using the Solid-State Pressure Catheter and recorded using PowerLab data acquisition system (AD Instruments, Colorado Springs, CO) at a sampling rate of 1 kilo samples per second. The data were analyzed using LabChart software (V8.0; AD Instruments) as 1-minute averages taken at 1-minute interval across the whole recording period. The primary outcomes of the study were mortality and total blood loss during and after the hemostatic agents were applied. Secondary outcomes included number of applications required to achieve hemostasis, incidence of rebleeding during the range of motion exercise, and pathologic changes to the injured artery and hind limb. Continuous data are reported as means and SD. The sample size was determined by power analysis (power, 0.0; α = 0.05) using G*Power 3 (Dusseldorf, Germany) based on the means and SDs of published data in the study by Littlejohn et al.,9 which had a similar study design as the current protocol. Posttreatment blood loss was considered as the primary outcome, and the estimated sample size was n = 8 per group. Final experimental groups were limited to seven animals given several animals were excluded during the baseline monitoring phase for inability to maintain mean arterial pressures greater than 50 mm Hg. Other studies analyzing hemostatic dressings in swine models generally range from 7 to 12 per experimental group.9–13 Histological analyses were analyzed with Kruskal-Wallis nonparametric analyses, with Dunn's multiple comparisons test performed for comparison of individual gauzes. Group comparisons were completed with two-way analysis of variance and Dunnett's post hoc testing with survival data analyzed using Mantel-Cox testing. p < 0.05 was considered statistically significant.
RESULTS
A total of 63 animals were used with 14 animals in the control group (CG) and 7 animals for each of the novel hemostatic agents. The baseline characteristics following hemodilution are shown in Table 1. There was no significant difference in baseline characteristics between animals. All of the swine were found to be coagulopathic based on posthemodilution ROTEM data with a maximal clot firmness of 49.7 ± 3.64 mm and a clot formation time of 124.6 ± 23.4 seconds, which were outside of the accepted reference ranges.14 While there was a wide range of mean clot formation time for unclear reasons, all values were well outside the accepted mean ensuring coagulopathy as indicated in our methodology. In addition, the swine were noted to have a hematocrit of less that 15% and acidotic before intervention with an average pH of 7.2 ± 0.02.
TABLE 1.
Baseline Characteristic Data per Group With Hematocrit, pH, Clot Formation Time, and Maximum Clot Firmness Following Hemodilution, Before Intervention Compared With Control Agent, CG
| Hemostatic Agent | CG (Control) | BL-IX | TAC | CS | CR | EP | XR | XS |
|---|---|---|---|---|---|---|---|---|
| Weight, kg | 41.4 ± 4.7 | 36.5 ± 3.6 | 41.3 ± 4.8 | 38.5 ± 5.4 | 39.0 ± 3.3 | 42 ± 5.3 | 41.6 ± 5.2 | 40.6 ± 6.3 |
| Hematocrit, % | 12.1 ± 1.8 | 12.6 ± 1.1 | 11 ± 2.1 | 12.5 ± 1.8 | 12.6 ± 1.4 | 12.2 ± 1.4 | 12.1 ± 1.4 | 11.6 ± 0.9 |
| pH | 7.20 ± 0.1 | 7.19 ± 0.1 | 7.22 ± 0.1 | 7.19 ± 0.1 | 7.26 ± 0.1 | 7.16 ± 0.1 | 7.23 ± 0.1 | 7.22 ± 0.0 |
| Clot formation time, s | 115 ± 37.3 | 110 ± 21.7 | 133.6 ± 33.8 | 120.3 ± 42.1 | 99.7 ± 17.8 | 176.9 ± 206 | 125.0 ± 29.2 | 116.1 ± 30.5 |
| Maximum clot firmness, mm | 52.0 ± 7.6 | 51.1 ± 5.6 | 41.3 ± 17.1 | 50.0 ± 6.9 | 53.7 ± 6.0 | 49.4 ± 12.8 | 48.8 ± 5.9 | 51.9 ± 4.3 |
CS, ChitoSAM 100; EP, EVARREST Fibrin Sealant Patch; TAC, TAC Wrapping Gauze; XR, ChitoGauze XR Pro.
The outcomes for each novel hemostatic agent including average total blood loss, number of device applications, overall survival, device failure rate, and rebleeding rate are shown in Table 1. The BL-IX was associated with significantly increased blood loss compared with CG. There was no difference in blood loss compared with the other agents In addition, all devices showed similar survival or device failure rates except for BL-IX (Table 1). In the animals that survived to the end of the 180-minute observation period, there was no difference in extravasation, thrombosis, or embolization seen on angiographic assessment (Table 2).
TABLE 2.
Average Total Blood Loss (mL), Average Number of Gauze Applications, Overall Survival Completed Through the 180-Minute Observation Period, and Positive Angiographic Perfusion Demonstrated for Each Hemostatic Agent Compared With Control Agent, CG
| Hemostatic Agent | CG (Control) | BL-IX | TAC | CS | CR | EP | XR | XS | p |
|---|---|---|---|---|---|---|---|---|---|
| Average blood loss, mL | 260 ± 319 | 913 ± 515 | 135 ± 127 | 112 ± 115 | 545 ± 734 | 297 ± 662 | 554 ± 613 | 778 ± 660 | 0.02 |
| No. gauze applications | 1.6 | 2.6 | 1.4 | 1.6 | 2 | 2.1 | 1.9 | 2.4 | 0.03 |
| Overall survival, % | 86 | 29 | 71 | 100 | 86 | 71 | 71 | 100 | 0.02 |
| Angiographic perfusion, % | 62 | 50 | 20 | 43 | 60 | 100 | 100 | 43 | 0.09 |
CS, ChitoSAM 100; EP, EVARREST Fibrin Sealant Patch; TAC, TAC Wrapping Gauze; XR, ChitoGauze XR Pro.
Evaluation of the postmortem hind limb muscle and arterial samples were evaluated histologically. Most of the hind limb tissue section demonstrated moderate myocyte degeneration, which was characterized by sarcoplasmic vacuolation and segmental loss of myocytes (Fig. 2). There was a significant effect of gauze used upon statistical analysis (p = 0.0346), with post hoc Dunn's multiple comparison test showing a significantly elevated level of hind limb necrosis of TAC compared with CG (p = 0.0098) (Fig. 3). In addition, there were instances of inflammation and myocyte loss seen in the arterial samples (Fig. 4); however, there was no effect on gauze in the pathologist scoring levels (p = 0.5731 and p = 0.8585 for inflammation and myocyte loss, respectively, data not shown). Moreover, despite the acute timeframe of this study, fibrin deposition can be seen in the defect area of some samples.
Figure 2.
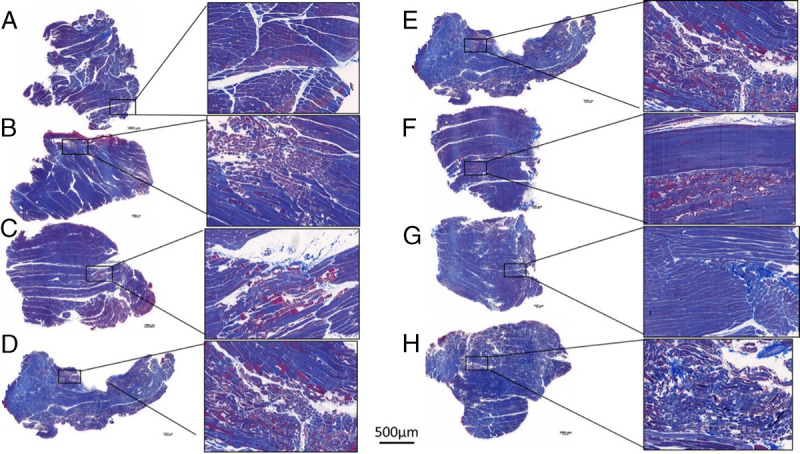
Histopathologic samples of each gauze demonstrating some degree of myocyte degeneration. QuikClot Combat Gauze (A), BL-IX (B), TAC (C), ChitoSAM 100 (D), CR (E), EVARREST Fibrin Sealant Patch (F), ChitoGauze XR Pro (G), XS (H). Scale bars on low magnification images are 1 mm, while one scale bar of 500 μm is used for all higher magnification images.
Figure 3.
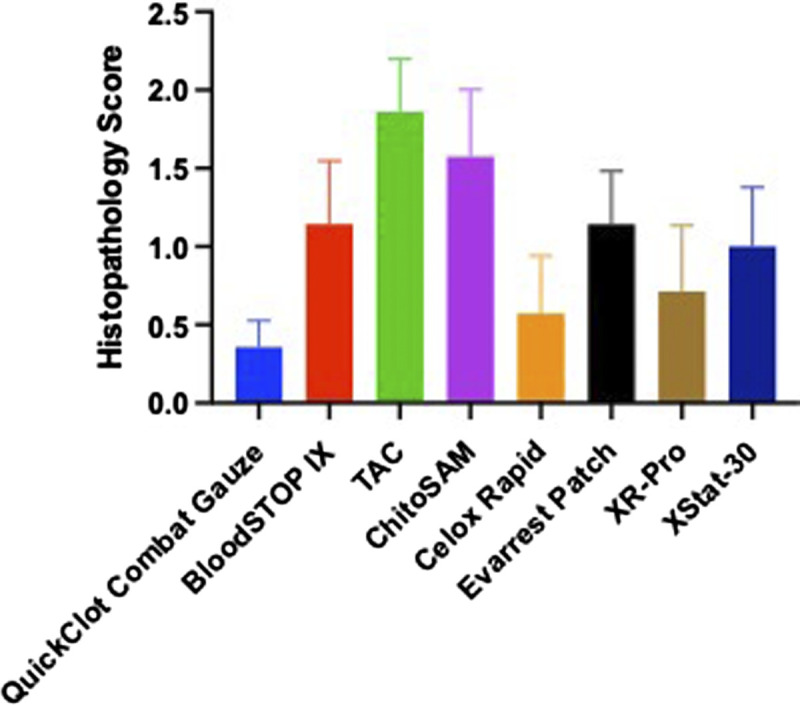
Foci of myocyte degeneration seen on postmortem hind limb muscle samples taken from each of the hemostatic agents demonstrating statistically significant increase in myocyte degeneration only in the TAC group.
Figure 4.
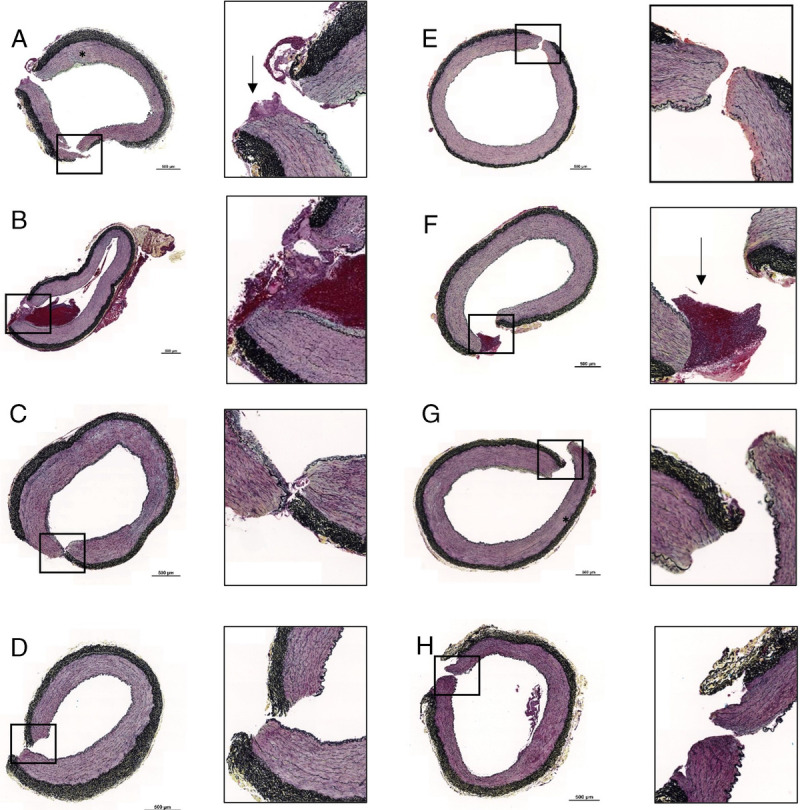
Histopathologic samples of each gauze demonstrating some degree of myocyte degeneration (black asterisk) and fibrin deposition (black arrows). QuikClot Combat Gauze (A), BL-IX (B), TAC (C), ChitoSAM 100 (D), CR (E), EVARREST Fibrin Sealant Patch (F), ChitoGauze XR Pro (G), XS (H). Scale bars on low magnification images are 0.5 mm.
DISCUSSION
The aim of this study was to evaluate the efficacy of seven commercially available novel hemostatic agents compared with the current military standard (CG) in a clinically relevant swine model of hemorrhage, acidosis, and coagulopathy. Before injury, there were no significant differences among the groups with regard to baseline characteristics (Table 1). In addition, the groups' physiologic and laboratory parameters were consistent with the “lethal triad” commonly seen in trauma patients as demonstrated by the acidosis and ROTEM results. Two critical observations were identified as the result of this experiment. During rigorous testing, the majority of these topical agents (CR, ChitoSAM 100, EVARREST Fibrin Sealant Patch, TAC, ChitoGauze XR Pro, XS) demonstrated no difference in efficiency in all parameters other than BL-IX, which allowed for higher blood loss and decreased survival. Second, only TAC demonstrated higher necrosis in the hind limb muscle samples. All other agents did not show a significant difference in inflammation, fibrosis, and myocyte degeneration of the hind limb muscle and arterial sample (Fig. 3).
Massive bleeding resulting from combat trauma is the leading cause of preventable death on the battlefield. Effective treatment options for injuries that are not amenable to tourniquet application (i.e., noncompressible or junctional injuries) are lacking. As such, the need for additional adjuncts in treating traumatic hemorrhage is obvious. Particularly in the military prehospital environment, reliable, easy-to-use hemostatic agents are of the utmost importance.15 Prior studies have compared hemostatic agents similar to those included in the experiment; however, most did not test under the same conditions or target the acidotic and coagulopathic condition as seen in our swine. QuikClot Combat Gauze is currently the mostly widely used in the military setting. There have been several studies demonstrating the efficacy of CG in achieving hemostasis, although its superiority compared with newer agents is inconclusive.13,16–18 Rall et al.17 showed nonsuperiority of the hemostatic dressings they tested, including CR (tested in this study) compared with CG with a similar overall mortality to our study. As such, several new agents have been brought to market including the ones in the Rall et al. study; however, there have been few head-to-head comparisons of each agent under the same methods of physiologic stress. QuikClot Combat Gauze is a nonwoven gauze coated in kaolin and is currently the recommended hemostatic agent. The novel agents testing in our study have different mechanisms for the hemostatic properties. ChitoSAM 100 is a nonwoven gauze made directly from chitosan precipitating red-blood cell cross-linking, platelet activation, and aggregation. Comparatively, CG and CR are nonwoven gauzes coated with chitosan. EVARREST Fibrin Sealant Patch consists of human fibrinogen and thrombin in a flexible patch, which prompt hemostasis when applied. BloodSTOP IX is composed of water soluble cellulose that forms and adhesive gel when coming into contact with blood. X-Stat 30 is also composed of cellulose, but it is consisted of small sponges directly injected into junctional wounds and expected to be removed during surgical exploration. Finally, TAC a nonwoven gauze consisted of several fibers and proprietary cotton product. This study provided a rigorous environment for testing of the hemostatic dressings, as early coagulopathy worsens outcomes in combat casualties.19 This study focused on arterial injuries that were not amenable to tourniquet application in an environment without readily available surgical capabilities. This was intended to provide an extreme testing environment for each of the novel agents.
The majority of the hemostatic agents that were tested demonstrated similar overall blood loss and survival. Notably, BL-IX had a higher overall blood loss and increased number of applications required to achieve hemostasis, which correlated to the decreased overall survival. Interestingly, XS trended toward a higher number of applications required to achieve hemostasis, but this was not statistically significant. Despite this trend, there was 100% survival in the swine tested in this group. This XS application device is the most novel from a user standpoint with its injector-like applicator. The novel applicator may be the source of increasing applications required to ultimately achieve hemostasis but, unlike the BL-IX, did not have an effect on overall survival. However, ease of use was not specifically tested in this model and can only be inferred. This study evaluated several commercially available products and ultimately demonstrated that there was no significant difference in primary outcomes (survival and blood loss) compared with CG except for BL-IX. We evaluated these agents in a head-to-head comparison under the same physiologic stress and coagulopathic conditions that may be encountered in the traumatically injured patient. This study was performed after generating the lethal triad in the swine. This rigorous testing environment is most similar to real-world combat scenarios. Future studies would need to include ease of use from novice and experienced users, storage requirements, and cost related to number of applications.
In addition, this study demonstrated that the histologic changes to both the site of injury and the surrounding muscle were similar among each of the agents, suggesting that there is no benefit of any single agent that would affect long-term healing on a histologic standpoint. Only TAC wrapping gauze demonstrated an increase in myocyte degeneration. This was only seen in the hind-limb muscles samples, however, and the degree of myocyte loss is of uncertain significance. Ultimately, if TAC is able to prevent blood loss and promote survival, it should still be considered for use even in the face of some distal loss of skeletal muscle myocytes. Similarly, while there were some instances of inflammation and myocyte loss in arterial samples, there were no differences because of gauze used, and the clinical significance of this is not established. This would require further investigation from a functional standpoint.
This study was not without limitations. Our initial power analysis was performed using similar methodology with an expected n = 8; however, given unexpected preinjury mortality in several swine, our total swine per group was limited.9 In addition, our blood loss was significantly less compared with the aforementioned study, which is unsurprising given that they performed transection of both the femoral artery and vein. We only used a positive control in this study—CG, the current military standard. A negative control, application of gauze alone, was considered to be unnecessary given that prior studies have shown similar efficacy compared with CG and would have been redundant.9,20 While this study attempted to simulate the physiologic stress seen in profoundly injured trauma patients, by nature of the experimental design, the standardization of arterial injury and external pressure does not translate to real-world scenarios where complex difficult-to-access injuries are the norm. Some degree of variation in use is expected based on the experience of the user and associated injuries, which may change the true effectiveness of each hemostatic agent. Future studies should involve testing regarding ease of use given the wide range of medical knowledge and capabilities under which these dressings are applied in combat scenarios. In addition, this study did not compare storage requirements for each individual hemostatic agent, which would be necessary particularly in the austere environment where additional supplies are not readily available. Despite the limitations of this study, it suggests that CG may not be the only hemostatic dressing available to effectively address hemorrhage in the trauma patient. For now, with the exception of BL-IX, the ideal hemostatic agent to use in life-threatening hemorrhage is the one that is readily available.
CONCLUSION
This study demonstrated the comparable outcomes seen in seven novel hemostatic agents compared with the standard military dressing—CG. Except for BL-IX, all of the agents demonstrated similar results in terms of overall survival, blood loss, and rebleeding rates. The similar efficacy rates in most of the agents suggest that further work in ease-of-use and cost analysis would be necessary to delineate the ideal hemostatic agent for control of arterial hemorrhage in the prehospital combat setting.
Supplementary Material
AUTHORSHIP
J.M.W. and P.W.W. conceived the study and were in charge of overall direction and planning. J.M.W., K.A.G., A.L.L., A.J.K., J.A.M., J.D.H., B.A., and D.M.B. carried out the experiments and processed and analyzed the data. K.A.G., J.M.W., and D.M.B. wrote the manuscript in consultation and critical revision by B.P. All authors discussed the results and contributed to the final manuscript.
ACKNOWLEDGMENT
This study was funded by an Interagency Agreement with US Army Medical Materiel Development Activity.
DISCLOSURE
The authors declare no conflicts of interest.
The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense, or The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. The authors have no financial interest related to the conduct of this research or the products tested or discussed during the project. In conducting research using animals, the investigators adhere to the laws of the United States and regulations of the Department of Agriculture.
Footnotes
This study was presented at the 2022 Military Health System Research Symposium, September 13, 2022, in Kissimmee, Florida.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Alexander J. Kersey, Email: aleckersey@gmail.com.
Alexis L. Lauria, Email: alexis.lauria625@gmail.com.
John A. Mares, Email: john.mares.ctr@usuhs.edu.
Justin D. Hutzler, Email: justin.hutzler.ctr@usuhs.edu.
Paul W. White, Email: whitepw7@gmail.com.
Biebele Abel, Email: Biebele.abel.ctr@usuhs.edu.
David M. Burmeister, Email: david.burmeister@usuhs.edu.
Brandon Propper, Email: bpropper@mac.com.
Joseph M. White, Email: jwhit189@jhmi.edu.
REFERENCES
- 1.Eastridge BJ Mabry RL Seguin P Cantrell J Tops T Uribe P Mallett O Zubko T Oetjen-Gerdes L Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–S437. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JF Ritenour AE McLaughlin DF Bagg KA Apodaca AN Mallak CT Pearse L Lawnick MM Champion HR Wade CE, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl):S21–S26; discussion S26–7. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb J Caruso J McMullin N Wade CE Pearse L Oetjen-Gerdes L Champion HR Lawnick M Farr W Rodriguez S, et al. Causes of death in US Special Operations Forces in the global war on terrorism: 2001–2004. US Army Med Dep J. 2007;24–37. [PubMed] [Google Scholar]
- 4.Gegel B, Burgert J, Gasko J, Campbell C, Martens M, Keck J, Reynolds H, Loughren M, Johnson D. The effects of QuikClot Combat Gauze and movement on hemorrhage control in a porcine model. Mil Med. 2012;177(12):1543–1547. [DOI] [PubMed] [Google Scholar]
- 5.Johnson D, Agee S, Reed A, Gegel B, Burgert J, Gasko J, Loughren M. The effects of QuikClot Combat Gauze on hemorrhage control in the presence of hemodilution. US Army Med Dep J. 2012;36–39. [PubMed] [Google Scholar]
- 6.Johnson D, Westbrook DM, Phelps D, Blanco J, Bentley M, Burgert J, Gegel B. The effects of QuikClot Combat Gauze on hemorrhage control when used in a porcine model of lethal femoral injury. Am J Disaster Med. 2014;9(4):309–315. [DOI] [PubMed] [Google Scholar]
- 7.Tactical Combat Casualty Care (TCCC) Guidelines for Medical Personnel 15 December 2021. 2022. Available at: https://books.allogy.com/web/tenant/8/books/b729b76a-1a34-4bf7-b76b-66bb2072b2a7/. Accessed July 13, 2023. [DOI] [PubMed]
- 8.Edwards J, Abdou H, Patel N, Madurska MJ, Poe K, Bonin JE, Richmond MJ, Rasmussen TE, Morrison JJ. The functional vascular anatomy of the swine for research. Vascular. 2022;30(2):392–402. [DOI] [PubMed] [Google Scholar]
- 9.Littlejohn LF, Devlin JJ, Kircher SS, Lueken R, Melia MR, Johnson AS. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad Emerg Med. 2011;18(4):340–350. [DOI] [PubMed] [Google Scholar]
- 10.Arnaud F Parreño-Sadalan D Tomori T Delima MG Teranishi K Carr W McNamee G McKeague A Govindaraj K Beadling C, et al. Comparison of 10 hemostatic dressings in a groin transection model in swine. J Trauma. 2009;67(4):848–855. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja N, Ostomel TA, Rhee P, Stucky GD, Conran R, Chen Z, Al-Mubarak GA, Velmahos G, Demoya M, Alam HB. Testing of modified zeolite hemostatic dressings in a large animal model of lethal groin injury. J Trauma. 2006;61(6):1312–1320. [DOI] [PubMed] [Google Scholar]
- 12.Sambasivan CN, Cho SD, Zink KA, Differding JA, Schreiber MA. A highly porous silica and chitosan-based hemostatic dressing is superior in controlling hemorrhage in a severe groin injury model in swine. Am J Surg. 2009;197(5):576–580; discussion 80. [DOI] [PubMed] [Google Scholar]
- 13.Kheirabadi BS, Scherer MR, Estep JS, Dubick MA, Holcomb JB. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J Trauma. 2009;67(3):450–459; discussion 9-60. [DOI] [PubMed] [Google Scholar]
- 14.Hoareau GL, Barthélemy A, Goy-Thollot I, Pouzot-Nevoret C, Beyer CA, Walker LE, Stewart IJ, Grayson JK. Reference intervals for and the effects of sample handling and sex on rotational thromboelastometry in healthy adult pigs. J Am Assoc Lab Anim Sci. 2020;59(3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam HB, Burris D, DaCorta JA, Rhee P. Hemorrhage control in the battlefield: role of new hemostatic agents. Mil Med. 2005;170(1):63–69. [DOI] [PubMed] [Google Scholar]
- 16.Ward KR, Tiba MH, Holbert WH, Blocher CR, Draucker GT, Proffitt EK, Bowlin GL, Ivatury RR, Diegelmann RF. Comparison of a new hemostatic agent to current combat hemostatic agents in a swine model of lethal extremity arterial hemorrhage. J Trauma. 2007;63(2):276–283; discussion 83-4. [DOI] [PubMed] [Google Scholar]
- 17.Rall JM, Cox JM, Songer AG, Cestero RF, Ross JD. Comparison of novel hemostatic dressings with QuikClot combat gauze in a standardized swine model of uncontrolled hemorrhage. J Trauma Acute Care Surg. 2013;75(2 Suppl 2):S150–S156. [DOI] [PubMed] [Google Scholar]
- 18.Granville-Chapman J, Jacobs N, Midwinter MJ. Pre-hospital haemostatic dressings: a systematic review. Injury. 2011;42(5):447–459. [DOI] [PubMed] [Google Scholar]
- 19.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–1463; discussion 63-5. [DOI] [PubMed] [Google Scholar]
- 20.Devlin JJ, Kircher S, Kozen BG, Littlejohn LF, Johnson AS. Comparison of ChitoFlex®, CELOX™, and QuikClot® in control of hemorrhage. J Emerg Med. 2011;41(3):237–245. [DOI] [PubMed] [Google Scholar]


