Background:
It remains uncertain how surgeons can safely pass the learning curve of laparoscopic pancreatoduodenectomy (LPD) without potentially harming patients. We aimed to develop a difficulty scoring system (DSS) to select an appropriate patient for surgeons.
Materials and Methods:
A total of 773 elective pancreatoduodenectomy surgeries between July 2014 and December 2019, including 346 LPD and 427 open pancreatoduodenectomy cases, were included. A 10-level DSS for LPD was developed, and an additional 77 consecutive LPD surgeries which could provide information of the learning stage I of LPD externally validated its performance between December 2019 and December 2021.
Results:
The incidences of postoperative complications (Clavien–Dindo≥III) gradually decreased from the learning curve stage I–III (20.00, 10.94, 5.79%, P=0.008, respectively). The DSS consisted of the following independent risk factors: (1) tumor location, (2) vascular resection and reconstruction, (3) learning curve stage, (4) prognostic nutritional index, (5) tumor size, and (6) benign or malignant tumor. The weighted Cohen’s κ statistic of concordance between the reviewer’s and calculated difficulty score index was 0.873. The C-statistics of DSS for postoperative complication (Clavien–Dindo≥III) were 0.818 in the learning curve stage I. The patients with DSS<5 had lower postoperative complications (Clavien–Dindo≥III) than those with DSS≥5 (4.35–41.18%, P=0.004) in the training cohort and had a lower postoperative pancreatic fistula (19.23–57.14%, P=0.0352), delayed gastric emptying (19.23–71.43%, P=0.001), and bile leakage rate (0.00–21.43%, P=0.0368) in validation cohort in the learning curve stage I.
Conclusion:
We developed and validated a difficulty score model for patient selection, which could facilitate the stepwise adoption of LPD for surgeons at different stages of the learning curve.
Keywords: complications, difficulty scoring system, laparoscopic pancreatoduodenectomy, learning curve
Highlights
The comprehensive data comparison of laparoscopic pancreatoduodenectomy (LPD) in different stages of learning curve versus open pancreatoduodenectomy (OPD).
A difficulty scoring system (DSS) for LPD were established and validated.
The patients with DSS<5 had lower postoperative complications than those with DSS≥5 in the learning curve stage I.
It could facilitate the stepwise adoption of LPD for surgeons at initial stage of the learning curve.
Introduction
Pancreatoduodenectomy (PD), or the Whipple procedure, is a complex surgical procedure that has been accepted as the gold-standard treatment for resectable lesions of the pancreatic head and periampullary region1. Since Gagner introduced a laparoscopic approach to PD in 19942, LPD has been a generally accepted surgical treatment by high-volume pancreatic surgery centers. In addition, according to the Miami international evidence-based guidelines on minimally invasive pancreatic resections3, minimally invasive pancreatectomy is preferred over OPD due to better surgical outcomes (i.e. shorter hospital stay, decreased blood loss, reduced pain, and comparable complication rates, oncological safety, and overall outcomes)4–6.
Previous studies have demonstrated the safety and feasibility of LPD7–9. However, the procedure was considered a feasible, safe, and oncological equivalent approach only in high-volume centers and experienced hands3,10. The experience, surgical skills, and completion of the learning stages are still major obstacles to the extensive application of LPD11. Our previous multicentre research revealed three learning phases of LPD, which were phase I (1–40 cases), phase II (41–104 cases), and phase III (>104 cases), and more than 104 procedures were needed to reach the learning curve plateau12. Despite the long training time for surgeons, there is no formal, universal, or standardized training program or certified curriculum for LPD. Previous studies that investigated LPD’s learning curve used different metrics as their endpoint to measure proficiency13–16. For example, the primary endpoints used to measure operative expertise included the operative time, conversion rate, estimated blood loss, morbidity, and length of hospital stay. These outcomes also raise the question of how surgeons can safely pass the learning curve without potentially harming patients.
Furthermore, a complex surgical procedure can be influenced by different factors, such as patient characteristics, laparoscopic technical issues, lesion characteristics and location, and surgeon’s skills and experience, which are significantly associated with perioperative and long-term outcomes of LPD. A difficulty scoring system (DSS) was used to graphically depict a statistical prognostic model to select an appropriate patient for a surgeon with different laparoscopic experience17–19. In this study, we aimed to develop and validate a DSS for patient selection by analyzing the clinical characteristics of patients and perioperative outcomes of LPD procedures, which could facilitate the safe and stepwise adoption of LPD for surgeons at a different stage of the learning curve. To the best of our knowledge, this is the first attempt to determine the patient selection, which can help surgeons navigate the steep learning curve of LPD.
Methods
Patients’ data collection
From July 2014 to December 2019, 773 consecutive patients underwent PD for various diagnoses performed by a single surgeon at a Biliary-Pancreatic Surgery Department. Of which 346 and 427 were LPD and OPD cases, respectively. The 427 OPD cases were all in the mature stage. Whereas the 346 LPD cases covered the learning curve, with 1–40 cases being stage I, 41–104 cases being stage II, 105–346 cases being stage III. From December 2018 to December 2019, 77 consecutive LPD cases performed by another surgeon who had not reached the mature stage of the learning curve at the same department were collected for external validation. In this study, we focused on the learning stage I of LPD. Therefore, a surgeon who can provide his data of stage I could provide the validation performance of DSS. The patients’ demographic and perioperative data were reviewed retrospectively. This study was approved by the Institutional Research Ethics committee of Tongji Hospital. The experiments were registered at ClinicalTrials (NCT05520606) and undertaken with the understanding and appropriate informed consent of each. Besides, the work has been reported in line with the STROCSS (Supplemental Digital Content 1, http://links.lww.com/JS9/A39) criteria20.
Definitions of variables
Postoperative complications and morbidity were defined and categorized according to the Clavien–Dindo (CD) classification; a CD≥III was defined as severe. Postoperative comorbidities, such as postpancreatectomy hemorrhage (PPH), postoperative pancreatic fistula (POPF), and delayed gastric emptying (DGE), were defined according to definitions outlined by the International Study Group of Pancreatic Surgery (ISGPF). The prognostic nutritional index (PNI) was calculated as 10×serum albumin (g/dl)+0.005×total lymphocytes count (per mm3)21. The neutrophil-to-lymphocyte ratio was calculated by dividing neutrophil count by lymphocyte count. The platelet-to-lymphocyte ratio was estimated as platelet count divided by lymphocyte count. The validation cohort data was collected in the same way as the training data.
Difficulty index definitions
A 10-level difficulty index was developed using a scale of 1–10, with 1 being the least difficult and 10 being the most difficult. The index was subsequently divided into the following three subgroups: 1–3 (low difficulty), 4–6 (intermediate difficulty), and 7–10 (high difficulty). The difficulty of each LPD surgery was determined using individual slides of the patient’s profile and operation videos and scored by three experienced surgeons, who skilled in pancreatic surgery and has completed more than 500 OPD procedures, using the 10-level difficulty index. Open discussion between the surgeon and reviewers was allowed before assigning the score. The idiographic surgical information, such as surgical approach, surrounding condition of the tumor, pancreatic characteristics (i.e. soft or hard), anastomotic methods, and other details, were used to confirm the difficulty level. Each expert reviewer made a separate score evaluation, and the median of their scores were computed as the difficulty score (DS).
Statistical analysis
Data from all cases were combined to perform univariable and subsequent multiple linear regression analyses to assess the clinical parameters of DS and develop a DSS that reflects clinical practice. Continuous variables were presented as a median and interquartile range, whereas categorical variables were presented as percentages. Continuous variables were compared using the Student’s t-test (two independent groups) or analysis of variance analysis (three independent groups), and categorical data were compared using the χ2 test or Fisher’s exact test. The interrater concordance between the reviewer’s DS and the calculated DS index was computed using the weighted Cohen’s κ, which evaluates the degree of disagreement by accounting for the differences in the importance of disagreement.
Multivariable linear regression analysis was used to determine the independent factors associated with technical difficulty. Factors with P-value less than 0.1 in the univariate regression analysis were included in the multivariate analysis. Subsequently, the final linear regression model was constructed using factors with P-value less than 0.05 in the multivariate regression analysis. Then, a scoring system was developed by assigning a weight to each risk factor based on the β coefficients from the final linear regression model, referred to as the Framingham study risk-score functions22. For the internal validation of the risk-scoring system, bootstrap resampling was performed by fitting the risk-scoring system in a bootstrap sample of 1000 subjects, which was drawn with a replacement from the original sample. For missing data analysis, a variable was excluded if it had missing data more than 20%. For the remaining data, the missingness was less than 10% and the complete analysis was used without any imputation. All calculations were performed with a R statistical software, version 4.1.2 and SAS 9.4, (http://www.r-project.org/) and a P-value less than 0.05 was considered statistically significant in a two-tailed test.
Results
Characteristics of patients in the laparoscopic and open pancreatoduodenectomy groups
The baseline and perioperative characteristics of 346 LPD and 427 OPD cases are described in Table 1 and Supplementary Table 1 (Supplemental Digital Content 2, http://links.lww.com/JS9/A40). There were no differences between the two groups for age, sex, BMI, American Society of Anaesthesiologists physical status score, American Joint Committee on Cancer (AJCC) stage, and medical or surgical history (P>0.05). The length of stay, tumor diameter, level of cancer antigen 19-9, and rate of vascular resection and reconstruction in the LPD group were significantly lower than those of the OPD group. The most common tumor types of the LPD and OPD groups were tumor in the duodenal papilla and pancreatic head, respectively. In addition, there were no differences between the groups for the number of harvested and positive lymph nodes, pancreatojejunostomy methods, rate of positive resected margin and R0 resection, postoperative complication (CD≥III), and mortality within 30 and 90 days. The operative time, estimated intraoperative blood loss, and intraoperative infusion quantity of the LPD group were lower than in the OPD group.
Table 1.
Characteristics of patients in the laparoscopic and open pancreatoduodenectomy groups
| n (%) | |||
|---|---|---|---|
| Variables | LPD (N=346) | OPD (N=427) | P |
| Age [mean (SD)] (years) | 55.99 (10.87) | 55.25 (10.55) | 0.3366 |
| Female | 147 (42.49) | 163 (38.17) | 0.2238 |
| BMI>24 kg/m2 | 242 (69.94) | 245 (57.38) | |
| ASA | 0.4066 | ||
| I | 36 (10.40) | 41 (9.60) | |
| II | 248 (71.68) | 293 (68.62) | |
| III | 62 (17.92) | 93 (21.78) | |
| Diabetes | 20 (5.78) | 22 (5.15) | 0.7017 |
| Surgical history | 101 (29.19) | 144 (33.72) | 0.178 |
| Alb [median (IQR)] (g/l) | 38.30 (35.60–40.80) | 37.90 (34.90–41.00) | 0.3909 |
| Tbil [median (IQR)] (μmol/l) | 27.00 (10.80–133.80) | 44.90 (11.10–151.40) | 0.2358 |
| CA19-9 [median (IQR)] (U/ml) | 36.29 (12.37–180.10) | 55.32 (13.20–307.80) | 0.0132 |
| PTCD | 126 (36.42) | 156 (36.53) | 0.973 |
| Vascular resection | 11 (3.18) | 54 (12.65) | <0.0001 |
| Tumor location | <0.0001 | ||
| Distal biliary duct | 27 (7.80) | 42 (9.84) | |
| Ampullary | 36 (10.40) | 40 (9.37) | |
| Duodenal papillary | 136 (39.31) | 74 (17.33) | |
| Pancreatic head | 147 (42.49) | 166 (38.87) | |
| AJCC | 0.2913 | ||
| IA | 90 (36.14) | 91 (28.26) | |
| IB | 66 (26.51) | 99 (30.75) | |
| IIA | 19 (7.63) | 25 (7.76) | |
| IIB | 58 (23.29) | 88 (27.33) | |
| III | 15 (6.02) | 15 (4.66) | |
| IV | 1 (0.40) | 4 (1.24) | |
| Tumor size [median (IQR)] (cm) | 2.50 (1.90–3.30) | 2.90 (2.00–4.00) | 0.0001 |
| Operative time [median (IQR)] (min) | 300.00 (250.00–380.00) | 370.00 (290.00–430.00) | <0.0001 |
| EIBL [median (IQR)] (ml) | 100.00 (50.00–300.00) | 300.00 (200.00–600.00) | <0.0001 |
| Lymph nodes harvested [median (IQR)] | 17.00 (6.00–25.00) | 18.00 (7.00–28.00) | 0.1821 |
| R0 resection | 324 (93.64) | 388 (90.87) | 0.1547 |
| 30-day mortality | 13 (3.76) | 17 (3.98) | 0.8726 |
| 90-day mortality | 17 (4.91) | 28 (6.56) | 0.3317 |
| Clavien–Dindo≥III | 29 (8.38) | 40 (9.37) | 0.6325 |
| POPF of B/C grade | 30 (8.67) | 19 (4.45) | 0.0166 |
| PPH of B/C grade | 24 (6.94) | 26 (6.09) | 0.6339 |
| DGE of B/C grade | 79 (22.83) | 115 (26.93) | 0.1911 |
| Bile leakage | 18 (5.20) | 31 (7.26) | 0.243 |
| Abdominal infection | 33 (9.54) | 21 (4.92) | 0.0122 |
| Hepatic failure | 0 (0.00) | 5 (1.17) | 0.0434 |
| Renal failure | 2 (0.58) | 5 (1.17) | 0.3869 |
| Pulmonary infection | 11 (3.18) | 10 (2.34) | 0.4765 |
| Cardiac dysfunction | 4 (1.16) | 10 (2.34) | 0.4765 |
| LOS [median (IQR)] (days) | 18.00 (15.00–22.00) | 20.00 (16.00–26.00) | 0.0002 |
AJCC, American Joint Committee on Cancer; Alb, albumin; ASA, American Society of Anesthesiologists physical status classes; CA19-9, cancer antigen 19-9; DGE, delayed gastric emptying; EIBL, estimated intraoperative blood loss; IQR, interquartile range; LOS, length of stay; LPD, laparoscopic pancreatoduodenectomy; OPD, open pancreatoduodenectomy; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage; PTCD, percutaneous transhepatic cholangial drain; Tbil, total bilirubin
Patient’s characteristics of the three-level difficulty stages of the learning curve
To investigate the differences between the learning stages of LPD, 346 LPD cases were categorized according to the definition of the learning curve of LPD, and patients’ characteristics between the three stages were compared. The results are shown in Table 2 and Supplementary Table 2 (Supplemental Digital Content 3, http://links.lww.com/JS9/A41). There were no differences between the three stages for age, sex, BMI, American Society of Anaesthesiologists score, length of stay, level of cancer antigen 19-9, and AJCC stage (P>0.05). The tumor diameter, red cell volume distribution width, PNI, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, controlling nutritional status, creatinine, total bilirubin, and history of diabetes in LPD cases were statistically significant between the three stages. The incidences of postoperative complications (CD≥III) gradually decreased from stage I to III (20.00, 10.94, 5.79%, respectively, P=0.008), including POPF of B/C grade (20.00, 15.63, 4.96%, respectively, P=0.001), PPH of B/C grade (20.00, 7.81, 4.55%, respectively, P=0.002), biliary leakage (15.00, 7.81, 2.89%, respectively, P=0.004), and DGE of B/C grade (37.50, 26.56, 19.42%, respectively, P=0.030).
Table 2.
Characteristics of patients according to three learning stages of laparoscopic pancreatoduodenectomy
| n (%) | ||||
|---|---|---|---|---|
| Variables | Stage I (N=40) | Stage II (N=64) | Stage III (N=242) | P |
| Age [mean (SD)] (years) | 57.78 (9.63) | 54.89 (12.31) | 55.99 (10.66) | 0.4215 |
| Female | 16 (40.00) | 28 (43.75) | 103 (42.56) | 0.9307 |
| BMI [mean (SD)] (kg/m2) | 21.46 (2.43) | 21.11 (2.76) | 22.17 (2.82) | 0.0148 |
| Tumor size [median (IQR)] (cm) | 2.10 (1.50–3.15) | 2.15 (1.80–3.00) | 2.50 (2.00–3.50) | 0.0139 |
| RDW_CV [median (IQR)] (%) | 14.40 (13.35–15.55) | 14.70 (13.45–15.90) | 13.90 (13.10–15.00) | 0.0174 |
| PNI [median (IQR)] ((%)) | 44.13 (39.43–46.58) | 44.35 (40.28–47.63) | 46.95 (43.45–50.30) | 0.0002 |
| NLR [median (IQR)] | 41.92 (33.34–60.10) | 46.96 (30.70–57.62) | 38.24 (29.94–51.13) | 0.0424 |
| PLR [median (IQR)] | 167.38 (113.12–222.35) | 163.94 (126.25–237.47) | 136.11 (96.93–184.35) | 0.0011 |
| CONUT [median (IQR)] | 3.00 (1.00–4.00) | 2.00 (1.00–3.50) | 2.00 (1.00–3.00) | 0.0248 |
| Creatinine [median (IQR)] (μmol/l) | 60.00 (52.00–67.50) | 63.00 (53.00–77.00) | 65.50 (56.00–77.00) | 0.0342 |
| TG [median (IQR)] (mmol/l ) | 0.00 (0.00–0.95) | 0.00 (0.00–0.94) | 0.54 (0.00–1.55) | 0.0129 |
| Plt [median (IQR)] (109/l) | 224.50 (171.50–289.00) | 236.50 (194.50–320.00) | 211.00 (169.00–266.00) | 0.0189 |
| ALT [median (IQR)] (U/l) | 92.50 (28.00–260.00) | 81.00 (16.50–216.00) | 53.00 (18.00–154.00) | 0.1472 |
| AST [median (IQR)] (U/l) | 68.00 (25.00–151.00) | 54.00 (20.50–154.00) | 47.00 (19.00–106.00) | 0.1499 |
| Alb [median (IQR)] (g/l) | 36.65 (33.90–38.45) | 37.45 (34.40–40.10) | 38.75 (36.30–41.20) | 0.0005 |
| Tbil [median (IQR)] (μmol/l) | 63.30 (15.95–159.60) | 57.10 (12.35–173.45) | 22.10 (9.90–118.70) | 0.0328 |
| CA19-9 [median (IQR)] (U/ml) | 27.91 (8.16–198.74) | 35.26 (11.64–150.25) | 39.17 (13.05–184.90) | 0.6302 |
| PTCD | 15 (37.50) | 25 (39.06) | 86 (35.54) | 0.863 |
| Vascular resection | 0 (0.00) | 0 (0.00) | 11 (4.55) | 0.0871a |
| Tumor location | 0.2213 | |||
| Distal biliary duct | 4 (13.33) | 6 (9.38) | 32 (13.22) | |
| Ampullary | 5 (16.67) | 16 (25.00) | 34 (14.05) | |
| Duodenal papillary | 16 (53.33) | 24 (37.50) | 96 (39.67) | |
| Pancreatic head | 5 (16.67) | 18 (28.13) | 80 (33.06) | |
| ASA | 0.3467 | |||
| I | 3 (7.50) | 11 (17.19) | 22 (9.09) | |
| II | 31 (77.50) | 43 (67.19) | 174 (71.90) | |
| III | 6 (15.00) | 10 (15.63) | 46 (19.01) | |
| AJCC | 0.8568a | |||
| IA | 15 (42.86) | 15 (35.71) | 60 (34.88) | |
| IB | 12 (34.29) | 10 (23.81) | 44 (25.58) | |
| IIA | 3 (8.57) | 4 (9.52) | 12 (6.98) | |
| IIB | 4 (11.43) | 10 (23.81) | 44 (25.58) | |
| III | 1 (2.86) | 3 (7.14) | 11 (6.40) | |
| IV | 0 (0.00) | 0 (0.00) | 1 (0.58) | |
| Operative time [median (IQR)] (min) | 460.00 (400.00–540.00) | 338.50 (260.00–425.00) | 280.00 (250.00–330.00) | <0.0001 |
| EIBL [median (IQR)] (ml) | 100.00 (50.00–200.00) | 100.00 (80.00–200.00) | 125.00 (50.00–300.00) | 0.3261 |
| Lymph nodes harvested [median (IQR)] | 18.00 (7.0–21.00) | 18.00 (7.0–25.0) | 19.0 (6.0–28.0) | 0.6752 |
| R0 resection | 2 (5.00) | 6 (9.38) | 14 (5.79) | 0.5391a |
| 30-day mortality | 2 (5.00) | 1 (1.56) | 10 (4.13) | 0.5719a |
| 90-day mortality | 2 (5.00) | 1 (1.56) | 14 (5.79) | 0.3805a |
| Clavien–Dindo≥III | 8 (20.00) | 7 (10.94) | 14 (5.79) | 0.0078 |
| POPF of B/C grade | 8 (20.00) | 10 (15.63) | 12 (4.96) | <0.001 |
| PPH of B/C grade | 8 (20.00) | 5 (7.81) | 11 (4.55) | 0.0017 |
| DGE of B/C grade | 15 (37.50) | 17 (26.56) | 47 (19.42) | 0.0304 |
| Bile leakage | 6 (15.00) | 5 (7.81) | 7 (2.89) | 0.0035 |
| Abdominal infection | 10 (25.00) | 8 (12.50) | 15 (6.20) | 0.0006a |
| Hepatic failure | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Renal failure | 0 (0.00) | 0 (0.00) | 2 (0.83) | 0.649a |
| Pulmonary infection | 3 (7.50) | 2 (3.13) | 6 (2.48) | 0.2452a |
| Cardiac dysfunction | 0 (0.00) | 0 (0.00) | 4 (1.65) | 0.2452a |
| LOS [median (IQR)] (days) | 21.00 (14.00–31.00) | 18.00 (14.50–24.00) | 18.00 (15.00–21.00) | 0.1156 |
AJCC, American Joint Committee on Cancer; Alb, albumin; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists physical status classes; AST, aspartate aminotransferase; CA19-9, cancer antigen 19-9; CONUT, Controlling Nutritional status score; DGE, delayed gastric emptying; EIBL, estimated intraoperative blood loss; IQR, interquartile range; LOS, length of stay; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; Plt, platelet; PNI, prognostic nutritional index; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage; PTCD, percutaneous transhepatic cholangial drainage; RDW_CV, red blood cell distribution width CV; Tbil, total bilirubin; TG, triglyceride.
Fisher exact test.
Difficulty scoring system for inchoate learning curve phase
Based on the reviewer’s difficulty 10-level index evaluation, 346 LPD cases were assigned a different value corresponding to their difficulty level. Using the automatic linear modeling tool, the following six clinical factors that significantly affected the difficulty level of LPD were identified: (1) tumor location, (2) vascular resection and reconstruction, (3) learning curve stage, (4) PNI, (5) tumor size, and (6) benign or malignant tumor (Table 3). The concordance between the reviewer’s 10-level difficulty index and DS calculated by the linear modeling index is shown in Figure 1. From the linear modeling DS system, we developed a simpler and more practical scoring system, wherein index scores were assigned based on the difference between the computed values in a category and rounded off to the closest whole number. The short-term postoperative outcomes of the cases were postoperative complications (CD≥III). We used the median as the cutoff value (e.g. DSS=5 for stage I and DSS=6 for stage II). The areas under the receiver operating characteristic curve of DSS to CD were 0.818 and 0.675 in learning curve stages I and II, respectively (Supplementary Table 3, Supplemental Digital Content 4, http://links.lww.com/JS9/A42). To further evaluate the outcomes of the learning cohort, we divided the 40 LPD cases in stage I into two subgroups (i.e. 23 cases, DSS<5; 17 cases, DSS≥5). The perioperative data and postoperative outcomes of these cases are shown in Table 4. The patients with DSS<5 had lower postoperative complications (CD≥III) than those with DSS≥5 (4.35–41.18%, P=0.004).
Table 3.
Linear modeling of the 10-level difficulty index for laparoscopic pancreatoduodenectomy
| Factors | Coefficient (95% CI) | P | Importance | Score |
|---|---|---|---|---|
| Intercept | –0.033 (–0.088 to 0.155) | 0.5933 | ||
| Location | ||||
| Other | 0 | 0.1942 | 1 | |
| Pancreatic | 1.214 (1.150–1.278) | <0.0001 | 2 | |
| Cut artery | ||||
| No | 0 | 0.0113 | 0 | |
| Yes | 1.036 (0.868–1.205) | <0.0001 | 1 | |
| Stage | ||||
| 1 | 0 | 0.5804 | 1 | |
| 2 | 2.632 (2.523–2.742) | <0.0001 | 3 | |
| 3 | 3.548 (3.454–3.641) | <0.0001 | 4 | |
| PNI | ||||
| PNI≥49 | 0 | 0.0741 | 0 | |
| PNI<49 | 0.669 (0.603–0.734) | <0.0001 | 1 | |
| Tumor size(cm) | ||||
| 0–2 | 0 | 0.0247 | 0 | |
| >2 | 0.661 (0.599–0.723) | <0.0001 | 1 | |
| Malignant | ||||
| 0 | 0 | 0.1153 | 0 | |
| 1 | 1.062 (0.992–1.133) | <0.0001 | 1 | |
Weighted κ coefficient (95% CI): 0.873 (0.844–0.901), R 2=0.966.
Correlation index, r=0.9558 (P<0.0001).
CI, confidence interval; PNI, prognostic nutritional index.
Figure 1.
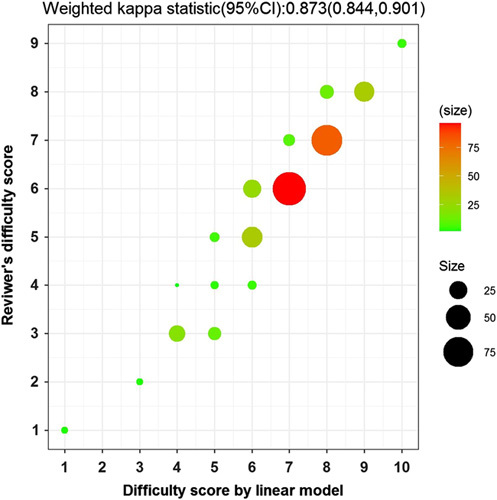
The κ value of the calculated and reviewer’s difficulty score. Weighted κ coefficient (95% CI): 0.873 (0.844–0.901), R 2=0.966, r=0.9558 (P<0.0001). CI, confidence interval.
Table 4.
Outcomes according to the difficulty scoring system in learning curve stage I in the training cohort
| n (%) | |||
|---|---|---|---|
| Variables | DSS<5 (N=23) | DSS≥5 (N=17) | P |
| Age [mean (SD)] (years) | 56.83 (11.07) | 59.06 (7.38) | 0.4756 |
| Female | 9 (39.13) | 7 (41.18) | 0.8961 |
| ASA | 0.2944 | ||
| 1 | 3 (13.04) | 0 (0.00) | |
| 2 | 17 (73.91) | 14 (82.35) | |
| 3 | 3 (13.04) | 3 (17.65) | |
| BMI [mean (SD)] (kg/m2) | 21.32 (2.07) | 21.65 (2.90) | 0.6697 |
| LOS [median (IQR)] (days) | 21.00 (14.00–25.00) | 25.00 (17.00–32.00) | 0.6252 |
| Tumor size [median (IQR)] (cm) | 1.80 (1.20–2.00) | 2.90 (2.50–3.50) | 0.0005 |
| Lym [median (IQR)] (109/l) | 1.55 (1.15–1.81) | 1.28 (0.86–1.50) | 0.0835 |
| RDW [median (IQR)] | 13.80 (13.00–14.40) | 15.40 (14.50–16.50) | 0.0277 |
| PNI [median (IQR)] | 45.40 (43.65–49.35) | 39.70 (37.40–43.50) | 0.0012 |
| NLR [median (IQR)] | 36.39 (31.36–47.93) | 50.81 (40.54–71.15) | 0.2077 |
| PLR [median (IQR)] | 151.32 (112.90–211.88) | 176.19 (113.33–239.53) | 0.4282 |
| CONUT [median (IQR)] | 2.00 (1.00–3.00) | 3.00 (1.00–4.00) | 0.1227 |
| Creatinine [median (IQR)] (mmol/l) | 65.00 (58.00–72.00) | 52.00 (47.00–58.00) | 0.0071 |
| BUN [median (IQR)] (mmol/l) | 4.21 (2.99–5.41) | 4.73 (3.96–5.51) | 0.6838 |
| CRP [median (IQR)] (mg/l) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.9315 |
| TC [median (IQR)] (mmol/l) | 4.47 (3.70–5.62) | 5.69 (4.47–6.46) | 0.0453 |
| WBC [median (IQR)] (109/l) | 5.35 (4.71–6.36) | 5.64 (4.65–6.83) | 0.3251 |
| Neu [median (IQR)] (%) | 57.30 (52.90–63.90) | 62.80 (58.20–66.90) | 0.1283 |
| RBC [median (IQR)] (1012/l) | 4.04 (3.82–4.43) | 3.89 (3.57–4.21) | 0.1212 |
| Hb [median (IQR)] (g/l) | 124.00 (117.00–136.00) | 111.00 (108.00–118.00) | 0.0115 |
| ALT [median (IQR)] (U/l) | 67.00 (16.00–277.00) | 136.00 (68.00–253.00) | 0.5951 |
| AST [median (IQR)] (U/l) | 51.00 (17.00–138.00) | 97.00 (51.00–174.00) | 0.2418 |
| γ-GT [median (IQR)] (U/l) | 239.00 (22.00–546.00) | 633.00 (365.00–1082.00) | 0.0099 |
| Alb [median (IQR)] (g/l) | 37.50 (35.90–41.40) | 34.50 (30.90–36.70) | 0.0024 |
| Tbil [median (IQR)] (μmol/l) | 19.80 (11.50–78.60) | 133.80 (69.40–202.60) | 0.0191 |
| Dbil [median (IQR)] (μmol/l) | 10.90 (4.10–63.80) | 109.40 (51.90–153.30) | 0.0168 |
| CA19-9 [median (IQR)] (U/ml) | 14.88 (7.24–166.82) | 80.19 (20.16–210.31) | 0.9972 |
| CEA [median (IQR)] (ng/l) | 2.51 (1.29–3.45) | 3.03 (1.87–4.22) | 0.2859 |
| CA125 [median (IQR)] (U/ml) | 17.50 (9.60–22.37) | 14.70 (9.10–19.00) | 0.8509 |
| AFP [median (IQR)] (ng/ml) | 3.17 (1.76–4.97) | 2.46 (2.15–3.16) | 0.080 |
| Operative time [median (IQR)] (min) | 480.00 (390.00–600.00) | 431.00 (400.00–530.00) | 0.3571 |
| EIBL [median (IQR)] (ml) | 100.00 (50.00–400.00) | 100.00 (30.00–200.00) | 0.1927 |
| IFIV [median (IQR)] (ml) | 3500.00 (3000.00–4500.00) | 3500.00 (2500.00–4500.00) | 0.283 |
| Intraoperative urine [median (IQR)] (ml) | 1400.00 (1000.00–2000.00) | 1200.00 (1000.00–1500.00) | 0.7652 |
| Lymph nodes harvested [median (IQR)] | 16.00 (10.00–21.00) | 17.00 (11.00–20.00) | 0.3176 |
| Positive lymph nodes [median (IQR)] | 0.00 (0.00–1.00) | 0.00 (0.00–1.00) | 0.8812 |
| PTCD | 5 (21.74) | 10 (58.82) | 0.0166 |
| Vascular resection | 23 (100.00) | 17 (100.00) | |
| Tumor location | 0.4074a | ||
| Distal biliary duct | 4 (17.39) | 1 (5.88) | |
| Ampullary | 3 (13.04) | 2 (11.76) | |
| Duodenal papillary | 12 (52.17) | 8 (47.06) | |
| Pancreatic head | 4 (17.39) | 6 (35.29) | |
| Tumor characteristic | 0.0877a | ||
| Benign | 5 (21.74) | 1 (5.88) | |
| Malignant | 21 (78.26) | 16 (94.11) | |
| AJCC | <0.0001 | ||
| IA | 15 (78.95) | 0 (0.00) | |
| IB | 0 (0.00) | 12 (75.00) | |
| IIA | 1 (5.26) | 2 (12.50) | |
| IIB | 2 (10.53) | 2 (12.50) | |
| III | 1 (5.26) | 0 (0.00) | |
| R0 resection | 0.826a | ||
| R0 | 22 (95.65) | 16 (94.12) | |
| R1 | 1 (4.35) | 1 (5.88) | |
| 30-day mortality | 0 (0.00) | 2 (11.76) | 0.092a |
| 90-day mortality | 0 (0.00) | 2 (11.76) | 0.092a |
| Clavien–Dindo≥III | 1 (4.35) | 7 (41.18) | 0.004a |
| POPF of B/C grade | 5 (21.74) | 3 (17.65) | 0.749a |
| PPH of B/C grade | 4 (17.39) | 4 (23.53) | 0.631a |
| DGE of B/C grade | 10 (43.48) | 5 (29.41) | 0.364a |
| Bile leakage | 2 (8.70) | 4 (23.53) | 0.194a |
| Abdominal infection | 2 (8.70) | 8 (47.06) | 0.006a |
| Hepatic failure | 0 (0.00) | 0 (0.00) | – |
| Renal failure | 0 (0.00) | 0 (0.00) | – |
| Pulmonary infection | 1 (4.35) | 2 (11.76) | 0.379a |
| Cardiac dysfunction | 0 (0.00) | 0 (0.00) | – |
AFP, alpha-fetoprotein; Alb, albumin; AJCC, American Joint Committee on Cancer; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists physical status classes; AST, aspartate aminotransferase; BUN; blood urea nitrogen; CA19-9, cancer antigen 19-9; CA125, carbohydrate antigen 125; CEA, carcinoembryonic antigen; CONUT, Controlling Nutritional status score; CRP, C-reactive protein; Dbil, direct bilirubin; DGE, delayed gastric emptying; DSS, difficulty scoring system; EIBL, estimated intraoperative blood loss; γ-GT, gamma-glutamyl transpeptidase; Hb, hemoglobin; IFIV, intraoperative fluid infusion volume; IQR, interquartile range; LOS, length of stay; Lym, lymphocytes; Neu, neutrophils; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage; PTCD, percutaneous transhepatic cholangial drainage; RBC, red blood cell; RDW, red blood cell distribution width; Tbil, total bilirubin; TC, serum total cholesterol; WBC; white blood cell.
Fisher exact test.
Validation of difficulty scoring system
To verify DSS’s accuracy and clinical application, we reviewed 77 LPD cases of another surgeon who had not reached the mature learning curve stage at the same center. Supplementary Table 4 (Supplemental Digital Content 5, http://links.lww.com/JS9/A43) summarizes the patients’ baseline demographic, clinicopathologic, and perioperative and postoperative data across the two learning curve stages. Supplementary Table 5 (Supplemental Digital Content 6, http://links.lww.com/JS9/A44) summarizes the characteristics of LPD surgeries according to difficulty groups. Twenty-eight cases were classified as low, 41 as intermediate, and eight as high difficulty. There were no significant differences in sex, age, BMI, and preoperative laboratory testing results between the three groups. Operative time [390.00 (300.00–435.00), 400.00 (358.00–435.00), 489.50 (413.00–522.00) min, P=0.0197], estimated blood loss [175.00 (50.00–300.00), 200.00 (150.00–300.00), 350.00 (300.00–900.00) ml, P=0.0046] were progressively increased from low to high difficulty groups. The PPH of B/C grade rate (3.57, 9.76, 37.50%, respectively, P=0.0474) and DGE of B/C grade rate (0.00, 17.50, 37.50%, respectively, P=0.0166) were progressively increased from low to high difficulty groups. However, it was not statistically significant. The postoperative complications (CD≥III) rate was increased from low to high difficulty groups (3.57, 12.20, 25.00%, respectively, P=0.1316).To further clarify the clinical application value, the 40 LPD cases in the first stage of the learning curve were divided into two groups according to the DSS score (26 cases for DSS<5 and 14 cases for DSS≥5). The POPF rate (19.23–57.14%, P=0.0352), DGE rate (19.23–71.43%, P=0.001), and bile leakage rate (0.00–21.43%, P=0.0368) were increased as the DSS increases. The postoperative complications (CD≥III) rate in DSS≥5 group was higher than those in DSS<5 group (3.85–14.29%, P=0.2763). There were no significant differences in other perioperative outcomes, such as postoperative morbidity, readmission rate, and hospitalization (Table 5).
Table 5.
Outcomes according to the difficulty scoring system in learning curve stage I in the validation cohort
| n (%) | |||
|---|---|---|---|
| Variables | DSS<5 (N=26) | DSS≥5 (N=14) | P |
| Age [mean (SD)] (years) | 54.92 (12.17) | 59.71 (6.14) | 0.1769 |
| Female | 11 (42.31) | 8 (57.14) | 0.3702 |
| ASA | 0.1306 | ||
| 1 | 1 (3.85) | 1 (7.14) | |
| 2 | 21 (80.77) | 7 (50.00) | |
| 3 | 4 (15.38) | 6 (42.86) | |
| BMI [mean (SD)] (kg/m2) | 23.25 (2.85) | 21.33 (2.33) | 0.0369 |
| LOS [median (IQR)] (days) | 19.50 (15.00–25.00) | 18.50 (17.00–26.00) | 0.9202 |
| Tumor size [median (IQR)] (cm) | 1.65 (1.30–2.00) | 2.50 (2.50–3.00) | 0.0006 |
| Lym [median (IQR)] (109/l) | 1.55 (1.16–1.91) | 1.21 (1.07–1.40) | 0.0453 |
| PNI [median (IQR)] | 46.20 (40.90–49.90) | 44.65 (38.40–48.15) | 0.1573 |
| WBC [median (IQR)] (109/l) | 6.11 (4.94–6.90) | 5.70 (5.09–6.28) | 0.7543 |
| Neu [median (IQR)] (%) | 60.20 (55.80–68.20) | 66.45 (63.30–69.90) | 0.0662 |
| RBC [median (IQR)] (1012/l) | 4.14 (4.00–4.28) | 3.80 (3.23–4.10) | 0.0016 |
| Hb [median (IQR)] (g/l) | 127.00 (115.00–134.00) | 116.50 (94.00–122.00) | 0.0114 |
| Alb [median (IQR)] (g/l) | 39.45 (34.50–40.80) | 38.50 (31.30–42.80) | 0.6054 |
| Tbil [median (IQR)] (μmol/l) | 26.75 (8.50–201.80) | 55.10 (18.00–125.90) | 0.9236 |
| Operative time [median (IQR)] (min) | 395.00 (300.00–450.00) | 400.00 (360.00–430.00) | 0.2424 |
| EIBL [median (IQR)] (ml) | 200.00 (50.00–300.00) | 200.00 (150.00–300.00) | 0.3161 |
| IFIV [median (IQR)] (ml) | 2500.00 (2000.00–3000.00) | 2250.00 (2000.00–3000.00) | 0.8304 |
| Intraoperative urine [median (IQR)] (ml) | 1000.00 (800.00–1200.00) | 900.00 (700.00–1200.00) | 0.843 |
| Lymph nodes harvested [median (IQR)] | 12 (9–14) | 13 (9–15) | 0.3461 |
| Vascular resection | 0 (0.00) | 1 (7.14) | 0.1675a |
| Tumor location | |||
| Distal biliary duct | 3 (11.54) | 0 (0.00) | 0.0042 |
| Ampullary | 3 (11.54) | 2 (14.29) | |
| Duodenal papillary | 14 (53.85) | 1 (7.14) | |
| Pancreatic head | 6 (23.08) | 11 (78.57) | |
| Tumor characteristic | 0.0344 | ||
| Benign | 10 (38.46) | 1 (7.14) | |
| Malignant | 16 (61.54) | 13 (92.86) | |
| AJCC | 0.0022 | ||
| IA | 17 (65.38) | 0 (0.00) | |
| IB | 4 (15.38) | 5 (35.71) | |
| IIA | 0 (0.00) | 1 (7.14) | |
| IIB | 4 (15.38) | 6 (42.86) | |
| III | 1 (3.85) | 2 (14.29) | |
| Clavien–Dindo≥III | 1 (3.85) | 2 (14.29) | 0.2763a |
| Pancreatic fistula | 5 (19.23) | 8 (57.14) | 0.0352a |
| POPF of B/C grade | 2 (7.69) | 3 (21.43) | 0.3223a |
| PPH | 4 (15.38) | 6 (42.86) | 0.0520a |
| PPH of B/C grade | 0 (0.00) | 2 (14.29) | 0.1167a |
| DGE | 5 (19.23) | 10 (71.43) | 0.0010 |
| DGE of B/C grade | 1 (3.85) | 2 (14.29) | 0.2763a |
| Bile leakage | 0 (0.00) | 3 (21.43) | 0.0368a |
| Abdominal infection | 0 (0.00) | 2 (14.29) | 0.1167a |
| Hepatic failure | 0 (0.00) | 0 (0.00) | – |
| Renal failure | 0 (0.00) | 0 (0.00)) | – |
| Pulmonary infection | 0 (0.00) | 0 (0.00) | – |
| Cardiac dysfunction | 0 (0.00) | 0 (0.00) | – |
AJCC, American Joint Committee on Cancer; Alb, albumin; ASA, American Society of Anesthesiologists physical status classes; DGE, delayed gastric emptying; DSS, difficulty scoring system; EIBL, estimated intraoperative blood loss; Hb, hemoglobin; IFIV, intraoperative fluid infusion volume; IQR, interquartile range; LOS, length of stay; Lym, lymphocytes; Neu, neutrophils; PNI, prognostic nutritional index; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage; RBC, red blood cell; Tbil, total bilirubin; WBC, white blood cell.
Fisher exact test.
Discussion
LPD has been accepted as a conventional surgical procedure for pancreatic head and ampullary tumors in large pancreatic surgery centers23,24. Owing to the complicated surgical procedure and high technical requirements of surgeons, LPD is considered the “Everest” of abdominal minimally invasive surgery. The initial phase of the learning curve is a prerequisite for inexperienced surgeons25,26, and it has been shown that more than 30–50 cases are needed at this phase27–29. Many institutions select patients with periampullary disease without vascular invasion for surgeons in the initial learning stage of LPD30. However, the precise patient selection criteria for the initial learning curve stage of LPD has not been demonstrated, which is critical in reducing postoperative complications and facilitating the steep learning curve of LPD14.
This study aimed to develop a DSS for LPD that can be used to select surgery cases based on the surgeon’s experience and expertise, ensuring safe stepwise adoption of LPD. The difficulty of LPD depends not only on the technical complexity of resection and reestablishment but also on various factors, such as a patient’s general condition, tumor size and location, and the degree of blood vessel invasion31. Moreover, ‘difficulty’ is a relative, subjective term that varies among individuals with different surgical skills. Several studies have attempted to grade the difficulty of a surgery and relevant risk factors, and the validation or clinical application of those models or risk factors has demonstrated that patient selection based on objective variables could improve the short-term outcome after surgery32,33. Unfortunately, only a few studies have examined patient selection during the initial stage of the learning curve of LPD.
In the present study, the difficulty levels of 346 LPD cases were assessed by experts who had mastered all LPD surgical procedures. Furthermore, we have identified a few known surgical difficulty indexes. First, tumor diameter larger than 2 cm. Tumor size is an independent risk factor for the prognosis of pancreatic ductal adenocarcinoma (PDAC) after surgery. The AJCC eighth edition staging system for PDAC defined a maximum tumor diameter of 2 cm as the boundary point of T1 and T2 stages. A smaller tumor size indicates that the tumor is at an early stage of differentiation. Moreover, a previous study showed that tumor size more than 3 cm is independently associated with long-term survival34. Second, surgical procedures involving vascular resection and reconstruction. Patients with superior mesenteric vein (SMV)/portal vein (PV) or hepatic artery invasion should be categorized as a more difficult case. The AJCC eighth edition staging system for PDAC has defined a tumor involving the celiac axis or superior mesenteric artery as a boundary point of T4 stage, regardless of tumor size. Furthermore, the ISGPS suggests that partial resection of the PV or SMV should be performed in case of their suspected involvement in tumor progression to achieve radical resection. However, postoperative complications after a venous resection appear to be contradictory. A recent observational study showed that venous resection is associated with increased mortality and poor survival35; however, another study examining 229 venous resections showed no differences in morbidity, mortality, and survival among the types of venous resection36. Third, pancreatic and malignant tumors. Tumors located on the pancreatic head or uncinate process are considered a more difficult surgery than those with tumors forming in other sites because the pancreatic head tumor can invade the SMV/PV more easily, especially if the tumor is malignant. The PNI, initially proposed by Buzby et al. 37 and refined by Onodera et al. 21, is calculated using the serum albumin concentration and total lymphocytes count in peripheral blood and is used to evaluate preoperative nutritional conditions and surgical complications in patients with gastrointestinal cancers. The significance of PNI as a prognostic predictor has been demonstrated in various types of human cancers38–40; low PNI may indicate hypoalbuminemia and/or lymphocytopenia. Hypoalbuminemia in patients after PD contributes to impaired liver function due to biliary obstruction. It is also associated with a sustained systemic inflammatory response from the tumor itself or host reaction. Contrarily, lymphocytes play important roles in the host immune response, postoperative tissue repair, and digestive system functional recovery. In most cases, the clinical and empirical patient selection based on PNI and other inflammatory indices do not reduce the complication rate. In our study, a linear model was generated by analyzing all clinical information and difficulty indexes, and a subsequent simpler 10-level scoring system was developed from the linear model. The weighted κ statistic was 0.873 for the 10-level difficulty index, showing an excellent agreement between the assessment by the reviewer and 10-level scoring system.
Furthermore, the clinical practice, usefulness, and applicability of the developed DSS were evaluated internally and externally. Compared with subjective and partial experiential selection criteria, the DSS developed in our study could provide a more accurate and objective-based selection for a surgeon who has been at the initial stage of the learning curve of LPD. The tumor’s location, size, and characteristics could be assessed accurately before surgery. Moreover, factors such as PNI and the learning curve stage were considered objective clinical data. In stage I of the learning curve of LPD (0–40 cases), patients with DSS<5 had lower postoperative complications (CD≥III) than those with DSS≥5 after PD, including POPF, DGE, and bile leakage rate. Although due to sample size limitations of the validation cohort, there were no significant differences in the postoperative complications rate (CD≥III). Based on these results, we still recommend selecting cases with DSS<5 in the learning curve stage I for surgeons to reduce postoperative complications and improve the outcomes. In addition, the six identified surgical difficulty indexes could aid surgeons in selecting the appropriate surgical method to improve patient fitness for surgery and the corresponding prognosis. Furthermore, it can also be used as a guide to build a useful curriculum for surgeons acquiring the technical skills of LPD in a stepwise manner.
This study has several limitations. First, although the learning and validation cohort data were from different surgeons, it was still a single-center study. The clinical application, rationality, and practicality of this DSS need to be verified in future studies. Besides, we developed the DSS only suitable for learning stage I of LPD. Therefore, in our future plans, the multicentric validation and more experience regarding learning stage I, learning stage II, and learning stage III will be conducted. Second, individual surgeons might have had some technical differences, including the surgical approach and strategy. Therefore, all the above were also risk factors for postoperative complications, and the influence of these subjective differences on our results needs to be verified further.
In conclusion, we developed a validated risk-scoring model for predicting the technical difficulty of LPD with satisfactory clinical usefulness. These results were based on objective variables, which could reduce postoperative complications and effectively replace the conventional subjective patient selection. We believe that our constructed DSS model can select more appropriate patients for surgeons at their initial learning stage of LPD, contributing to safer and more reliable short-term outcomes for surgeons.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
The study was approved by the institutional review board at each participating hospital, and the need for informed consent was waived due to the retrospective nature of the study.
Sources of funding
This study was supported by grants from The National Natural Science Foundation of China (81402443 to F.P.; 81772950 to R.Q.; 81773160 to M.W.), the HUBEI Natural Science Foundation (2017CFB467 to M.W.), the Tongji Hospital Science Fund for Distinguished Young Scholars (2016YQ08 to M.W.), and the Wuhan applied basic research project (2016060101010070 to R.Q.).
Authors’ contribution
F.P.: conceptualization, methodology, data curation, investigation. R.H.: conceptualization, methodology, writing – original draft. H.W. and M.W.: conceptualization, methodology, software, formal analysis. H.Z.: conceptualization, methodology, software, formal analysis, visualization. T.Q.: writing – review and editing, project administration, resources, supervision. R.Q.: conceptualization, writing – review and editing, project administration, funding acquisition, supervision.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Guarantor
Renyi Qin.
Supplementary Material
Acknowledgements
The authors thank Editage (www.editage.com) for English language editing.
Footnotes
Feng Peng and Ruizhi He contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 3 April 2023
Contributor Information
Feng Peng, Email: fpeng@hust.edu.cn.
Ruizhi He, Email: heruizhi1020@qq.com.
Hebin Wang, Email: whb8310@163.com.
Hang Zhang, Email: 172356995@qq.com.
Min Wang, Email: 68227369@qq.com.
Tingting Qin, Email: qintingting77@163.com.
Renyi Qin, Email: ryqin@tjh.tjmu.edu.cn.
References
- 1. Tung S, Davis LE, Hallet J, et al. Population-level symptom assessment following pancreaticoduodenectomy for adenocarcinoma. JAMA Surg 2019;154:e193348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408–410. [DOI] [PubMed] [Google Scholar]
- 3. Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 2020;271:1–14. [DOI] [PubMed] [Google Scholar]
- 4. Tan HL, Syn N, Goh BKP. Systematic review and meta-analysis of minimally invasive pancreatectomies for solid pseudopapillary neoplasms of the pancreas. Pancreas 2019;48:1334–1342. [DOI] [PubMed] [Google Scholar]
- 5. Torphy RJ, Friedman C, Halpern A, et al. Comparing short-term and oncologic outcomes of minimally invasive versus open pancreaticoduodenectomy across low and high volume centers. Ann Surg 2019;270:1147–1155. [DOI] [PubMed] [Google Scholar]
- 6. Shin SH, Kim YJ, Song KB, et al. Totally laparoscopic or robot-assisted pancreaticoduodenectomy versus open surgery for periampullary neoplasms: separate systematic reviews and meta-analyses. Surg Endosc 2017;31:3459–74. [DOI] [PubMed] [Google Scholar]
- 7. Poves I, Burdio F, Morato O, et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: The PADULAP randomized controlled trial. Ann Surg 2018;268:731–739. [DOI] [PubMed] [Google Scholar]
- 8. Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg 2017;104:1443–1450. [DOI] [PubMed] [Google Scholar]
- 9. Nassour I, Wang SC, Christie A, et al. Minimally invasive versus open pancreaticoduodenectomy: a propensity-matched study from a national cohort of patients. Ann Surg 2018;268:151–157. [DOI] [PubMed] [Google Scholar]
- 10. Qin R, Kendrick ML, Wolfgang CL, et al. International expert consensus on laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg Nutr 2020;9:464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adam MA, Thomas S, Youngwirth L, et al. Defining a hospital volume threshold for minimally invasive pancreaticoduodenectomy in the United States. JAMA Surg 2017;152:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang M, Peng B, Liu J, et al. Practice patterns and perioperative outcomes of laparoscopic pancreaticoduodenectomy in China: a retrospective multicenter analysis of 1029 patients. Ann Surg 2021;273:145–153. [DOI] [PubMed] [Google Scholar]
- 13. Morato O, Poves I, Burdio F, et al. Evaluation of the learning curve for laparoscopic pancreatoduodenectomy by CUSUM analyses. Cohort study. Int J Surg 2020;80:61–67. [DOI] [PubMed] [Google Scholar]
- 14. Choi M, Hwang HK, Lee WJ, et al. Total laparoscopic pancreaticoduodenectomy in patients with periampullary tumors: a learning curve analysis. Surg Endosc 2021;35:2636–2644. [DOI] [PubMed] [Google Scholar]
- 15. Kuroki T, Kitasato A, Adachi T, et al. Learning curve for laparoscopic pancreaticoduodenectomy: a single surgeon’s experience with consecutive patients. Hepatogastroenterology 2014;61:838–841. [PubMed] [Google Scholar]
- 16. Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95–103. [DOI] [PubMed] [Google Scholar]
- 17. Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745–753. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Otero Luppi C, Targarona Soler EM, Balague Ponz C, et al. Clinical, anatomical, and pathological grading score to predict technical difficulty in laparoscopic splenectomy for non-traumatic diseases. World J Surg 2017;41:439–448. [DOI] [PubMed] [Google Scholar]
- 19. Iwashita Y, Ohyama T, Honda G, et al. What are the appropriate indicators of surgical difficulty during laparoscopic cholecystectomy? Results from a Japan-Korea-Taiwan multinational survey. J Hepatobiliary Pancreat Sci 2016;23:533–547. [DOI] [PubMed] [Google Scholar]
- 20. Agha R, Abdall-Razak A, Crossley E, et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156–165. [DOI] [PubMed] [Google Scholar]
- 21. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–1005. [PubMed] [Google Scholar]
- 22. Wolf PA, D’Agostino RB, Belanger AJ, et al. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 23. Palanivelu C, Rajan PS, Rangarajan M, et al. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16:731–740. [DOI] [PubMed] [Google Scholar]
- 24. Zhang H, Lan X, Peng B, et al. Is total laparoscopic pancreaticoduodenectomy superior to open procedure? A meta-analysis. World J Gastroenterol 2019;25:5711–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Hilst J, de Rooij T, Abu Hilal M, et al. Worldwide survey on opinions and use of minimally invasive pancreatic resection. HPB (Oxford) 2017;19:190–204. [DOI] [PubMed] [Google Scholar]
- 26. Tsamalaidze L, Stauffer JA. Pancreaticoduodenectomy: minimizing the learning curve. J Vis Surg 2018;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Speicher PJ, Nussbaum DP, White RR, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014;21:4014–4019. [DOI] [PubMed] [Google Scholar]
- 28. Nagakawa Y, Nakamura Y, Honda G, et al. Learning curve and surgical factors influencing the surgical outcomes during the initial experience with laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2018;25:498–507. [DOI] [PubMed] [Google Scholar]
- 29. Kuroki T, Fujioka H. Training for laparoscopic pancreaticoduodenectomy. Surg Today 2019;49:103–107. [DOI] [PubMed] [Google Scholar]
- 30. Ausania F, Landi F, Martinez-Perez A, et al. A meta-analysis of randomized controlled trials comparing laparoscopic vs open pancreaticoduodenectomy. HPB (Oxford) 2019;21:1613–1620. [DOI] [PubMed] [Google Scholar]
- 31. Kang CM, Lee SH, Chung MJ, et al. Laparoscopic pancreatic reconstruction technique following laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2015;22:202–210. [DOI] [PubMed] [Google Scholar]
- 32. Ohtsuka T, Ban D, Nakamura Y, et al. Difficulty scoring system in laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Sci 2018;25:489–497. [DOI] [PubMed] [Google Scholar]
- 33. Goh BKP, Kabir T, Koh YX, et al. External validation of the Japanese difficulty scoring system for minimally-invasive distal pancreatectomies. Am J Surg 2019;218:967–971. [DOI] [PubMed] [Google Scholar]
- 34. Benassai G, Quarto G, Perrotta S, et al. Long-term survival after curative resection for pancreatic ductal adenocarcinoma – surgical treatment. Int J Surg 2015;21(suppl 1):S1–S3. [DOI] [PubMed] [Google Scholar]
- 35. Giovinazzo F, Turri G, Katz MH, et al. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179–191. [DOI] [PubMed] [Google Scholar]
- 36. Ravikumar R, Sabin C, Abu Hilal M, et al. Impact of portal vein infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br J Surg 2017;104:1539–1548. [DOI] [PubMed] [Google Scholar]
- 37. Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160–167. [DOI] [PubMed] [Google Scholar]
- 38. Mohri Y, Inoue Y, Tanaka K, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg 2013;37:2688–2692. [DOI] [PubMed] [Google Scholar]
- 39. Mohri T, Mohri Y, Shigemori T, et al. Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J Surg Oncol 2016;14:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum 2015;58:1048–1057. [DOI] [PubMed] [Google Scholar]


