Background:
Neoadjuvant therapy (NAT) has become common worldwide for resectable advanced esophageal cancer and frequently involves weight loss. Although failure to rescue (death after major complications) is known as an emerging surgical quality measure, little is known about the impact of weight loss during NAT on failure to rescue. This retrospective study aimed to investigate the association of weight loss during NAT and short-term outcomes, including failure to rescue after esophagectomy.
Materials and methods:
Patients who underwent esophagectomy after NAT between July 2010 and March 2019 were identified from a Japanese nationwide inpatient database. Based on quartiles of percent weight change during NAT, patients were grouped into four categories of gain, stable, small loss, and loss (>4.5%). The primary outcomes were failure to rescue and in-hospital mortality. The secondary outcomes were major complications, respiratory complications, anastomotic leakage, and total hospitalization costs. Multivariable regression analyses were used to compare outcomes between the groups, adjusting for potential confounders, including baseline BMI.
Results:
Among 15 159 eligible patients, in-hospital mortality and failure to rescue occurred in 302 (2.0%) and 302/5698 (5.3%) patients, respectively. Weight loss (>4.5%) compared to gain was associated with increased failure to rescue and in-hospital mortality [odds ratios 1.55 (95% CI: 1.10–2.20) and 1.53 (1.10–2.12), respectively]. Weight loss was also associated with increased total hospitalizations costs, but not with major complications, respiratory complications, and anastomotic leakage. In subgroup analyses, regardless of baseline BMI, weight loss (>4.8% in nonunderweight or >3.1% in underweight) was a risk factor for failure to rescue and in-hospital mortality.
Conclusion:
Weight loss during NAT was associated with failure to rescue and in-hospital mortality after esophagectomy, independent of baseline BMI. This emphasizes the importance of weight loss measurement during NAT to assess the risk for a subsequent esophagectomy.
Keywords: failure to rescue, mortality, neoadjuvant therapy, esophageal cancer, oesophagectomy, weight loss
Introduction
Highlights
A quarter of patients had weight loss (>4.5%) during neoadjuvant therapy.
Weight loss during neoadjuvant therapy was associated with mortality after esophagectomy.
Weight loss was also associated with failure to rescue (death after complications).
These associations were independent of baseline BMI.
Subtotal esophagectomy, the mainstay curative treatment for resectable esophageal cancer, is one of the most invasive surgical procedures, and results in high proportions of postoperative mortality (4.5%) and complications (59%)1. Because postoperative complications may be inevitable, even with appropriate surgical procedures and perioperative care, quality improvement efforts have shifted from preventing complications to preventing deaths after the development of complications2. Risk factors for ‘failure to rescue’, defined as death among patients with major complications, have thus received increasing attention3–5. In particular, modifiable patient-level risk factors for failure to rescue could be appealing targets for improving the surgical outcomes across all hospitals2. Although age and pre-existing comorbidities, such as renal failure and liver disease have been reported as patient-level risk factors for failure to rescue after esophagectomy6, most of them seem to be unmodifiable.
Esophageal cancer has the highest median weight loss before diagnosis among all cancers7. Nearly half of the patients undergoing esophagectomy experience weight loss before diagnosis8,9 and further weight loss occurs frequently during neoadjuvant therapy (NAT)10,11, which has become common worldwide recently for advanced esophageal cancer12. The cause of weight loss is multifactorial, including tumor-associated symptoms (e.g. dysphagia) and abnormal metabolism, therapy-associated toxicities, and muscle atrophy due to patient inactivity13. Consequently, preoperative severe weight loss (>10% during the 6 months before surgery) occurred in 9–20% of esophageal cancer patients14–16 and was associated with postoperative mortality after esophagectomy14.
Although weight loss before diagnosis is unmodifiable, weight loss after diagnosis to surgery is potentially modifiable, especially in patients who receive NAT because they generally have sufficient time (2–3 months or more) for preoperative optimization. Indeed, previous studies showed that prehabilitation (i.e. preoperative exercise and nutritional intervention) during NAT resulted in a relative preoperative weight gain (4.6–4.8%) compared to the control group17,18. However, the impact of weight change during NAT on the surgical outcomes, including failure to rescue and mortality, remains unknown.
In the present study, we aimed to determine the association between weight loss during NAT and short-term outcomes after oncologic esophagectomy, using a Japanese nationwide inpatient database.
Materials and methods
Data source
This retrospective cohort study was conducted using the Diagnosis Procedure Combination database, a Japanese nationwide inpatient database19. More than 8 million hospital administrative claims data and discharge abstracts from more than 1200 hospitals are collected annually in this database. All university hospitals are required to participate in the database, while other hospitals participate on a voluntary basis. The requirement for informed consent was waived because of the anonymous nature of the data. This study was approved by the institutional review board with a unique identifying number of the registration.
The database includes the following information: sex; age at admission; height and weight at admission; smoking history (including both current smoker and ex-smoker); diagnosis and comorbidities at admission and complications after admission recorded using the International Classification of Diseases, 10th Revision (ICD-10) codes; clinical cancer stage based on the seventh edition of the Union for International Cancer Control Tumor, Node, Metastasis classification; preoperative chemotherapy and radiotherapy; interventional and surgical procedures according to the original Japanese codes; unique hospital identifier; length of stay; discharge status; and total hospitalization costs. All discharge abstracts for each patient were recorded at discharge by the attending physician. Previous validation studies have shown high accuracy for cancer diagnosis20, surgical procedures21, comorbidities22, and postoperative complications23.
Study protocol
We identified patients who underwent esophagectomy with two-field (thoraco-abdominal) or three-field (cervico-thoraco-abdominal) lymph node dissection for esophageal cancer between July 2010 and March 2019. Among them, we included those who started 5-flurouracil-based NAT (chemotherapy or chemoradiotherapy) 4–20 weeks prior to esophagectomy. Patients who underwent trans-hiatal esophagectomy and two-stage reconstruction were not included in this study. We used the original Japanese procedure codes for surgery to identify patients who underwent these procedures.
The exclusion criteria were as follows: patients aged less than 18 years; patients who underwent combined surgery for laryngeal or hypopharyngeal cancer; and patients who were outliers for height (<100 or >200 cm), weight (<20 or >200 kg), BMI (<12.5 or >60 kg/m2), or weight change during NAT (>30% gain or >30% loss). Weight change during NAT was defined as the relative percent change in weight between the weight at admission for the initial NAT (baseline weight) and the weight at admission for surgery (presurgery weight); that is, weight change=(presurgery weight – baseline weight) / baseline weight×100. We also excluded patients who underwent NAT and esophagectomy during the same hospitalization because data on their presurgery weight were not available in the database.
Weight change during NAT was categorized into quartiles (Q) from the highest weight gain to the highest weight loss: Q1, weight gain (≥2.0% gain); Q2, weight stable (<2.0% gain or ≤0.9% loss); Q3, small weight loss (0.8–4.5% loss); and Q4, weight loss (>4.5% loss).
The primary outcomes were failure to rescue and in-hospital mortality. Failure to rescue was defined as the proportion of mortality in patients with at least one major complication24. The secondary outcomes were major complications, respiratory complications, anastomotic leakage, length of stay after surgery, and total hospitalization costs. We defined major complications as respiratory complications, anastomotic leakage, pneumothorax, chylothorax, empyema, peritonitis, ileus/bowel obstruction/symptomatic hernia (hiatal or diaphragmatic), pulmonary embolism, acute coronary syndrome, heart failure, stroke, acute kidney injury, sepsis, and others, resulting in unplanned intubation or death24,25. The ICD-10 codes and procedure codes used to define these postoperative complications are shown in Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JS9/A156.
We examined patient background characteristics, including sex, age (presurgery), baseline BMI (preNAT), smoking history (presurgery), comorbidities (presurgery), and clinical T, N, and M factors. Baseline BMI was categorized into four groups based on the criteria for Asia-Pacific populations by the World Health Organization according to our previous study: less than 18.5 kg/m2 (underweight), 18.5–22.9 kg/m2 (normal), 23.0–27.4 kg/m2 (overweight), and greater than or equal to 27.5 kg/m2 (obese)24,26. Comorbidities were scored by the Charlson comorbidity index based on ICD-10 codes27 and classified into three groups: 2, 3–4, and greater than or equal to 5. The clinical T factor was divided into three categories: T0–2, T3–4, and TX/missing. The clinical N factor was divided into three categories: N0, N1–3, and NX/missing. The clinical M factor was divided into three categories: M0, M1, and missing.
We also investigated treatment characteristics, including the regimen and cycles of chemotherapy, radiotherapy, the interval from NAT to surgery, preoperative tube feeding, the field of lymph node dissection, the thoracic approach, and hospital volume. Chemotherapy regimens were categorized into three groups: cisplatin plus 5-fluorouracil; docetaxel, cisplatin plus 5-fluorouracil (DCF); and others (5-fluorouracil-based). The cycles of chemotherapy were categorized into two groups; 1 and greater than or equal to 2. In Japan, preoperative chemotherapy with the cisplatin plus 5-fluorouracil regimen (two courses) was the standard NAT for locally advanced esophageal cancer (clinical stage ≥II) during the study period, while intensive chemotherapy with the DCF regimen (three courses) and chemoradiotherapy were evaluated in a clinical trial28–30. The interval from NAT to surgery (defined as the number of days from the initiation of NAT to the surgery) was divided into two groups by the median (65 days). Preoperative tube feeding was defined as enteral nutrition (via jejunostomy, gastrostomy, nasojejunal tube, or nasogastric tube) at least once from the day of admission for the initial NAT to the day before surgery. The field of lymph node dissection was either two fields (thoracic and abdominal approaches) or three fields (cervical, thoracic, and abdominal approaches; with or without supraclavicular lymph node dissection). The thoracic approach for esophagectomy was divided into open and minimally invasive (i.e. thoracoscopic, mediastinoscopy-assisted, or robotic-assisted esophagectomy) approaches. Whether the thoracic approach was open or minimally invasive was not recorded before March 2014. The database also did not include information on whether an abdominal approach was an open laparotomy or a laparoscopy. Hospital volume was defined as the number of esophagectomies performed per year in each hospital and was categorized into Q.
Statistical analysis
We conducted the following multivariable regression analyses fitted with generalized estimating equations: logistic regression analyses for failure to rescue, in-hospital mortality, major complications, respiratory complications, and anastomotic leakage; and linear regression analyses for length of stay and total hospitalization costs. The generalized estimating equations enabled us to adjust the analyses for clustering of variables within the same hospital, such as patient background characteristics and physician practice patterns31. The explanatory variables in the multivariable analyses were patient background characteristics (sex, age, Charlson comorbidity index, baseline BMI category, smoking history, clinical T, N, and M factors), and treatment characteristics (regimens and cycles of chemotherapy, radiotherapy, interval from NAT to surgery, preoperative tube feeding, field of lymph node dissection, thoracic approach, and hospital volume). The odds ratios (ORs) and their 95% CI were calculated in the logistic regression analyses, and the coefficients and their 95% CI were calculated in the linear regression analyses for quartile categories of weight change during NAT. The weight gain group (Q1) was used as a reference because we considered that patients in this group were closest to their preillness weight. That is, esophageal cancer patients who require NAT often experience weight loss due to dysphasia and other causes before the diagnosis8,9,13, and they can regain their preillness weight during NAT through prehabilitation17,18 or tumor shrinkage.
In subgroup analyses, we performed multivariable logistic regression analyses fitted with generalized estimating equations stratified by baseline BMI (underweight or nonunderweight [normal/overweight/obese]) for the primary outcomes. Q of weight change during NAT in nonunderweight and underweight patients were calculated and categorized separately for each baseline BMI. The ORs in the subgroup analyses were adjusted for sex, age, and the Charlson comorbidity index.
In the summary statistics, the Kruskal–Wallis H test was used for comparisons of continuous variables and the χ 2-test was used for comparisons of categorical variables between the groups. Statistical significance was accepted at P less than 0.05. All statistical analyses were conducted using statistics and data (STATA) version 16 (StataCorp LLC). This study was conducted in line with the strengthening the reporting of cohort, cross-sectional and case-control studies in surgery (STROCSS) criteria32, Supplemental Digital Content 2, http://links.lww.com/JS9/A157.
Results
Overall, 40 245 esophageal cancer patients who underwent esophagectomy between July 2010 and March 2019 were identified. Among them, 16 498 patients received 5-flurouracil-based NAT 4–20 weeks prior to esophagectomy. There were no patients younger than 18 years of age. We excluded patients with combined surgery for laryngeal/hypopharyngeal cancer (n=151), patients who were outliers for height (n=118), weight (n=14), BMI (n=12), or weight change (n=36), and patients who underwent NAT and esophagectomy during the same hospitalization (n=1008). Finally, 15 159 patients were analyzed in the study. The median age was 67 (interquartile range, 61–72) years, and 12 661 patients (84%) were male.
The patient and treatment characteristics were compared among the quartile categories of weight change during NAT (Table 1). The weight gain group was more likely to be young and underweight at baseline, to receive chemotherapy with DCF, to receive greater than or equal to 2 cycles of chemotherapy, and to have a longer interval from NAT to surgery than the other groups. The weight loss (>4.5%) group was more likely to be obese at baseline, to be diagnosed with clinical T3–4, to receive preoperative tube feeding, and to receive radiotherapy compared to the other groups.
Table 1.
Patient background and treatment characteristics categorized by quartiles of weight change during neoadjuvant therapy.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
|---|---|---|---|---|---|---|
| Factor | Category | Gain (n=3789) | Stable (n=3789) | Small loss (n=3791) | Loss (>4.5%) (n=3790) | P |
| Patient background | ||||||
| Sex | Male | 3111 (82) | 3162 (83) | 3174 (84) | 3214 (85) | 0.017 |
| Age, years | 65 (59–70) | 67 (61–71) | 68 (62–73) | 68 (63–73) | <0.001 | |
| Baseline BMI category | Underweight | 1043 (28) | 624 (16) | 530 (14) | 493 (13) | <0.001 |
| Normal weight | 2041 (54) | 2029 (54) | 1983 (52) | 2031 (54) | ||
| Overweight | 658 (17) | 1020 (27) | 1136 (30) | 1097 (29) | ||
| Obese | 47 (1.2) | 116 (3.1) | 142 (3.8) | 169 (4.5) | ||
| Charlson comorbidity index | 2 | 3005 (79) | 3054 (81) | 3030 (80) | 2999 (79) | 0.63 |
| 3–4 | 463 (12) | 448 (12) | 468 (12) | 484 (13) | ||
| ≥ 5 | 321 (8.5) | 287 (7.6) | 293 (7.7) | 307 (8.1) | ||
| Smoking history | 3044 (80) | 2865 (76) | 2806 (74) | 2836 (75) | <0.001 | |
| Clinical T factor | T0–2 | 1194 (32) | 1318 (35) | 1389 (37) | 1059 (28) | <0.001 |
| T3–4 | 2291 (60) | 2173 (57) | 2129 (56) | 2436 (64) | ||
| TX/missing | 304 (8.0) | 298 (7.9) | 273 (7.2) | 295 (7.8) | ||
| Clinical N factor | N0 | 910 (24) | 895 (24) | 1056 (28) | 884 (23) | <0.001 |
| N1–3 | 2594 (68) | 2592 (68) | 2465 (65) | 2629 (69) | ||
| NX/missing | 285 (7.5) | 302 (8.0) | 270 (7.1) | 277 (7.3) | ||
| Clinical M factor | M0 | 3338 (88) | 3363 (89) | 3351 (88) | 3360 (89) | 0.68 |
| M1 | 183 (4.8) | 149 (3.9) | 171 (4.5) | 165 (4.4) | ||
| Missing | 268 (7.1) | 277 (7.3) | 269 (7.1) | 265 (7.0) | ||
| Treatments | ||||||
| Chemotherapy regimen | CF | 2259 (60) | 2580 (68) | 2656 (70) | 2643 (70) | <0.001 |
| DCF | 1331 (35) | 983 (26) | 918 (24) | 930 (25) | ||
| Others | 199 (5.3) | 226 (6.0) | 217 (5.7) | 217 (5.7) | ||
| Cycles of chemotherapy | ≥ 2 | 3293 (87) | 2931 (77) | 3032 (80) | 3017 (80) | <0.001 |
| Radiotherapy (with chemotherapy) | 366 (9.7) | 400 (11) | 449 (12) | 561 (15) | <0.001 | |
| Interval from NAT to surgery ≥ 65 days | 2356 (62) | 1830 (48) | 1804 (48) | 1884 (50) | <0.001 | |
| Preoperative tube feeding | 265 (7.0) | 219 (5.8) | 238 (6.3) | 430 (11) | <0.001 | |
| Three-field lymph node dissection | 3429 (90) | 3409 (90) | 3384 (89) | 3375 (89) | 0.14 | |
| Thoracic approach | Unspecified (to March 2014) | 1230 (32) | 1336 (35) | 1281 (34) | 1259 (33) | 0.034 |
| Open | 1010 (27) | 1047 (28) | 1002 (26) | 1012 (27) | ||
| Minimally invasive | 1549 (41) | 1406 (37) | 1508 (40) | 1519 (40) | ||
| Hospital volume, cases per year | < 7 | 849 (22) | 800 (21) | 822 (22) | 799 (21) | <0.001 |
| 7–19 | 910 (24) | 926 (24) | 914 (24) | 1036 (27) | ||
| 20–42 | 969 (26) | 1061 (28) | 878 (23) | 749 (20) | ||
| ≥ 43 | 1061 (28) | 1002 (26) | 1177 (31) | 1206 (32) | ||
Data are presented as n (%) or median (interquartile range).
CF, cisplatin plus 5-fluorouracil; DCF, Docetaxel, cisplatin plus 5-fluorouracil; NAT, neoadjuvant therapy.
The crude outcomes of the patients categorized by the quartile categories of weight change during NAT are shown in Table 2 and the detailed postoperative complications are shown in Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/JS9/A156. In-hospital mortality and failure to rescue occurred in 302 (2.0%) and 302/5698 (5.3%) patients, respectively. The proportions of failure to rescue, in-hospital mortality, length of stay after surgery, and total hospitalization costs differed significantly among the groups (P=0.004, 0.005, <0.001, and <0.001, respectively).
Table 2.
Crude outcomes of patients categorized by quartiles of weight change during neoadjuvant therapy.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|
| Variable | Gain (n=3789) | Stable (n=3789) | Small loss (n=3791) | Loss (>4.5%) (n=3790) | P |
| Failure to rescue | 59/1450 (4.1) | 67/1417 (4.7) | 76/1390 (5.5) | 100/1441 (6.9) | 0.004 |
| In-hospital mortality | 59 (1.6) | 67 (1.8) | 76 (2.0) | 100 (2.6) | 0.005 |
| Major complications | 1450 (38) | 1417 (37) | 1390 (37) | 1441 (38) | 0.48 |
| Respiratory complications | 971 (26) | 940 (25) | 940 (25) | 987 (26) | 0.51 |
| Anastomotic leakage | 459 (12) | 484 (13) | 449 (12) | 464 (12) | 0.66 |
| Length of stay after surgery, days | 23 (17–35) | 23 (17–36) | 23 (17–36) | 24 (18–40) | <0.001 |
| Total hospitalization costs, US$ | 28 744 (25 588–33 982) | 28 728 (25 625–34 464) | 28 942 (25 684–34 663) | 29 625 (26 323–36 229) | <0.001 |
Data are presented as n (%) or median (interquartile range).
Table 3 shows the associations between weight change during NAT and short-term outcomes using multivariable regression analyses. Patients with weight loss greater than 4.5% were significantly associated with a higher occurrence of failure to rescue (ORs, 1.55 [95% CI, 1.10–2.20]), higher in-hospital mortality (1.53 [1.11–2.12]), longer length of stay after surgery, and increased hospitalization costs, compared to those with weight gain. Small weight loss as well as stable weight were not associated with the outcomes. The proportions of major complications, respiratory complications, and anastomotic leakage were similar among the groups.
Table 3.
Multivariable regression analyses for short-term outcomes.
| Quartile 2 | Quartile 3 | Quartile 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stable | Small loss | Loss (>4.5%) | |||||||
| Variable | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Failure to rescue | 1.09 | 0.76–1.56 | 0.64 | 1.27 | 0.87–1.85 | 0.22 | 1.55 | 1.10–2.20 | 0.013 |
| In-hospital mortality | 1.10 | 0.77–1.57 | 0.61 | 1.23 | 0.85–1.77 | 0.27 | 1.53 | 1.10–2.12 | 0.011 |
| Major complications | 0.98 | 0.89–1.09 | 0.74 | 0.95 | 0.86–1.04 | 0.27 | 1.00 | 0.90–1.12 | 0.94 |
| Respiratory complications | 0.97 | 0.88–1.07 | 0.51 | 0.94 | 0.86–1.04 | 0.23 | 1.02 | 0.91–1.14 | 0.76 |
| Anastomotic leakage | 1.05 | 0.91–1.22 | 0.49 | 0.98 | 0.85–1.12 | 0.74 | 0.97 | 0.84–1.12 | 0.69 |
| Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | |
| Length of stay after surgery, days | 0.4 | −1.3–2.1 | 0.66 | 0.1 | −1.6–1.8 | 0.93 | 1.8 | 0.1–3.5 | 0.042 |
| Total hospitalization costs, US$ | 458 | −347–1263 | 0.27 | 44 | −667–756 | 0.90 | 973 | 233–1713 | 0.010 |
OR and Coef are with reference to Quartile 1 (Gain). The explanatory variables were patient background characteristics (sex, age, Charlson comorbidity index, baseline BMI category, smoking history, clinical T factor, clinical N factor, clinical M factor) and treatment characteristics (chemotherapy regimen, cycles of chemotherapy, radiotherapy, interval from neoadjuvant therapy to surgery, preoperative tube feeding, field of lymph node dissection, thoracic approach, and hospital volume). A generalized estimating equation was used to adjust for within-hospital clustering.
Coef, coefficient; OR, odds ratio.
Figure 1 shows subgroup analyses for the primary outcomes stratified by nonunderweight and underweight at baseline. The median weight change during NAT was 1.2% loss (interquartile range, 1.5% gain to 4.8% loss) in nonunderweight patients and 0.2% gain (4.5% gain to 3.1% loss) in underweight patients. Regardless of baseline BMI, weight loss (>4.8% in the nonunderweight and >3.1% in the underweight) was significantly associated with a higher occurrence of failure to rescue and in-hospital mortality compared to weight gain.
Figure 1.
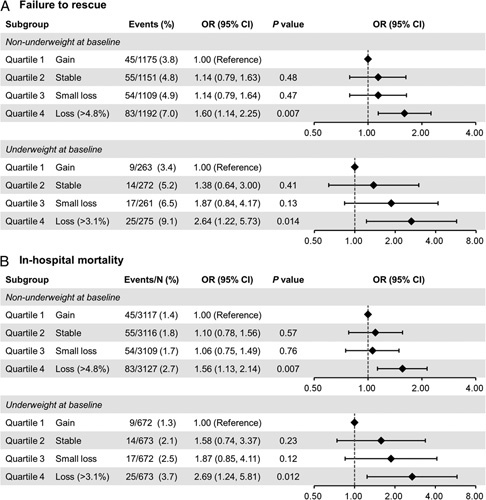
Subgroup multivariable regression analyses of primary endpoints stratified by baseline BMI. Odds ratios of respiratory failure (A) and in-hospital mortality associated with weight change during neoadjuvant therapy (B). The explanatory variables were sex, age, and Charlson comorbidity index. OR, odds ratio.
Discussion
In this nationwide retrospective cohort study, we investigated the association between weight loss during NAT and short-term outcomes after esophagectomy in patients with esophageal cancer after adjustment for potential confounders. The key findings of the present study were as follows: weight loss greater than 4.5% was associated with increased failure to rescue and in-hospital mortality but not with major complications, compared to weight gain; and weight loss (>4.8% group in nonunderweight patients or >3.1% in underweight patients) was a risk factor for failure to rescue and in-hospital mortality regardless of baseline BMI. To the best of our knowledge, this is the first study to analyze the association between weight loss during NAT and short-term outcomes after esophagectomy.
Both weight loss and low BMI are well-known markers of malnutrition33, and the guidelines on nutrition in cancer patients strongly recommend BMI measurement at cancer diagnosis as well as repeated evaluation of weight change after diagnosis13. We previously reported that low BMI was a risk factor for failure to rescue as well as in-hospital mortality after oncologic esophagectomy24. However, although weight loss frequently occurs during the NAT period (i.e. after diagnosis to surgery) in esophageal cancer patients10,11, its impact on short-term outcomes after esophagectomy remains unknown.
In the current study, we revealed that a weight loss greater than 4.5% during NAT was a risk factor for failure to rescue and in-hospital mortality after esophagectomy. Our findings suggest that the increased in-hospital mortality in patients with weight loss was attributed to an increased risk of death after major complications (i.e. failure to rescue) rather than an increased risk of complications. Previous randomized controlled trials have shown that NAT does not increase postoperative mortality after esophagectomy34–36. However, patients with weight loss during NAT may have an increased risk of failure to rescue presumably due to decreased physiological reserve (e.g. immune, endocrine, musculoskeletal, and respiratory systems)37. Therefore, esophageal surgeons should devote more attention to patients who experienced weight loss during NAT. When these patients develop a major complication, early interventions for the complication (e.g. intensive care unit admission and concentrating human resources) could reduce mortality38,39.
In subgroup analysis according to baseline BMI, the weight loss group (>4.8% in nonunderweight patients and >3.1% in underweight patients) demonstrated poor primary outcomes regardless of baseline BMI. Impact of weight change on the general condition may differ depending on baseline BMI. Indeed, some guidelines recommend low BMI patient-specific cut-off value of weight change in diagnosis of cancer cachexia and malnutrition: cancer cachexia criteria: weight loss greater than 5% over the last 6 months, or BMI less than 20 kg/m2 and ongoing weight loss greater than 2%40; malnutrition criteria: weight loss greater than 10%, or low BMI (<20 kg/m2 if < 70 years or <22 kg/m2 if ≥70 years) and weight loss greater than 5% over the last 3 months41. For accurate risk assessment, surgeons should consider baseline BMI when assessing the weight change during NAT.
We previously showed that low BMI was associated with an increased risk of major complications after esophagectomy24. However, the current study results revealed that a weight loss greater than 4.5% during NAT was not associated with major or specific complications (such as respiratory complications and anastomotic leakage) after oncologic esophagectomy. Likewise, previous studies showed that neither a preoperative weight loss greater than 10% during 3 months before diagnosis15 nor a skeletal muscle loss greater than 5% during NAT42 was associated with postoperative complications. Although weight loss and low BMI are both markers of malnutrition33, they would have different effects on surgical outcomes. Therefore, our results emphasize the importance of assessing weight loss during NAT for risk stratification in patients undergoing esophagectomy as well as the preoperative BMI.
Moreover, weight loss during NAT could be an appealing target for preoperative optimization because this is potentially modifiable through a preoperative exercise program and weekly nutritional counseling17,18, preoperative jejunostomy tube feeding43, or proper management of chemotherapy-induced toxicities (e.g. nausea, vomiting, and oral mucositis)44. In recent enhanced recovery after surgery programs, the focus has shifted from postoperative care to preoperative optimization45. The recent adoption of intensive NAT has increased toxicity, which might contribute to weight loss, but the time period for preoperative optimization (the interval from diagnosis to surgery) has been extended29,46,47. We recommend repeated weight measurements during NAT because theoretically this allows early detection of patients with a small weight loss in addition to providing opportunities for intervention to prevent further weight loss. Because the current study showed that a small weight loss was not associated with poor outcomes, minimizing weight loss during NAT may improve surgical outcomes.
This study has several limitations. First, information on weight loss before diagnosis was unavailable in the database. However, because weight change before diagnosis is unmodifiable and can be clinically unclear due to the self-reported nature, we consider that BMI at diagnosis and weight change after diagnosis are more reliable and pivotal factors for patients undergoing esophagectomy following NAT. Second, because only a small number of obese patients were included, the statistical power of the association between weight change and primary outcomes for this group was insufficient. However, we do not recommend indifference to weight loss during NAT in obese patients because it may lead to sarcopenic obesity and poor short-term postoperative outcomes48,49. Third, information on the thoracic approach was unavailable in the database for patients before April 2014 because minimally invasive esophagectomy has been reimbursed separately from open esophagectomy since April 2014 by the publicly provided universal insurance system. Therefore, during adjustment in the present analyses, the thoracic approach was classified as unspecified, open, or minimally invasive. Fourth, our database did not contain information on histopathological findings (squamous cell carcinoma or adenocarcinoma). In a previous report using data from 344 institutions in Japan, squamous cell carcinoma accounted for 88% and adenocarcinoma for 7.1%50. Although baseline BMI may differ between squamous cell carcinoma and adenocarcinoma patients51,52, baseline BMI was adjusted for in the analyses; therefore, the loss of information on histopathological findings would not skew the results. Finally, information on jejunostomy tube placement during esophagectomy was also not available from the database. Jejunostomy or nasojejunal tube feeding would have been generally used after esophagectomy in Japan because Japanese esophageal cancer practice guidelines recommend postoperative tube feeding28,53.
Conclusions
Weight loss during NAT was associated with increased failure to rescue and in-hospital mortality after esophagectomy for esophageal cancer, independent of baseline BMI. We consider that weight loss during NAT may be a principal indicator that needs to be evaluated regularly for preoperative optimization (e.g. prehabilitation and jejunostomy tube feeding) and a safe operation.
Ethical approval
This study was approved by the Institutional Review Board of the University of Tokyo [approval number: 3501-(5)].
Sources of funding
This work was supported by grants from the Ministry of Health, Labor, and Welfare, Japan (21AA2007 and 22AA2003) and the Ministry of Education, Culture, Sports, Science, and Technology, Japan (20H03907).
Author contribution
Y.H.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing – original draft; T.K.: data curation, methodology, validation, writing – review and editing; H.K.: data curation, methodology, writing – review and editing; H.I.: data curation, validation, writing – review and editing; S.M.: methodology, writing – review and editing; H.K.: methodology, writing – review and editing; K.U.: data curation, resources, writing – review and editing; H.M.: data curation, resources, writing – review and editing; K.F.: data curation, resources, writing – review and editing; H.D.: supervision, writing – review and editing; O.I.: supervision, writing – review and editing; H.Y.: conceptualization, funding acquisition, resources, supervision, writing – review and editing; Y.K.: conceptualization, supervision, writing – review and editing.
Conflicts of interest disclosure
Dr Kitagawa has received grants and personal fees from Asahi Kasei Pharma Corporation, grants, personal fees and others from Ono Pharmaceutical Co. Ltd., grants and personal fees from Otsuka Pharmaceutical Factory Inc., grants and personal fees from Nippon Covidien Inc., grants, personal fees and others from Taiho Pharmaceutical Co. Ltd., grants, personal fees and others from Chugai Pharmaceutical Co. Ltd., grants and personal fees from Kaken Pharmaceutical Co. Ltd., personal fees from AstraZeneca K.K., personal fees from Ethicon Inc., personal fees from Olympus Corporation, personal fees from Shionogi & Co. Ltd., personal fees and others from Bristol-Myers Squibb K.K., personal fees from MSD K.K., personal fees from Smith & Nephew K.K., personal fees from Aska Pharmaceutical Co. Ltd., personal fees from Miyarisan Pharmaceutical Co. Ltd., personal fees from Toray Industries, Inc., personal fees from Daiichi Sankyo Co. Ltd., personal fees from Chugai Foundation for Innovative Drug Discovery Science, personal fees from Nippon Kayaku Co. Ltd., grants from Yakult Honsha Co. Ltd., grants from Otsuka Pharmaceutical Co. Ltd., grants from Tsumura & Co., grants from Sumitomo Pharma Co. Ltd., grants and personal fees from EA Pharma Co. Ltd., grants from Eisai Co. Ltd., grants from Kyouwa Hakkou Kirin Co. Ltd., grants from Medicon Inc., grants from Takeda Pharmaceutical Co. Ltd., grants from Teijin Pharma Ltd., and personal fees from Intuitive Surgical G.K., outside the submitted work. There are no other conflicts of interest to disclose.
Research registration unique identifying number (UIN)
Name of the registry: UMIN
Unique Identifying number or registration ID: UMIN000049208
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://center6.umin.ac.jp/cgi-openbin/ctr_e/ctr_view.cgi?recptno=R000056046
Guarantor
Yuki Hirano.
Data availability statement
Because the data in this study was extracted from a nationwide database, data use requires approval of the Ministry of Health, Labor, and Welfare, Japan.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, http://www.journal-surgery.net.
Published online 3 April 2023
Contributor Information
Yuki Hirano, Email: yukihirano1@gmail.com.
Takaaki Konishi, Email: takaakonishi-ncd@umin.ac.jp.
Hidehiro Kaneko, Email: kanekohidehiro@gmail.com.
Hidetaka Itoh, Email: hitoh.ggl@gmail.com.
Satoru Matsuda, Email: s.matsuda.a8@keio.jp.
Hirofumi Kawakubo, Email: hkawakubo@keio.jp.
Kazuaki Uda, Email: udakazuaki-tky@umin.ac.jp.
Hiroki Matsui, Email: ptmatsui-tky@umin.ac.jp.
Kiyohide Fushimi, Email: kfushimi.hci@tmd.ac.jp.
Hiroyuki Daiko, Email: hdaiko@ncc.go.jp.
Osamu Itano, Email: itano@iuhw.ac.jp.
Hideo Yasunaga, Email: yasunagah@m.u-tokyo.ac.jp.
Yuko Kitagawa, Email: kitagawa.a3@keio.jp.
References
- 1. Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291–298. [DOI] [PubMed] [Google Scholar]
- 2. Portuondo JI, Shah SR, Singh H, et al. Failure to rescue as a surgical quality indicator: current concepts and future directions for improving surgical outcomes. Anesthesiology 2019;131:426–437. [DOI] [PubMed] [Google Scholar]
- 3. Ervin JN, Vitous CA, Wells EE, et al. Rescue improvement conference: a novel tool for addressing failure to rescue. Ann Surg 2023;277:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ward ST, Dimick JB, Zhang W, et al. Association between hospital staffing models and failure to rescue. Ann Surg 2019;270:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah R, Attwood K, Arya S, et al. Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surg 2018;153:e180214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdelsattar ZM, Habermann E, Borah BJ, et al. Understanding failure to rescue after esophagectomy in the United States. Ann Thorac Surg 2020;109:865–871. [DOI] [PubMed] [Google Scholar]
- 7. Bozzetti F. SCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer 2009;17:279–284. [DOI] [PubMed] [Google Scholar]
- 8. Zhang HL, Yang YS, Duan JN, et al. Prognostic value of preoperative weight loss-adjusted body mass index on survival after esophagectomy for esophageal squamous cell carcinoma. World J Gastroenterol 2020;26:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang S, Tan Y, Cai X, et al. Preoperative weight loss is associated with poorer prognosis in operable esophageal cancer patients: a single-center retrospective analysis of a large cohort of Chinese patients. J Cancer 2020;11:1994–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujihata S, Ogawa R, Nakaya S, et al. The impact of skeletal muscle wasting during neoadjuvant chemotherapy on postoperative anastomotic leakage in patients with esophageal cancer. Esophagus 2021;18:258–266. [DOI] [PubMed] [Google Scholar]
- 11. Jain R, Shaikh T, Yee JL, et al. Impact of clinical markers of nutritional status and feeding jejunostomy use on outcomes in esophageal cancer patients undergoing neoadjuvant chemoradiotherapy. Nutrients 2020;12:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuppusamy MK, Low DE. International Esodata Study Group (IESG). Evaluation of international contemporary operative outcomes and management trends associated with esophagectomy: a 4-year study of >6000 patients using ECCG definitions and the online Esodata database. Ann Surg 2022;275:515–525. [DOI] [PubMed] [Google Scholar]
- 13. Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr 2021;40:2898–2913. [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259–266. [DOI] [PubMed] [Google Scholar]
- 15. van der Schaaf MK, Tilanus HW, van Lanschot JJ, et al. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg 2014;147:490–495. [DOI] [PubMed] [Google Scholar]
- 16. Liou DZ, Serna-Gallegos D, Mirocha J, et al. Predictors of failure to rescue after esophagectomy. Ann Thorac Surg 2018;105:871–878. [DOI] [PubMed] [Google Scholar]
- 17. Xu YJ, Cheng JC, Lee JM, et al. A walk-and-eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Oncologist 2015;20:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ligthart-Melis GC, Weijs PJ, te Boveldt ND, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587–593. [DOI] [PubMed] [Google Scholar]
- 19. Yasunaga H. Real world data in Japan: chapter II the diagnosis procedure combination database. Ann Clin Epidemiol 2019;1:76–79. [Google Scholar]
- 20. Shigemi D, Morishima T, Yamana H, et al. Validity of initial cancer diagnoses in the diagnosis procedure combination data in Japan. Cancer Epidemiol 2021;74:102016. [DOI] [PubMed] [Google Scholar]
- 21. Konishi T, Yoshimoto T, Fujiogi M, et al. Validity of operative information in Japanese administrative data: a chart review-based analysis of 1221 cases at a single institution. Surg Today 2022;52:1484–1490. [DOI] [PubMed] [Google Scholar]
- 22. Yamana H, Moriwaki M, Horiguchi H, et al. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 2017;27:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamana H, Tsuchiya A, Horiguchi H, et al. Validity of a model using routinely collected data for identifying infections following gastric, colon, and liver cancer surgeries. Pharmacoepidemiol Drug Saf 2022;31:452–460. [DOI] [PubMed] [Google Scholar]
- 24. Hirano Y, Kaneko H, Konishi T, et al. Impact of body mass index on major complications, multiple complications, in-hospital mortality, and failure to rescue after esophagectomy for esophageal cancer: a nationwide inpatient database study in Japan. Ann Surg 2023;277:e785–e792. [DOI] [PubMed] [Google Scholar]
- 25. Hirano Y, Kaneko H, Konishi T, et al. Short-term outcomes of epidural analgesia in minimally invasive esophagectomy for esophageal cancer: nationwide inpatient data study in Japan. Ann Surg Oncol 2022;29:8225–8234. [DOI] [PubMed] [Google Scholar]
- 26. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 27. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 28. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 2022;40(suppl):238. [Google Scholar]
- 30. Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752–755. [DOI] [PubMed] [Google Scholar]
- 31. Hanley JA, Negassa A, Edwardes MD, et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157:364–375. [DOI] [PubMed] [Google Scholar]
- 32. Mathew G, Agha R. STROCSS Group. Strocss 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Ann Med Surg (Lond) 2021;72:103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen GL, Cederholm T, Correia M, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the Global Clinical Nutrition Community. JPEN J Parenter Enteral Nutr 2019;43:32–40. [DOI] [PubMed] [Google Scholar]
- 34. Ge L, Wang HJ, Yin D, et al. Effectiveness of 5-flurouracil-based neoadjuvant chemotherapy in locally-advanced gastric/gastroesophageal cancer: a meta-analysis. World J Gastroenterol 2012;18:7384–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirao M, Ando N, Tsujinaka T, et al. Influence of preoperative chemotherapy for advanced thoracic oesophageal squamous cell carcinoma on perioperative complications. Br J Surg 2011;98:1735–1741. [DOI] [PubMed] [Google Scholar]
- 36. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907. Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 37. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferraris VA, Bolanos M, Martin JT, et al. Identification of patients with postoperative complications who are at risk for failure to rescue. JAMA Surg 2014;149:1103–1108. [DOI] [PubMed] [Google Scholar]
- 39. Yasunaga H, Hashimoto H, Horiguchi H, et al. Variation in cancer surgical outcomes associated with physician and nurse staffing: a retrospective observational study using the Japanese diagnosis procedure combination database. BMC Health Serv Res 2012;12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 41. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr 2015;34:335–340. [DOI] [PubMed] [Google Scholar]
- 42. den Boer RB, Jones KI, Ash S, et al. Impact on postoperative complications of changes in skeletal muscle mass during neoadjuvant chemotherapy for gastro-oesophageal cancer. BJS Open 2020;4:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huddy JR, Huddy FMS, Markar SR, et al. Nutritional optimization during neoadjuvant therapy prior to surgical resection of esophageal cancer—a narrative review. Dis Esophagus 2018;31:1–11. [DOI] [PubMed] [Google Scholar]
- 44. Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol 2005;6:93–102. [DOI] [PubMed] [Google Scholar]
- 45. Ljungqvist O, de Boer HD, Balfour A, et al. Opportunities and challenges for the next phase of enhanced recovery after surgery: a review. JAMA Surg 2021;156:775–784. [DOI] [PubMed] [Google Scholar]
- 46. Matsuda S, Kitagawa Y, Takemura R, et al. Real-world evaluation of the efficacy of neoadjuvant DCF over CF in esophageal squamous cell carcinoma: propensity score matched analysis from 85 authorized institutes for esophageal cancer in Japan. Ann Surg 2022. 10.1097/SLA.0000000000005533 [DOI] [PubMed] [Google Scholar]
- 47. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–1957. [DOI] [PubMed] [Google Scholar]
- 48. Fehrenbach U, Wuensch T, Gabriel P, et al. CT body composition of sarcopenia and sarcopenic obesity: predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers (Basel) 2021;13:2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao Q, Hu K, Gao J, et al. Prevalence and prognostic value of sarcopenic obesity in patients with cancer: a systematic review and meta-analysis. Nutrition 2022;101:111704. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe M, Toh Y, Ishihara R, et al. Comprehensive registry of esophageal cancer in Japan, 2014. Esophagus 2022;19:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi YJ, Lee DH, Han KD, et al. Joint effects of low body mass index and alcohol consumption on developing esophageal squamous cell cancer: a Korean nationwide population-based cohort study. Asian Pac J Cancer Prev 2017;18:1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130:883–890. [DOI] [PubMed] [Google Scholar]
- 53. Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because the data in this study was extracted from a nationwide database, data use requires approval of the Ministry of Health, Labor, and Welfare, Japan.


