Background:
Microsurgical treatment options for lymphedema consist mainly of lymphovenous anastomosis (LVA) and vascularized lymph node transfers (VLNTs). There are no standard measurements of the effectiveness of these interventions and reported outcomes vary among studies.
Methods:
A systematic review and meta-analysis were performed based on a structured search in Embase, Medline, PubMed, Cinahl, Cochrane, and ProQuest in October 2020, with an update in February 2022. Firstly, a qualitative summary of the main reported outcomes was performed, followed by a pooled meta-analysis of the three most frequently reported outcomes using a random effects model. Randomized controlled trials, prospective cohorts, retrospective cohorts, and cross-sectional and case–control studies that documented outcomes following microsurgery in adult patients were included. Studies of other surgical treatments (liposuction, radical excision, lymphatic vessel transplantation) or without reported outcomes were excluded. The study protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews) (ID: CRD42020202417). No external funding was received for this review.
Results:
One hundred fifty studies, including 6496 patients, were included in the systematic review. The qualitative analysis highlighted the three most frequently reported outcomes: change in circumference, change in volume, and change in the number of infectious episodes per year. The overall pooled change in excess circumference across 29 studies, including 1002 patients, was −35.6% [95% CI: −30.8 to −40.3]. The overall pooled change in excess volume across 12 studies including 587 patients was −32.7% [95% CI: −19.8 to −45.6], and the overall pooled change in the number of cutaneous infections episodes per year across 8 studies including 248 patients was −1.9 [95% CI: −1.4 to −2.3]. The vast majority of the studies included were case series and cohorts, which were intrinsically exposed to a risk of selection bias.
Conclusion:
The currently available evidence supports LVA and vascularized lymph node transfers as effective treatments to reduce the severity of secondary lymphedema. Standardization of staging method, outcomes measurements, and reporting is paramount in future research in order to allow comparability across studies and pooling of results.
Keywords: lymphedema, lymphovenous anastomosis, microsurgery, vascularized lymph node transfer
Introduction
Highlights
The vast majority of published studies focus on female patients and on secondary lymphedema.
Pooled reduction of 35% in excess circumference and 32% in excess volume after microsurgery.
Heterogeneity persists in measurement methods; standardization is of paramount importance.
Rationale
The term lymphedema describes any pathologic lymph fluid accumulation in the interstitial compartment of the human body, regardless of whether it is primary lymphedema caused by congenital malformation of the lymph drainage system or secondary lymphedema caused by infectious diseases or by injury to lymph vessels (traumatism, radiotherapy, surgery). Primary lymphedema is a rare disease and accounts for ∼1–10% of all lymphedema cases1,2. Secondary lymphedema is more frequent, with the leading cause in low to middle-income countries being infection by nematodes, while the main etiology in high-income countries is an injury to lymph vessels during surgical or radiological cancer treatment1,3. Classical management is based on compressive dressings and manual drainage [compressive decongestive therapy (CDT)]. For cases refractory to conservative strategies, microsurgical interventions appeared in the 1960s when several different teams described lymphovenous anastomosis (LVA) in animal models4, followed later by reports on humans5–10. LVA techniques were later refined and are nowadays applied worldwide. Vascularized lymph node transfers (VLNT) were first described in animals in 1979 by Shesol et al.11 and in humans in 1982 by Clodius et al.12 as pedicled flaps. This technique was later refined as free flap surgery and was widely adopted as a second line of treatment or as an adjunction to LVA.
Published literature on both techniques increased drastically in the last 10 years, describing very satisfying results. However, there is no standard measurement of the effectiveness of these interventions and reported outcomes vary significantly among studies. This lack of standardization prevented the production of good-quality evidence about the efficacy and safety of these procedures. In this systematic review of the literature, we aimed to determine how the outcomes of microsurgical treatment for lymphedema were assessed in published studies. When enough studies reported similar assessment methods, we aimed to pool the results in a meta-analysis to determine the effectiveness of microsurgical therapy. In addition, data regarding adverse events associated with microsurgery for lymphedema was collected.
Methods
Methods of this review were determined in advance and published in a protocol on PROSPERO (ID: CRD42020202417). This systematic review followed the PRISMA (preferred reporting items for systematic reviews and meta-analyses), Supplemental Digital Content 1, http://links.lww.com/JS9/A343 and AMSTAR 2 (assessing the methodological quality of systematic reviews), Supplemental Digital Content 2, http://links.lww.com/JS9/A344 guidelines13,14. A self-performed rating according to AMSTAR 2 concluded that moderate confidence could be given in the results of this review.
Eligibility criteria
Randomized controlled trials, prospective cohort, retrospective cohort, and cross-sectional and case-series studies that documented outcomes following microsurgery for extremities lymphedema in adult (>18 years old) patients were included. We willingly renounced a systematic review and meta-analysis restricted to randomized controlled trials only as very few have been published so far, and such an analysis was unlikely to provide a comprehensive summary of the effects of microsurgical treatment of lymphedema. Microsurgery was defined as LVA or VLNT. Several variations of the LVA technique have been described, and in order to avoid unjustified exclusion of studies, all surgical techniques were included. We tried to categorize studies as using either single LVA (sLVA) or multiple LVA (mLVA, being defined as multiples lymphatics anastomosed to a single vein). Authors are, however, not always consistent in the use of these abbreviations. Studies reporting a combined treatment using both microsurgical techniques or using the microsurgical technique in combination with a flap for soft tissue reconstruction were included. Studies comparing both microsurgical techniques were included as well. Studies reporting procedures in which patients underwent microsurgical treatment followed, secondarily, by other surgical or nonsurgical treatments were included if they reported details of the assessments before and after microsurgery.
Studies of other surgical treatments (liposuction, radical excision) or without reported outcomes were excluded. Lymphatic vessel transplantation or transfer, despite being microsurgical by definition, was excluded as it is noticeably less widespread than the two other techniques. Studies about parasitic lymphedema were excluded as well. Studies reported in languages other than English were excluded.
Information sources
To identify articles, a systematic search strategy was designed by a medical librarian, using a combination of controlled vocabulary terms and free text terms covering the two overarching concepts of the research: lymphedema and microsurgery. The search strategy was translated for a range of databases: Embase.com, Medline Ovid SP, PubMed (not Medline), Cinahl Full Text – Ebsco, Cochrane Central Register of Controlled Trials – Wiley and ProQuest Dissertations & Theses A&I. The search strategies were peer-reviewed by a second medical librarian before the initial searches were conducted in October 2020 and repeated in February 2022. The searches were performed without limits for publication date or languages. The search equations for all the databases are provided as Supplementary material 3, Supplemental Digital Content 6, http://links.lww.com/JS9/A348. All references identified through the searches were downloaded into EndNote and duplicates were removed.
Selection process
The resulting records were uploaded to the Rayyan QCRI software. This software was used to perform screening for inclusion by two authors who graded articles for levels of evidence during the same process. When uncertainty about inclusion was present, a supplementary evaluation and grading of evidence was performed by a senior author. All disagreements were resolved by consensus among the three reviewers.
Data collection process
Data collection was performed by two authors under the supervision of one senior author. When needed, study investigators were contacted by e-mail to obtain or confirm data.
Data items
In the qualitative part of the review, the assessment methods used in the included studies were retrieved and classified according to these preselected categories: volume, circumference, number of CDT per defined period of time, use of compressive garments, number of infectious episodes per defined period of time, lymphoscintigraphy, lymphography, magnetic resonance imaging/computed tomography/single-photon emission computed tomography-computed tomography (MRI/CT/SPECT-CT), quality of life (QoL) questionnaires, and patient satisfaction. In the second part of the review, quantitative results for the three most frequently reported outcomes were retrieved from all concerned studies and pooled for a meta-analysis.
In parallel to these outcomes, data relative to study location, participants’ characteristics (sex, age, BMI), etiology of lymphedema, localization of lymphedema, preoperative conservative therapy attempts, staging [International Society of Lymphology (ISL) scale or other staging scales], duration of symptoms, surgical technique used, the mean number of anastomosis (for LVA) and mean follow-up duration was retrieved as well, Supplementary material 4, Supplemental Digital Content 7, http://links.lww.com/JS9/A349.
Study risk of bias assessment/study quality
For studies included in the meta-analysis, randomized controlled trials’ methodological quality was assessed with the Cochrane risk-of-bias tool. Cohort and case-series studies were assessed using a modified version of the Newcastle–Ottawa Scale (NOS) in which the items relating to controls and comparability were removed due to the type of studies included. This change from the protocol published on PROSPERO was decided in order to improve comparability across surgical specialties.
Effect measures
The three most frequently reported outcomes were the change in excess circumference, the change in excess volume, and the change in the number of cutaneous infections per year (see the ‘Results’ section). The effect size used in the meta-analysis for the change in excess circumference was the reduction, in percentage, of the circumference difference between the healthy limb and the affected contralateral limb. When cohort studies reported changes for different arms without reporting the change for the overall cohort, the reported variations and standard deviations were pooled. When studies reported changes with a range instead of standard deviation, we did not input the latter in accordance with the Cochrane Handbook recommendations, and studies were excluded. When studies reported raw data without summary for the entire set of patients, mean and standard deviations were calculated from the data available. For the change in excess volume, the selected effect size was the reduction, in percentage, of the volume difference between the healthy limb and the affected contralateral limb. The same methods were used to retrieve mean values and standard deviations if they were not directly provided in the paper. The effect size for the change in the number of cutaneous infections per year was the raw number of such episodes across 12 months.
Synthesis methods
Qualitative analysis
All studies were included in this analysis.
Quantitative analysis
Only studies that provided the adequate effect size and the necessary associated standard deviation were included in the meta-analysis for each outcome. All multi-arms designs (LVA vs. VLNT, lower vs. upper extremity; LVA-technique A vs. LVA-technique B) were treated by groups combination in order to avoid both double-counting and correlated effect sizes, according to the Cochrane recommendations15. We planned a subgroup analysis for each surgical technique if at least 10 studies could be included. The pooling of the effect sizes was performed using a random effects model in order to account for the considerable heterogeneity between the studies. The restricted maximum likelihood estimator16 was used to calculate the heterogeneity variance τ2. We used Knapp–Hartung adjustments17 to calculate the confidence interval around the pooled effect. An outlier and influence analysis was performed for all pooled effect sizes. All analyses were performed using the R Project for Statistical Computing version 3.6.2. A P value less than 0.05 was considered statistically significant.
Results
Study selection
The original search yielded 3181 results. Three articles were identified through other sources. After the removal of duplicates, 1891 records were screened and 219 full-text articles were assessed for eligibility. One thousand six hundred seventy-two records were excluded at this stage. After the full-text assessment, another 69 articles were excluded, leaving 150 articles for the qualitative analysis9,18–165. Eleven articles amongst these 69 formally met the inclusion criteria but were nonetheless excluded due to insufficient reporting of patients’ characteristics, insufficient reporting of outcomes and/or overlapping of patients’ populations (Fig. 1: PRISMA flowchart). Eight of those 11 studies, ranging from 2001 to 2016, encompassed a broad population of several thousand patients and played a significant role in the popularization and refinement of LVA techniques. The absence of precise population sizes and characteristics, as well as insufficient outcomes data, however, prevented their inclusion in our systematic review and meta-analysis. A list of all excluded studies with justification for the exclusion is available as Supplementary material 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A346.
Figure 1.
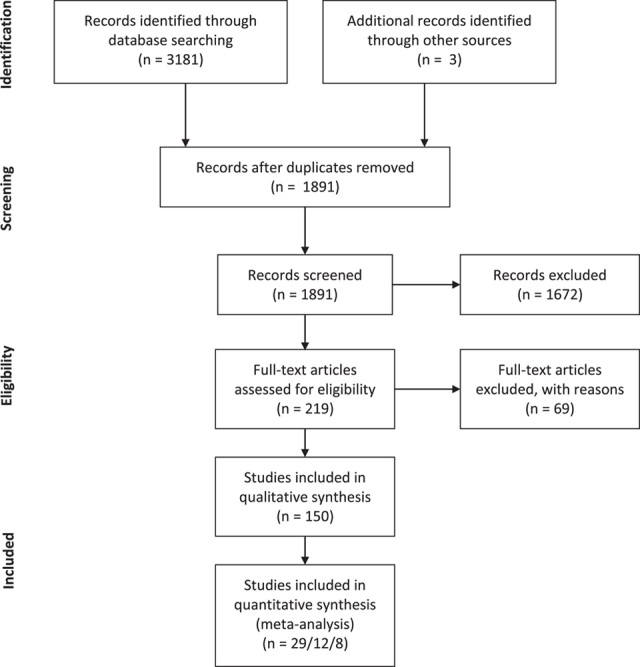
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart.
The 150 included articles reported on 6496 patients and covered a period from 1990 to 2022. The number of patients included in each study ranged from 5 to 664 (mean: 43 patients). Gender was not reported for 1751 patients. However, 4335 of the remaining 4745 patients (91%) were female and 410 were male (9%). The etiology of lymphedema was frequently reported (138/151 studies): 92% of the cases were secondary lymphedema, amongst which 58% were breast carcinoma-related lymphedema. Seven percent of the cases were primary lymphedema and 1% was of unreported etiology. The localization was almost always reported (149/150 studies) with a fairly equal repartition between the lower and upper extremities: 52% of the cases concerned the lower extremity and 48% the upper extremity. Duration of symptoms and duration of follow-up were reported in 96/150 (64%) and 127/150 (85%) studies. The average symptom duration was 66.8±32.6 months. The average follow-up was 20.4±16.5 months. There were two randomized controlled trials, one randomized trial, 29 prospective and 35 retrospective cohorts, 30 prospective case series, and 53 retrospective case series. Eighty studies included only secondary lymphedema, while 54 studies included a mix of primary and secondary lymphedema cases and one study focused on primary lymphedema only. The remaining 16 studies had either no available information or incomplete information about the etiology. The staging method used was highly variable with 62 studies using the International Society of Lymphology (ISL) scale, while 43 studies reported results using Campisi, Cheng, Yamamoto, indocyanine green leg dermal backflow (ICG-LDB/DBF) or custom staging systems and 45 studies reporting no stages for the included patients. Regarding the technique, 89 studies investigated LVA, 48 studies VLNT, and 13 a combination of both procedures. Eighty-eight percent (78/89) of the studies reporting about LVA included sLVA. Four percent (4/89) included mLVA and 8% included either a combination of both or an unspecified technique. The characteristics of all included studies are described in a table available as Supplementary material 1: included studies, Supplemental Digital Content 5, http://links.lww.com/JS9/A347.
Results of syntheses
Qualitative analysis
In the 150 articles included, the most frequently reported outcomes were the change in circumference (100/150 studies, 67%), the change in volume (54/150 studies, 36%), and the change in number of cutaneous infections (53/150 studies, 35%). Several studies reported more than one outcome. Changes in quality of life (QoL) questionnaire scores were reported in 22% of studies. Lymphoscintigraphic changes were reported in the same proportion as patient satisfaction (21% of studies) and changes in the use of compressive garments were reported in 19% of studies. Lastly, changes in the number of CDT sessions in lymphography and MRI/CT/SPECT-CT follow-up investigations were respectively reported in 11, 7, and 3% of all included studies.
Quantitative analysis
Primary outcome: change in excess circumference. Among the 100 studies that reported changes in circumference, 42 reported a change in excess circumference, 34 reported a change in upper extremity or lower extremity lymphedema index (UEL index or LEL index), 17 reported a change in absolute circumference, and 7 reported another type of change (e.g. arbitrary categorical cutoffs). In order to be able to pool effect sizes, we limited the meta-analysis to the most frequently reported effect, that is the change (in percentage) in excess circumference (Table 1: results of individual studies). Twenty-nine studies could be included and the remaining 13 had to be excluded because necessary data were missing. Twenty out of these 29 studies investigated VLNT, 8 investigated LVA, and 1 investigated a combination of both techniques. The overall pooled change in excess circumference for the 1002 patients in these 29 studies was −35.6% [95% CI: −30.9 to −40.3] (Fig. 2: forest plot). The between-study heterogeneity variance was estimated at τ 2=62.12 [95% CI: 4.13–160.31], with an I 2 value of 44.3% [95% CI: 13.5–64.1%]. The prediction interval ranged from −18.8% to −52.4%. An influence analysis confirmed that no study differed significantly from the others and/or contributed disproportionately to heterogeneity.
Table 1.
Change in excess circumference (%).
| Author | n | Mean (SD) | Origin of effect size | Technique |
|---|---|---|---|---|
| Agko et al., 201892 | 12 | −37.8 (1.8) | Supplied in paper | VLNT |
| Aljaaly et al., 2019111 | 15 | −30.1 (23.7) | Supplied in paper | VLNT |
| Cheng et al., 201345 | 10 | −40.4 (16.1) | Supplied in paper | VLNT |
| Cheng, 2012166 | 6 | −65.0 (22.3) | Calculated from data | VLNT |
| Ciudad et al., Apr 2020139 | 6 | −30.0 (5.1) | Supplied in paper | VLNT |
| Ciudad et al., Feb 2020140 | 83 | −24.0 (10.4) | Supplied in paper | VLNT |
| Ciudad et al., Oct 201772 | 7 | −43.7 (2.5) | Supplied in paper | VLNT |
| Ciudad et al., Mar 201771 | 10 | −39.5 (1.8) | Supplied in paper | VLNT |
| Di Taranto et al., 2020122 | 10 | −21.8 (5.5) | Calculated from subgroups | VLNT |
| Engel et al., 201889 | 72 | −26.1 (10.7) | Calculated from subgroups | VLNT+LVA |
| Gennaro et al., 201668 | 69 | −50.3 (20.0) | Calculated from data | LVA |
| Gustafsson et al., 201885 | 35 | −19.8 (9.2) | Supplied in paper | VLNT |
| Ho et al., 2019106 | 76 | −17.2 (7.0) | Supplied in paper | VLNT |
| Ho et al., 201886 | 43 | −52.0 (19.1) | Supplied in paper | VLNT |
| Ito et al., 201665 | 5 | −63.8 (20.2) | Supplied in paper | LVA |
| Kim et al., 2021157 | 133 | −8.0 (36.5) | Supplied in paper | LVA |
| Koshima et al., 200423 | 52 | −41.8 (31.2) | Supplied in paper | LVA |
| Koshima et al., 200322 | 13 | −55.6 (29.6) | Supplied in paper | LVA |
| Koshima et al., 200020 | 12 | −47.3 ( 24.3) | Calculated from data | LVA |
| Koshima et al., 199619 | 6 | −65.7 (21.2) | Supplied in paper | LVA |
| Lin et al., 200926 | 13 | −50.6 (19.2) | Supplied in paper | VLNT |
| Liu et al., 201894 | 30 | −47.0 (27.9) | Supplied in paper | VLNT |
| Maruccia et al., Nov 2019103 | 16 | −53.2 (8.6) | Calculated from subgroups | VLNT |
| Maruccia et al., Jan 2019104 | 21 | −42.6 (9.9) | Calculated from subgroups | VLNT |
| Patel et al., Jul 201547 | 25 | −28.7 (19.0) | Calculated from subgroups | VLNT |
| Patel, Jan 201548 | 20 | −40.5 (22.2) | Supplied in paper | VLNT |
| Roka-Palkovits et al., 2021153 | 70 | −38.9 (2.5) | Supplied in paper | VLNT |
| Viitanen et al., 201344 | 14 | −55.0 (164.1) | Calculated from data | VLNT |
| Yodrabum et al., 2021148 | 118 | −16.0 (8.9) | Calculated from subgroups | LVA |
LVA, lymphovenous anastomosis; VLNT. vascularized lymph node transfers.
Figure 2.
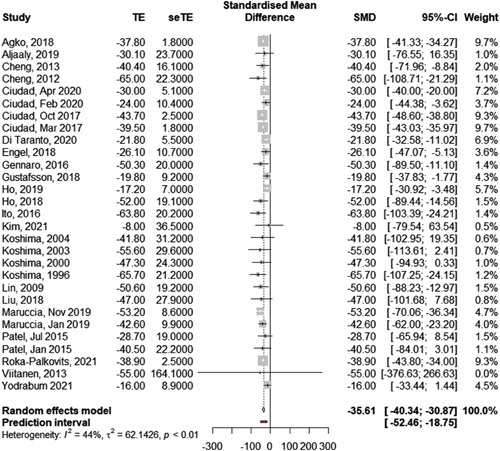
Pooled change in excess circumference after microsurgical therapy using a random effect model. Effect size = Change in excess circumference in percentage.
Secondary outcomes: change in excess volume and change in cutaneous infections. Data regarding volume variation was available in 54 out of 150 studies. Among these 54 studies, 39 reported a change in excess volume, 7 reported a change in absolute volume, and 8 studies reported another type of volume change. As for the primary outcome, we limited the meta-analysis to studies reporting the same effect size, namely the change, in percentage, in excess volume (Table 2: results of individual studies and Fig. 3: forest plot). Twelve studies provided sufficient data to be included. Five of these 12 studies investigated LVA, 6 investigated VLNT, and 1 investigated a combination of both techniques. The overall pooled change in excess volume for the 587 patients included in these studies was −32.7% [95% CI: −19.8 to −45.6] (Fig. 3: forest plot). The between-study heterogeneity variance was estimated at τ 2=265.86 [95% CI: 7.07–579.05], with an I 2 value of 54.7% [95% CI: 13.2–76.4%]. The prediction interval ranged from +5.9% to −71.3%. Akita et al.145 were an outlier with a significantly smaller change in excess circumference with a more limited standard deviation. When excluded, the overall pooled change in excess volume of the remaining 11 studies increased slightly to −38.4% [95% CI: −27.7 to −49.1], with a notable reduction in heterogeneity (I 2=0.0%, 95% CI: 0.0–60.2%) and in prediction interval (−15.5% to −63.2%).
Table 2.
Change in excess volume (%).
| Author | n | Mean (SD) | Origin of effect size | Method | Technique |
|---|---|---|---|---|---|
| Akita et al., 2021145 | 60 | −5.2 (5.8) | SD calculated from subgroups | Truncated cone formula | VLNT/LVA |
| Demirtas et al., 201029 | 78 | −57.6 (22.1) | Supplied in paper | Software calculation on serial measurements | LVA |
| Demirtas et al., 200928 | 42 | −59.3 (22.3) | Supplied in paper | Software calculation on serial measurements | LVA |
| Dionyssiou et al., 2021159 | 64 | −55.7 (23.0) | Supplied in paper | Truncated cone formula | VLNT |
| Ho et al., 2019106 | 76 | −27.0 (27.3) | SD calculated from subgroups | CT scan | VLNT |
| Johnson et al., 2019107 | 7 | −22.7 (33.2) | SD calculated from data | Not reported | VLNT |
| Khan et al., 201998 | 27 | −9.2 (71.8) | Supplied in paper | Perometry | LVA |
| Manrique et al., 2020130 | 26 | −20.6 (8.2) | SD calculated from subgroups | Truncated cone formula | VLNT |
| Montag et al., 2019117 | 24 | −20.1 (44.9) | Supplied in paper | Truncated cone formula | VLNT |
| Schaverien et al., 2021152 | 134 | −45.7 (8.7) | Supplied in paper | Perometry | VLNT |
| Yasunaga et al., 2020126 | 19 | −46.0 (35.8) | Supplied in paper | Bioimpedance | LVA |
| Yasunaga et al., 2019112 | 30 | −45.1 (36.3) | Supplied in paper | Bioimpedance | LVA |
CT, computed tomography, LVA, lymphovenous anastomosis; VLNT. vascularized lymph node transfers.
Figure 3.
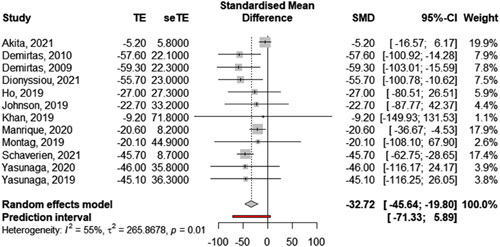
Pooled change in excess volume after microsurgical therapy using a random effect model. Effect size = Change in excess volume in percentage.
A change in cutaneous infections was reported in 48 out of 151 studies: 38 reported a change in the number of infectious episodes per year, 8 studies a change in the number of patients affected per year, and 2 used another form of a report. Eight out of the 40 studies provided sufficient data to be included in the meta-analysis (Table 3: results of individual studies and Fig. 4: forest plot). Five out of these eight studies investigated VLNT and three investigated LVA. The overall pooled change in the number of cutaneous infections episode per year for the 248 patients included was −1.9 [95% CI: −1.4 to −2.3] (Fig. 3: forest plot). The between-study heterogeneity variance was estimated at τ 2=0, with an I 2 value of 0.0% [95% CI: 0.0–67.6%]. The prediction interval ranged from −1.5 to −2.3. The heterogeneity and influence analysis did not highlight any significant outlier.
Table 3.
Change in number of infectious episodes per year (n).
| Author | n | Mean (SD) | Origin of effect size | Technique |
|---|---|---|---|---|
| Asuncion et al., 201888 | 15 | −2.5 (1.4) | SD calculated from data | VLNT |
| Cheng et al., 201345 | 10 | −1.3 (1.0) | Supplied in paper | VLNT |
| Ciudad, 2020140 | 83 | −2.2 (1.1) | Supplied in paper | VLNT |
| Ciudad, 201772 | 7 | −1.7 (0.8) | SD calculated from data | VLNT |
| Gennaro et al., 201668 | 69 | −2.6 (2.9) | SD calculated from data | LVA |
| Gratzon et al., 201776 | 50 | −2.8 (1.8) | SD calculated from data | VLNT |
| Ito et al., 201665 | 5 | −1.4 (1.9) | Supplied in paper | LVA |
| Matsubara et al., 200624 | 9 | −2.1 (1.6) | SD calculated from data | LVA |
LVA, lymphovenous anastomosis; VLNT. vascularized lymph node transfers.
Figure 4.
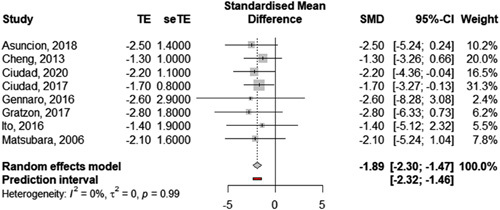
Pooled change in cutaneous infections using a random effect model. Effect size = Change in number of cutaneous infections per year (absolute value).
Discussion
The systematic review highlighted a certain homogeneity in the patients’ populations with a large majority of cases involving female patients and lymphedema of secondary etiology. Several key demographics were, however, frequently missing: the gender distribution was not specified for a quarter of the population and characteristics such as age and BMI were reported with their standard deviations in less than half of the studies. In addition, a large variety of staging systems was applied by the different authors (International Society of Lymphology, Campisi’s, Cheng’s, ICG-LDB). We observed a high heterogeneity between the studies in terms of measurement units, measurement methods as well as grouping distributions. For the most frequently reported outcome (changes in volume), some authors reported changes in excess volume, and some others reported a change in the absolute volume of the limb. Results were reported using several different techniques: serial circumferential measurements and calculations, perometry, water volumetry, CT scan, MRI, or bioimpedance. Variations in circumferences were as well reported, sometimes as changes in excess and sometimes as changes in absolute circumference. The measurement sites on the extremity were not standardized, and some authors preferred to report changes in percentages, in raw centimetric value, or using the LEL and UEL indexes. We observed the same variability on data assessment for the other outcomes such as frequency of infectious episodes or CDT sessions. This heterogeneity was expected but nonetheless limited the number of studies that could eventually be included in the meta-analysis. Another example of such variability is found in the reporting of quality of life changes. The majority of studies (19/33) reported such changes using the LYMQOL scale or one of its components but some other scales were frequently used as well (Lymph-ICF, LeQOLIS, LLIS, and custom scales). Amongst studies using the LYMQOL scale, none reported values with standard deviation and a meta-analysis was therefore not possible. This is unfortunate given the dramatic variations reported by some authors: the 14 studies that included the overall QoL score of LYMQOL reported increases ranging from 16 to 240% with a preoperative score ranging from 2.2 to 5.8 and a postoperative score ranging from 6.3 to 8.2.
It is worth noting that the scoring system described in the original article from Keeley et al.167 does not provide a recommended way of summarizing results. As a consequence, some authors summed the scores of all questions while others reported only the score of the ‘overall QoL’ question. The fact that, in the first case, a higher score reflects a lower quality of life while it is the opposite in the second case only adds to the confusion. We therefore strongly advocate for future studies to use only the overall QoL score of the LYMQOL scale unless these studies are specifically focused on quality of life outcomes.
Our reported pooled reduction of 35% in excess circumference following microsurgical treatment for lymphedema is a promising result. The pooled reduction in excess volume of 33% and the significant reduction in the number of cutaneous infections per year are exciting results as well.
A similar systematic review and meta-analysis were recently published without previous publication of the protocol168. It included 66 studies with similar outcomes, despite the application of broader inclusion criteria (notably, the inclusion of liposuction therapies) and slightly different effect sizes. Thirty-nine of the 66 studies (59%) included in this review were included in our systematic review as well. The remaining 27 studies were excluded from our selection because they either reported results after liposuction (14 studies), reported the effects of preventive microsurgical therapy (6 studies), included cases of filariasis (2 studies), included pediatric cases (1 study), reported the treatment of lymphocele (1 study), were published in a language other than English and/or could not be accessed (3 studies). The pooled effect size of our meta-analysis cannot be directly compared with Chang et al.’s, but our findings are overall coherent with this previous work which reported a pooled reduction of 3.80 [95% CI: 2.93–4.67] cm in circumference after treatment with LVA and 1.64 cm [95% CI: 0.87–2.42] after treatment with VLNT.
These results should be approached with precaution as the validity of our meta-analysis is entirely based on the validity of the included studies. Only two of these were randomized controlled trials that can guarantee a superior level of evidence. The vast majority of the studies included in the systematic review and all studies included in the meta-analysis were nonrandomized studies of interventions (NRSI) which intrinsically exposed to a risk of selection bias. As recently demonstrated by the RE-ENERGIZE Trial, the pooling of results in a meta-analysis of NRSI may result in a statistically significant estimate of an effect of great magnitude that is however not reproducible in a randomized, placebo-controlled trial169,170. Another issue is the pressure to publish positive results that might have precluded the publication of nonsignificant changes after surgery or, when published, might have precluded inclusion in the meta-analysis because necessary data were not reported27,155.
In spite of these limitations, this systematic review’s validity is supported by its extensive reach. It joins a growing set of literature171–175 that suggests LVA and vascularized lymph node transfers are effective treatments to reduce the severity of secondary lymphedema of the extremities.
The standardization of staging, outcomes measurements, and their reporting is a paramount next step in order to allow comparability between studies and the pooling of results. The most objective, reproducible, and standardized outcome available at the moment is the Kleinhans transport index calculated on lymphoscintigraphy176. The standardization of the procedure and publication of ‘standard protocols’ are recent achievements177,178 and offer researchers the opportunity to compare studies across centers and countries. Large randomized and case–control trials are underway for both surgical techniques (NCT03578380 at Sykehuset Telemark in Norway179, NCT03941756 at MD Anderson Cancer Center in the USA180, and NCT05064176 at Universitaire Ziekenhuizen Leuven in Belgium181 for LVA; NCT03248310 at Memorial Sloan Kettering Cancer Center in the USA182 for VLNT) and should provide further data between 2023 and 2025.
Hopefully, the results of these trials will provide high-quality evidence that can be translated into clinical-practice recommendations to guide healthcare providers in patient selection and in the choice of the adequate treatment.
Ethical approval
No ethical approval is necessary (systematic review and meta-analysis).
Sources of funding
The authors did not receive any funding for this study.
Author contribution
J.M., J.E., and P.D.S.: designed the study; J.M., J.E., and M.G.: collected the data; J.M.: analyzed the data; J.M., J.E., M.G., L.M., and P.D.S.: wrote the manuscript. All authors contributed and approved the final version of the manuscript.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique identifying number or registration ID: CRD42020202417.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=202417
Guarantor
Prof Pietro Di Summa, MD, PhD, Plastic and Hand Surgery Department, Lausanne University Hospital.
Conflicts of interest disclosure
The authors do not report any conflicts of interest.
Data statement
How effective is lymphatic microsurgery? A systematic review and meta-analysis of outcomes after microsurgical treatment of lymphedema
Research data for this article
Data extracted from included studies and data used for all analyses are available from the corresponding authors upon reasonable request. Analytic code is freely available in the R Project for Statistical Computing.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 17 April 2023
Contributor Information
Joachim N. Meuli, Email: jomeuli@gmail.com.
Martino Guiotto, Email: Martino.Guiotto@chuv.ch.
Jolanda Elmers, Email: Jolanda.Elmers@chuv.ch.
Lucia Mazzolai, Email: Lucia.Mazzolai@chuv.ch.
Pietro G. di Summa, Email: Pietro.Di-Summa@chuv.ch.
References
- 1. Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol 2017;77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 2. Lee JH, Chang DW. Surgical treatment of primary lymphedema. Lymphat Res Biol 2017;15:220–226. [DOI] [PubMed] [Google Scholar]
- 3. Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464–472. [DOI] [PubMed] [Google Scholar]
- 4. Danese C, Bower R, Howard J. Experimental anastomoses of lymphatics. Arch Surg 1962;84:6–9. [DOI] [PubMed] [Google Scholar]
- 5. Danese CA, Papaioannou AN, Morales LE, et al. Surgical approaches to lymphatic blocks. Surgery 1968;64:821–826. [PubMed] [Google Scholar]
- 6. O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg 1977;60:197–211. [DOI] [PubMed] [Google Scholar]
- 7. O’Brien BM, Shafiroff BB. Microlymphaticovenous and resectional surgery in obstructive lymphedema. World J Surg 1979;3:3–15; 121-123. [DOI] [PubMed] [Google Scholar]
- 8. Jamal S. Lymphovenous anastomosis in filarial lymphedema. Lymphology 1981;14:64–68. [PubMed] [Google Scholar]
- 9. Campisi C, Boccardo F, Alitta P, et al. Derivative lymphatic microsurgery: indications, techniques, and results. Microsurgery 1995;16:463–468. [DOI] [PubMed] [Google Scholar]
- 10. Campisi C, Boccardo F, Tacchella M. Reconstructive microsurgery of lymph vessels: the personal method of lymphatic-venous-lymphatic (LVL) interpositioned grafted shunt. Microsurgery 1995;16:161–166. [DOI] [PubMed] [Google Scholar]
- 11. Shesol BF, Nakashima R, Alavi A, et al. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast Reconstr Surg 1979;63:817–823. [PubMed] [Google Scholar]
- 12. Clodius L, Smith PJ, Bruna J, et al. The lymphatics of the groin flap. Ann Plast Surg 1982;9:447–458. [DOI] [PubMed] [Google Scholar]
- 13. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg Lond Engl 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT Thomas J Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2. Published online February 2021.
- 16. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 2005;30:261–293. [Google Scholar]
- 17. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003;22:2693–2710. [DOI] [PubMed] [Google Scholar]
- 18. O’Brien BM, Mellow CG, Khazanchi RK, et al. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg 1990;85:562–572. [DOI] [PubMed] [Google Scholar]
- 19. Koshima I, Kawada S, Moriguchi T, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg 1996;97:397–405. [DOI] [PubMed] [Google Scholar]
- 20. Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437–442. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto Y, Horiuchi K, Sasaki S, et al. Follow-up study of upper limb lymphedema patients treated by microsurgical lymphaticovenous implantation (MLVI) combined with compression therapy. Microsurgery 2003;23:21–26. [DOI] [PubMed] [Google Scholar]
- 22. Koshima I, Nanba Y, Tsutsui T, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg 2003;19:209–215. [DOI] [PubMed] [Google Scholar]
- 23. Koshima I, Nanba Y, Tsutsui T, et al. Minimal invasive lymphaticovenular anastomosis under local anesthesia for leg lymphedema: is it effective for stage III and IV? Ann Plast Surg 2004;53:261–266. [DOI] [PubMed] [Google Scholar]
- 24. Matsubara S, Sakuda H, Nakaema M, et al. Long-term results of microscopic lymphatic vessel-isolated vein anastomosis for secondary lymphedema of the lower extremities. Surg Today 2006;36:859–864. [DOI] [PubMed] [Google Scholar]
- 25. Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema. Ann Surg 2006;243:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265–1275. [DOI] [PubMed] [Google Scholar]
- 27. Damstra RJ, Voesten HGJ, van Schelven WD, et al. Lymphatic venous anastomosis (LVA) for treatment of secondary arm lymphedema. A prospective study of 11 LVA procedures in 10 patients with breast cancer related lymphedema and a critical review of the literature. Breast Cancer Res Treat 2009;113:199–206. [DOI] [PubMed] [Google Scholar]
- 28. Demirtas Y, Ozturk N, Yapici O, et al. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery 2009;29:609–618. [DOI] [PubMed] [Google Scholar]
- 29. Demirtas Y, Ozturk N, Yapici O, et al. Comparison of primary and secondary lower-extremity lymphedema treated with supermicrosurgical lymphaticovenous anastomosis and lymphaticovenous implantation. J Reconstr Microsurg 2010;26:137–143. [DOI] [PubMed] [Google Scholar]
- 30. Narushima M, Mihara M, Yamamoto Y, et al. The intravascular stenting method for treatment of extremity lymphedema with multiconfiguration lymphaticovenous anastomoses. Plast Reconstr Surg 2010;125:935–943. [DOI] [PubMed] [Google Scholar]
- 31. Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg 2010;126:752–758. [DOI] [PubMed] [Google Scholar]
- 32. Gharb BB, Rampazzo A, di Spilimbergo SS, et al. Vascularized lymph node transfer based on the hilar perforators improves the outcome in upper limb lymphedema. Ann Plast Surg 2011;67:589–593. [DOI] [PubMed] [Google Scholar]
- 33. Mihara M, Hayashi Y, Murai N, et al. Regional diagnosis of lymphoedema and selection of sites for lymphaticovenular anastomosis using elastography. Clin Radiol 2011;66:715–719. [DOI] [PubMed] [Google Scholar]
- 34. Furukawa H, Osawa M, Saito A, et al. Microsurgical lymphaticovenous implantation targeting dermal lymphatic backflow using indocyanine green fluorescence lymphography in the treatment of postmastectomy lymphedema. Plast Reconstr Surg 2011;127:1804–1811. [DOI] [PubMed] [Google Scholar]
- 35. Lee BB, Laredo J, Neville R. Reconstructive surgery for chronic lymphedema: a viable option, but. Vascular 2011;19:195–205. [DOI] [PubMed] [Google Scholar]
- 36. Maegawa J, Yabuki Y, Tomoeda H, et al. Outcomes of lymphaticovenous side-to-end anastomosis in peripheral lymphedema. J Vasc Surg 2012;55:753–760. [DOI] [PubMed] [Google Scholar]
- 37. Maegawa J, Hosono M, Tomoeda H, et al. Net effect of lymphaticovenous anastomosis on volume reduction of peripheral lymphoedema after complex decongestive physiotherapy. Eur J Vasc Endovasc Surg 2012;43:602–608. [DOI] [PubMed] [Google Scholar]
- 38. Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468–473. [DOI] [PubMed] [Google Scholar]
- 39. Auba C, Marre D, Rodríguez‐Losada G, et al. Lymphaticovenular anastomoses for lymphedema treatment: 18 months postoperative outcomes. Microsurgery 2012;32:261–268. [DOI] [PubMed] [Google Scholar]
- 40. Mihara M, Hara H, Kikuchi K, et al. Scarless lymphatic venous anastomosis for latent and early-stage lymphoedema using indocyanine green lymphography and non-invasive instruments for visualising subcutaneous vein. J Plast Reconstr Aesthet Surg 2012;65:1551–1558. [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto T, Yoshimatsu H, Narushima M, et al. A modified side-to-end lymphaticovenular anastomosis. Microsurgery 2013;33:130–133. [DOI] [PubMed] [Google Scholar]
- 42. Ayestaray B, Bekara F, Andreoletti JB. Patent blue-enhanced lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg 2013;66:382–389. [DOI] [PubMed] [Google Scholar]
- 43. Boccardo F, De Cian F, Campisi CC, et al. Surgical prevention and treatment of lymphedema after lymph node dissection in patients with cutaneous melanoma. Lymphology 2013;46:20–26. [PubMed] [Google Scholar]
- 44. Viitanen TP, Visuri MT, Hartiala P, et al. Lymphatic vessel function and lymphatic growth factor secretion after microvascular lymph node transfer in lymphedema patients. Plast Reconstr Surg Glob Open 2013;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286–1298. [DOI] [PubMed] [Google Scholar]
- 46. Akita S, Mitsukawa N, Kuriyama M, et al. External valvuloplasty for subcutaneous small veins to prevent venous reflux in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg 2013;132:1008–1014. [DOI] [PubMed] [Google Scholar]
- 47. Patel KM, Lin CY, Cheng MH. A prospective evaluation of lymphedema-specific quality-of-life outcomes following vascularized lymph node transfer. Ann Surg Oncol 2015;22:2424–2430. [DOI] [PubMed] [Google Scholar]
- 48. Patel KM, Lin CY, Cheng MH. From theory to evidence: long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg 2015;31:26–30. [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto T, Yamamoto N, Azuma S, et al. Near-infrared illumination system-integrated microscope for supermicrosurgical lymphaticovenular anastomosis. Microsurgery 2014;34:23–27. [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto T, Yoshimatsu H, Narushima M, et al. Sequential anastomosis for lymphatic supermicrosurgery: multiple lymphaticovenular anastomoses on 1 venule. Ann Plast Surg 2014;73:46–49. [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg 2014;72:67–70. [DOI] [PubMed] [Google Scholar]
- 52. Yamamoto T, Yamamoto N, Numahata T, et al. Navigation lymphatic supermicrosurgery for the treatment of cancer-related peripheral lymphedema. Vasc Endovascular Surg 2014;48:139–143. [DOI] [PubMed] [Google Scholar]
- 53. Granzow JW, Soderberg JM, Kaji AH, et al. An effective system of surgical treatment of lymphedema. Ann Surg Oncol 2014;21:1189–1194. [DOI] [PubMed] [Google Scholar]
- 54. Ayestaray B, Bekara F. π-Shaped lymphaticovenular anastomosis: the venous flow sparing technique for the treatment of peripheral lymphedema. J Reconstr Microsurg 2014;30:551–560. [DOI] [PubMed] [Google Scholar]
- 55. Chen R, Mu L, Zhang H, et al. Simultaneous breast reconstruction and treatment of breast cancer-related upper arm lymphedema with lymphatic lower abdominal flap. Ann Plast Surg 2014;73:S12. [DOI] [PubMed] [Google Scholar]
- 56. Mihara M, Hara H, Furniss D, et al. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. BJS Br J Surg 2014;101:1391–1396. [DOI] [PubMed] [Google Scholar]
- 57. Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919–2924. [DOI] [PubMed] [Google Scholar]
- 58. Akita S, Mitsukawa N, Kuriyama M, et al. Comparison of vascularized supraclavicular lymph node transfer and lymphaticovenular anastomosis for advanced stage lower extremity lymphedema. Ann Plast Surg 2015;74:573–579. [DOI] [PubMed] [Google Scholar]
- 59. Chen WF, Yamamoto T, Fisher M, et al. The “octopus” lymphaticovenular anastomosis: evolving beyond the standard supermicrosurgical technique. J Reconstr Microsurg 2015;31:450–457. [DOI] [PubMed] [Google Scholar]
- 60. Yamamoto T, Yoshimatsu H, Yamamoto N, et al. Multisite lymphaticovenular anastomosis using vein graft for uterine cancer-related lymphedema after pelvic lymphadenectomy. Vasc Endovascular Surg 2015;49:195–200. [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto T, Chen WF, Yamamoto N, et al. Technical simplification of the supermicrosurgical side-to-end lymphaticovenular anastomosis using the parachute technique. Microsurgery 2015;35:129–134. [DOI] [PubMed] [Google Scholar]
- 62. Seki Y, Yamamoto T, Yoshimatsu H, et al. The superior-edge-of-the-knee incision method in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg 2015;136:665e. [DOI] [PubMed] [Google Scholar]
- 63. Mihara M, Hara H, Tange S, et al. Multisite lymphaticovenular bypass using supermicrosurgery technique for lymphedema management in lower lymphedema cases. Plast Reconstr Surg 2016;138:262–272. [DOI] [PubMed] [Google Scholar]
- 64. Masia J, Pons G, Nardulli ML. Combined surgical treatment in breast cancer-related lymphedema. J Reconstr Microsurg 2016;32:16–27. [DOI] [PubMed] [Google Scholar]
- 65. Ito R, Wu CT, Lin MCY, et al. Successful treatment of early-stage lower extremity lymphedema with side-to-end lymphovenous anastomosis with indocyanine green lymphography assisted. Microsurgery 2016;36:310–315. [DOI] [PubMed] [Google Scholar]
- 66. Shih HB, Shakir A, Nguyen DH. Use of indocyanine green-SPY angiography for tracking lymphatic recovery after lymphaticovenous anastomosis. Ann Plast Surg 2016;76:S232. [DOI] [PubMed] [Google Scholar]
- 67. Chen WF, Zhao H, Yamamoto T, et al. Indocyanine green lymphographic evidence of surgical efficacy following microsurgical and supermicrosurgical lymphedema reconstructions. J Reconstr Microsurg 2016;32:688–698. [DOI] [PubMed] [Google Scholar]
- 68. Gennaro P, Gabriele G, Mihara M, et al. Supramicrosurgical lymphatico-venular anastomosis (LVA) in treating lymphoedema: 36-months preliminary report. Eur Rev Med Pharmacol Sci 2016;20:4642–4653. [PubMed] [Google Scholar]
- 69. Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73–79. [DOI] [PubMed] [Google Scholar]
- 70. De Brucker B, Zeltzer A, Seidenstuecker K, et al. Breast cancer-related lymphedema: quality of life after lymph node transfer. Plast Reconstr Surg 2016;137:1673–1680. [DOI] [PubMed] [Google Scholar]
- 71. Ciudad P, Maruccia M, Socas J, et al. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: technique and outcomes. Microsurgery 2017;37:197–205. [DOI] [PubMed] [Google Scholar]
- 72. Ciudad P, Manrique OJ, Date S, et al. Double gastroepiploic vascularized lymph node tranfers to middle and distal limb for the treatment of lymphedema. Microsurgery 2017;37:771–779. [DOI] [PubMed] [Google Scholar]
- 73. Coriddi M, Wee C, Meyerson J, et al. Vascularized jejunal mesenteric lymph node transfer: a novel surgical treatment for extremity lymphedema. J Am Coll Surg 2017;225:650–657. [DOI] [PubMed] [Google Scholar]
- 74. Cornelissen AJM, Kool M, Lopez Penha TR, et al. Lymphatico-venous anastomosis as treatment for breast cancer-related lymphedema: a prospective study on quality of life. Breast Cancer Res Treat 2017;163:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nguyen AT, Suami H, Hanasono MM, et al. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J Surg Oncol 2017;115:84–89. [DOI] [PubMed] [Google Scholar]
- 76. Gratzon A, Schultz J, Secrest K, et al. Clinical and psychosocial outcomes of vascularized lymph node transfer for the treatment of upper extremity lymphedema after breast cancer therapy. Ann Surg Oncol 2017;24:1475–1481. [DOI] [PubMed] [Google Scholar]
- 77. Inbal A, Teven CM, Chang DW. Latissimus dorsi flap with vascularized lymph node transfer for lymphedema treatment: technique, outcomes, indications and review of literature. J Surg Oncol 2017;115:72–77. [DOI] [PubMed] [Google Scholar]
- 78. Smith ML, Molina BJ, Dayan E, et al. Heterotopic vascularized lymph node transfer to the medial calf without a skin paddle for restoration of lymphatic function: proof of concept. J Surg Oncol 2017;115:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Poumellec MA, Foissac R, Cegarra-Escolano M, et al. Surgical treatment of secondary lymphedema of the upper limb by stepped microsurgical lymphaticovenous anastomoses. Breast Cancer Res Treat 2017;162:219–224. [DOI] [PubMed] [Google Scholar]
- 80. Gennaro P, Gabriele G, Salini C, et al. Our supramicrosurgical experience of lymphaticovenular anastomosis in lymphoedema patients to prevent cellulitis. Eur Rev Med Pharmacol Sci 2017;21:674–679. [PubMed] [Google Scholar]
- 81. Winters H, Tielemans HJP, Hameeteman M, et al. The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res Treat 2017;165:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gentileschi S, Servillo M, Albanese R, et al. Lymphatic mapping of the upper limb with lymphedema before lymphatic supermicrosurgery by mirroring of the healthy limb. Microsurgery 2017;37:881–889. [DOI] [PubMed] [Google Scholar]
- 83. Akita S, Tokumoto H, Yamaji Y, et al. Contribution of simultaneous breast reconstruction by deep inferior epigastric artery perforator flap to the efficacy of vascularized lymph node transfer in patients with breast cancer-related lymphedema. J Reconstr Microsurg 2017;33:571–578. [DOI] [PubMed] [Google Scholar]
- 84. Arrivé L, Derhy S, Dlimi C, et al. Noncontrast magnetic resonance lymphography for evaluation of lymph node transfer for secondary upper limb lymphedema. Plast Reconstr Surg 2017;140:806e. [DOI] [PubMed] [Google Scholar]
- 85. Gustafsson J, Chu SY, Chan WH, et al. Correlation between quantity of transferred lymph nodes and outcome in vascularized submental lymph node flap transfer for lower limb lymphedema. Plast Reconstr Surg 2018;142:1056–1063. [DOI] [PubMed] [Google Scholar]
- 86. Ho OA, Lin CY, Pappalardo M, et al. Comparisons of submental and groin vascularized lymph node flaps transfer for breast cancer-related lymphedema. Plast Reconstr Surg Glob Open 2018;6:e1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seki Y, Kajikawa A, Yamamoto T, et al. Single lymphaticovenular anastomosis for early-stage lower extremity lymphedema treated by the superior-edge-of-the-knee incision method. Plast Reconstr Surg Glob Open 2018;6:e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Asuncion MO, Chu SY, Huang YL, et al. Accurate prediction of submental lymph nodes using magnetic resonance imaging for lymphedema surgery. Plast Reconstr Surg Glob Open 2018;6:e1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Engel H, Lin CY, Huang JJ, et al. Outcomes of lymphedema microsurgery for breast cancer-related lymphedema with or without microvascular breast reconstruction. Ann Surg 2018;268:1076–1083. [DOI] [PubMed] [Google Scholar]
- 90. Akita S, Yamaji Y, Tokumoto H, et al. Improvement of the efficacy of vascularized lymph node transfer for lower-extremity lymphedema via a prefabricated lympho-venous shunt through lymphaticovenular anastomosis between the efferent lymphatic vessel and small vein in the elevated vascularized lymph node. Microsurgery 2018;38:270–277. [DOI] [PubMed] [Google Scholar]
- 91. Hayashi A, Hayashi N, Yoshimatsu H, et al. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J Surg Oncol 2018;117:290–298. [DOI] [PubMed] [Google Scholar]
- 92. Agko M, Ciudad P, Chen HC. Staged surgical treatment of extremity lymphedema with dual gastroepiploic vascularized lymph node transfers followed by suction-assisted lipectomy – a prospective study. J Surg Oncol 2018;117:1148–1156. [DOI] [PubMed] [Google Scholar]
- 93. Kenworthy EO, Nelson JA, Verma R, et al. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J Surg Oncol 2018;117:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu HL, Pang SY, Lee CC, et al. Orthotopic transfer of vascularized groin lymph node flap in the treatment of breast cancer-related lymphedema: clinical results, lymphoscintigraphy findings, and proposed mechanism. J Plast Reconstr Aesthet Surg 2018;71:1033–1040. [DOI] [PubMed] [Google Scholar]
- 95. Visconti G, Salgarello M, Hayashi A. The recipient venule in supermicrosurgical lymphaticovenular anastomosis: flow dynamic classification and correlation with surgical outcomes. J Reconstr Microsurg 2018;34:581–589. [DOI] [PubMed] [Google Scholar]
- 96. Pereira N, Lee YH, Suh Y, et al. Cumulative experience in lymphovenous anastomosis for lymphedema treatment: the learning curve effect on the overall outcome. J Reconstr Microsurg 2018;34:735–741. [DOI] [PubMed] [Google Scholar]
- 97. Salgarello M, Mangialardi ML, Pino V, et al. A prospective evaluation of health-related quality of life following lymphaticovenular anastomosis for upper and lower extremities lymphedema. J Reconstr Microsurg 2018;34:701–707. [DOI] [PubMed] [Google Scholar]
- 98. Khan AA, Hernan I, Adamthwaite JA, et al. Feasibility study of combined dynamic imaging and lymphaticovenous anastomosis surgery for breast cancer-related lymphoedema. BJS Br J Surg 2019;106:100–110. [DOI] [PubMed] [Google Scholar]
- 99. Chung JH, Baek SO, Park HJ, et al. Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema. Arch Plast Surg 2019;46:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suzuki Y, Sakuma H, Yamazaki S. Comparison of patency rates of lymphaticovenous anastomoses at different sites for lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord 2019;7:222–227. [DOI] [PubMed] [Google Scholar]
- 101. Hadiwattage S, Watawana L, Peiris M, et al. Impact of lymphovenous anastomosis on limb circumference in patients with lymphoedema tarda. J Lymphoedema 2019;14:29–31; Wounds International. [Google Scholar]
- 102. Seki Y, Kajikawa A, Yamamoto T, et al. The dynamic-lymphaticovenular anastomosis method for breast cancer treatment-related lymphedema: Creation of functional lymphaticovenular anastomoses with use of preoperative dynamic ultrasonography. J Plast Reconstr Aesthet Surg 2019;72:62–70. [DOI] [PubMed] [Google Scholar]
- 103. Maruccia M, Pezzolla A, Nacchiero E, et al. Efficacy and early results after combining laparoscopic harvest of double gastroepiploic lymph node flap and active physiotherapy for lower extremity lymphedema. Microsurgery 2019;39:679–687. [DOI] [PubMed] [Google Scholar]
- 104. Maruccia M, Elia R, Ciudad P, et al. Postmastectomy upper limb lymphedema: combined vascularized lymph node transfer and scar release with fat graft expedites surgical and patients’ related outcomes. A retrospective comparative study. J Plast Reconstr Aesthet Surg 2019;72:892–901. [DOI] [PubMed] [Google Scholar]
- 105. Leppäpuska IM, Suominen E, Viitanen T, et al. Combined surgical treatment for chronic upper extremity lymphedema patients: simultaneous lymph node transfer and liposuction. Ann Plast Surg 2019;83:308–317. [DOI] [PubMed] [Google Scholar]
- 106. Ho OA, Chu SY, Huang YL, et al. Effectiveness of vascularized lymph node transfer for extremity lymphedema using volumetric and circumferential differences. Plast Reconstr Surg Glob Open 2019;7:e2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Johnson AR, Bravo MG, Granoff MD, et al. Flow-through omental flap for vascularized lymph node transfer: a novel surgical approach for delayed lymphatic reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hara H, Mihara M. Postoperative changes in lymphoscintigraphic findings after lymphaticovenous anastomosis. Ann Plast Surg 2019;83:548–552. [DOI] [PubMed] [Google Scholar]
- 109. Hara H, Mihara M. Multi-area lymphaticovenous anastomosis with multi-lymphosome injection in indocyanine green lymphography: a prospective study. Microsurgery 2019;39:167–173. [DOI] [PubMed] [Google Scholar]
- 110. AlJindan FK, Lin CY, Cheng MH. Comparison of outcomes between side-to-end and end-to-end lymphovenous anastomoses for early-grade extremity lymphedema. Plast Reconstr Surg 2019;144:486–496. [DOI] [PubMed] [Google Scholar]
- 111. Aljaaly HA, Fries CA, Cheng MH. Dorsal wrist placement for vascularized submental lymph node transfer significantly improves breast cancer-related lymphedema. Plast Reconstr Surg Glob Open 2019;7:e2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yasunaga Y, Yanagisawa D, Ohata E, et al. Bioelectrical impedance analysis of water reduction in lower-limb lymphedema by lymphaticovenular anastomosis. J Reconstr Microsurg 2019;35:306–314. [DOI] [PubMed] [Google Scholar]
- 113. Visconti G, Tartaglione G, Bartoletti R, et al. Compartimental harvesting of dual lymph node flap from the right supraclavicular area for the treatment of lower extremity lymphedema: a case series. J Plast Reconstr Aesthet Surg 2019;72:211–215. [DOI] [PubMed] [Google Scholar]
- 114. Winters H, Tielemans HJP, Verhulst AC, et al. The long-term patency of lymphaticovenular anastomosis in breast cancer-related lymphedema. Ann Plast Surg 2019;82:196–200. [DOI] [PubMed] [Google Scholar]
- 115. Phillips GSA, Gore S, Ramsden A, et al. Lymphaticovenular anastomosis improves quality of life and limb volume in patients with secondary lymphedema after breast cancer treatment. Breast J 2019;25:859–864. [DOI] [PubMed] [Google Scholar]
- 116. Phillips GSA, Gore S, Ramsden A, et al. Lymphaticovenular anastomosis in the treatment of secondary lymphoedema of the legs after cancer treatment. J Plast Reconstr Aesthet Surg 2019;72:1184–1192. [DOI] [PubMed] [Google Scholar]
- 117. Montag E, Okada AY, Arruda EGP, et al. Influence of vascularized lymph node transfer (VLNT) flap positioning on the response to breast cancer-related lymphedema treatment. Rev Colégio Bras Cir 2019;46:e2156. [DOI] [PubMed] [Google Scholar]
- 118. Dionyssiou D, Demiri E, Sarafis A, et al. Functional lymphatic reconstruction with the “Selected Lymph Node” technique guided by a SPECT-CT lymphoscintigraphy. J Surg Oncol 2019;120:911–918. [DOI] [PubMed] [Google Scholar]
- 119. Gentileschi S, Albanese R, Pino V, et al. SPECT/CT and fusion ultrasound to target the efferent groin lymph node for lymphatic surgery. Microsurgery 2019;39:605–612. [DOI] [PubMed] [Google Scholar]
- 120. Klingelhoefer E, Hesse K, Taeger CD, et al. Factors affecting outcomes after supermicrosurgical lymphovenous anastomosis in a defined patient population. Clin Hemorheol Microcirc 2019;73:53–63. [DOI] [PubMed] [Google Scholar]
- 121. Scaglioni MF, Uyulmaz S, Arvanitakis M, et al. Intraoperatively detected but previously indocyanine green-negative lymphatic vessels may have misprized potentials and should not be neglected in lymphaticovenous bypass surgery. Ann Plast Surg 2019;83:69–72. [DOI] [PubMed] [Google Scholar]
- 122. Di Taranto G, Chen SH, Elia R, et al. Free gastroepiploic lymph nodes and omentum flap for treatment of lower limb ulcers in severe lymphedema: killing two birds with one stone. J Surg Oncol 2020;121:168–174. [DOI] [PubMed] [Google Scholar]
- 123. Lin CY, Liu HE, Cheng MH. Factors associated with professional healthcare advice seeking in breast cancer–related lymphedema. J Surg Oncol 2020;121:67–74. [DOI] [PubMed] [Google Scholar]
- 124. Yoshida S, Koshima I, Imai H, et al. Characteristics and outcomes of lymphaticovenular anastomosis in older patients with bilateral involvement versus younger patients with unilateral involvement in lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord 2020;8:646–657. [DOI] [PubMed] [Google Scholar]
- 125. Yoshida S, Koshima I, Imai H, et al. Lymphovenous anastomosis for morbidly obese patients with lymphedema. Plast Reconstr Surg Glob Open 2020;8:e2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yasunaga Y, Yanagisawa D, Nakajima Y, et al. Water reductive effect of lymphaticovenular anastomosis on upper-limb lymphedema: bioelectrical impedance analysis and comparison with lower-limb lymphedema. J Reconstr Microsurg 2020;36:660–666. [DOI] [PubMed] [Google Scholar]
- 127. Yang JCS, Wu SC, Lin WC, et al. Supermicrosurgical lymphaticovenous anastomosis as alternative treatment option for moderate-to-severe lower limb lymphedema. J Am Coll Surg 2020;230:216–227. [DOI] [PubMed] [Google Scholar]
- 128. Qiu SS, Pruimboom T, Cornelissen AJM, et al. Outcomes following lymphaticovenous anastomosis (LVA) for 100 cases of lymphedema: results over 24-months follow-up. Breast Cancer Res Treat 2020;184:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wolfs JAGN, de Joode LGEH, van der Hulst RRWJ, et al. Correlation between patency and clinical improvement after lymphaticovenous anastomosis (LVA) in breast cancer-related lymphedema: 12-month follow-up. Breast Cancer Res Treat 2020;179:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Manrique OJ, Bustos SS, Kapoor T, et al. Gastroepiploic vascularized lymph node transfer for the treatment of extremity lymphedema: comparison between middle and distal inset. Gland Surg 2020;9:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kristiansen M, Halle M, Pignatti M, et al. Evaluation and selection of lower limb lymphedema patients for lymphaticovenular anastomosis: a prospective study. Injury 2020;51:S108–S113. [DOI] [PubMed] [Google Scholar]
- 132. Koide S, Lin CY, Chen C, et al. Long-term outcome of lower extremity lymphedema treated with vascularized lymph node flap transfer with or without venous complications. J Surg Oncol 2020;121:129–137. [DOI] [PubMed] [Google Scholar]
- 133. Ngo QD, Munot S, Mackie H, et al. Vascularized lymph node transfer for patients with breast cancer-related lymphedema can potentially reduce the burden of ongoing conservative management. Lymphat Res Biol 2020;18:357–364. [DOI] [PubMed] [Google Scholar]
- 134. Mousavi SR, Akbari ME, Zarrintan S. Vascularized gastroepiploic lymph node transfer significantly improves breast cancer-related lymphedema. J Surg Oncol 2020;121:163–167. [DOI] [PubMed] [Google Scholar]
- 135. Tzou CHJ, Steinbacher J, Czedik‐Eysenberg M, et al. Institutionalization of reconstructive lymphedema surgery in Austria—single center experience. J Surg Oncol 2020;121:91–99. [DOI] [PubMed] [Google Scholar]
- 136. Beederman M, Garza R, Agarwal S, et al. Outcomes for physiologic microsurgical treatment of secondary lymphedema involving the extremity. Ann Surg 2020;276:e255–e263. [DOI] [PubMed] [Google Scholar]
- 137. Akita S, Yamaji Y, Tokumoto H, et al. Prevention of venous reflux with full utilization of venoplasty in lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg 2020;73:537–543. [DOI] [PubMed] [Google Scholar]
- 138. Chang EI, Ibrahim A, Liu J, et al. Optimizing quality of life for patients with breast cancer-related lymphedema: a prospective study combining DIEP flap breast reconstruction and lymphedema surgery. Plast Reconstr Surg 2020;145:676e–685e. [DOI] [PubMed] [Google Scholar]
- 139. Ciudad P, Manrique OJ, Bustos SS, et al. Combined microvascular breast and lymphatic reconstruction with deep inferior epigastric perforator flap and gastroepiploic vascularized lymph node transfer for postmastectomy lymphedema patients. Gland Surg 2020;9:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ciudad P, Manrique OJ, Bustos SS, et al. Comparisons in long-term clinical outcomes among patients with upper or lower extremity lymphedema treated with diverse vascularized lymph node transfer. Microsurgery 2020;40:130–136. [DOI] [PubMed] [Google Scholar]
- 141. Bianchi A, Visconti G, Hayashi A, et al. Ultra-high frequency ultrasound imaging of lymphatic channels correlates with their histological features: a step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg 2020;73:1622–1629. [DOI] [PubMed] [Google Scholar]
- 142. Onoda S, Nishimon K. The utility of surgical and conservative combination therapy for advanced stage lymphedema. J Vasc Surg Venous Lymphat Disord 2021;9:234–241. [DOI] [PubMed] [Google Scholar]
- 143. Drobot A, Bez M, Abu Shakra I, et al. Microsurgery for management of primary and secondary lymphedema. J Vasc Surg Venous Lymphat Disord 2021;9:226–233 e1. [DOI] [PubMed] [Google Scholar]
- 144. Cha HG, Oh TM, Cho MJ, et al. Changing the paradigm: lymphovenous anastomosis in advanced stage lower extremity lymphedema. Plast Reconstr Surg 2021;147:199–207. [DOI] [PubMed] [Google Scholar]
- 145. Akita S, Yamaji Y, Tokumoto H, et al. Correlation of the changes in physical activity and clinical results following lymphatic microsurgery. Microsurgery 2021;41:44–49. [DOI] [PubMed] [Google Scholar]
- 146. Yoshida S, Koshima I, Imai H, et al. Lymphaticovenous anastomosis for age-related lymphedema. J Clin Med 2021;10:5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yoshida S, Koshima I, Imai H, et al. Modified intraoperative distal compression method for lymphaticovenous anastomosis with high success and a low venous reflux rates. J Plast Reconstr Aesthet Surg 2021;74:2050–2058. [DOI] [PubMed] [Google Scholar]
- 148. Yodrabum N, Khaogate K, Chaikangwan I, et al. Lymphaticovenular anastomosis for patients with lymphedema of the upper extremity at Siriraj Hospital: a quantitative analysis study. J Med Assoc Thai 2021;104:620–628. [Google Scholar]
- 149. Yang JCS, Wu SC, Hayashi A, et al. Lower limb lymphedema patients can still benefit from supermicrosurgical lymphaticovenous anastomosis (LVA) after vascularized lymph node flap transfer (VLNT) as delayed lymphatic reconstruction—a retrospective cohort study. J Clin Med 2021;10:3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sutherland A, Wagner JL, Korentager S, et al. Is bioimpedance spectroscopy a useful tool for objectively assessing lymphovenous bypass surgical outcomes in breast cancer-related lymphedema? Breast Cancer Res Treat 2021;186:1–6. [DOI] [PubMed] [Google Scholar]
- 151. Schiltz D, Kiermeier N, Müller K, et al. Quality of life evaluation and lack of correlation with volumetric results after lymphovenous anastomoses in lymphedema therapy of the lower extremity. J Vasc Surg Venous Lymphat Disord 2022;10:436–444 e1. [DOI] [PubMed] [Google Scholar]
- 152. Schaverien MV, Asaad M, Selber JC, et al. Outcomes of vascularized lymph node transplantation for treatment of lymphedema. J Am Coll Surg 2021;232:982–994. [DOI] [PubMed] [Google Scholar]
- 153. Roka-Palkovits J, Lin MCY, Tzou CHJ, et al. Retrograde manual lymphatic drainage following vascularized lymph node transfer to distal recipient sites for extremity lymphedema: a retrospective study and literature review. Plast Reconstr Surg 2021;148:425e. [DOI] [PubMed] [Google Scholar]
- 154. Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305–1314. [DOI] [PubMed] [Google Scholar]
- 155. Rannikko EH, Suominen SH, Saarikko AM, et al. Long-term results of microvascular lymph node transfer: correlation of preoperative factors and operation outcome. Plast Reconstr Surg Glob Open 2021;9:e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kwon HR, Hwang JH, Mun GH, et al. Predictive role of lymphoscintigraphy undergoing lymphovenous anastomosis in patients with lower extremity lymphedema: a preliminary study. BMC Med Imaging 2021;21:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kim HO, Woo KJ, Kim BS, et al. Lymphoscintigraphic findings as indicators of lymphaticovenous anastomosis outcome in patients with extremity lymphedema: a retrospective cohort study. Clin Nucl Med 2021;46:549–555. [DOI] [PubMed] [Google Scholar]
- 158. Hara H, Mihara M. Lymphaticovenous anastomosis for advanced-stage lower limb lymphedema. Microsurgery 2021;41:140–145. [DOI] [PubMed] [Google Scholar]
- 159. Dionyssiou D, Sarafis A, Tsimponis A, et al. Long-term outcomes of lymph node transfer in secondary lymphedema and its correlation with flap characteristics. Cancers 2021;13:6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chung J-H, Hwang Y-J, Park S-H, et al. Preliminary outcomes of combined surgical approach for lower extremity lymphedema: supraclavicular lymph node transfer and lymphaticovenular anastomosis. J Plast Surg Hand Surg 2021;56:261–269. [DOI] [PubMed] [Google Scholar]
- 161. Brahma B, Putri RI, Reuwpassa JO, et al. Lymphaticovenular anastomosis in breast cancer treatment-related lymphedema: a short-term clinicopathological analysis from Indonesia. J Reconstr Microsurg 2021;37:643–654. [DOI] [PubMed] [Google Scholar]
- 162. Yasunaga Y, Kinjo Y, Nakajima Y, et al. Impact of magnetic resonance lymphography on lymphaticolvenular anastomosis for lower-limb lymphedema. J Reconstr Microsurg 2022;38:121–128. [DOI] [PubMed] [Google Scholar]
- 163. Yang JCS, Wu SC, Hayashi A, et al. Selection of optimal functional lymphatic vessel cutoff size in supermicrosurgical lymphaticovenous anastomosis in lower extremity lymphedema. Plast Reconstr Surg 2022;149:237–246. [DOI] [PubMed] [Google Scholar]
- 164. van Mulken TJM, Wolfs JAGN, Qiu SS, et al. One-year outcomes of the first human trial on robot-assisted lymphaticovenous anastomosis for breast cancer-related lymphedema. Plast Reconstr Surg 2022;149:151–161. [DOI] [PubMed] [Google Scholar]
- 165. Moon KC, Kim HK, Lee TY, et al. Vascularized lymph node transfer for surgical treatments of upper versus lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord 2022;10:170–178. [DOI] [PubMed] [Google Scholar]
- 166. Cheng MH, Huang JJ, Nguyen DH, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol 2012;126:93–98. [DOI] [PubMed] [Google Scholar]
- 167. Keeley V, Crooks S, Locke J, et al. A quality of life measure for limb lymphoedema (LYMQOL). J Lymphoedema 2010;5:26–37. [Google Scholar]
- 168. Chang DW, Dayan J, Greene AK, et al. Surgical treatment of lymphedema: a systematic review and meta-analysis of controlled trials. Results of a consensus conference. Plast Reconstr Surg 2021;147:975–993. [DOI] [PubMed] [Google Scholar]
- 169. Heyland DK, Wibbenmeyer L, Pollack JA, et al. A randomized trial of enteral glutamine for treatment of burn injuries. N Engl J Med 2022;387:1001–1010. [DOI] [PubMed] [Google Scholar]
- 170. van Zanten ARH, Dhaliwal R, Garrel D, et al. Enteral glutamine supplementation in critically ill patients: a systematic review and meta-analysis. Crit Care 2015;19:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Guiotto M, Bramhall RJ, Campisi C, et al. A systematic review of outcomes after genital lymphedema surgery: microsurgical reconstruction versus excisional procedures. Ann Plast Surg 2019;83:e85–e91. [DOI] [PubMed] [Google Scholar]
- 172. Carl HM, Walia G, Bello R, et al. Systematic review of the surgical treatment of extremity lymphedema. J Reconstr Microsurg 2017;33:412–425. [DOI] [PubMed] [Google Scholar]
- 173. Jarvis NR, Torres RA, Avila FR, et al. Vascularized omental lymphatic transplant for upper extremity lymphedema: a systematic review. Cancer Rep 2021;4:e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nacchiero E, Maruccia M, Elia R, et al. Lymphovenous anastomosis for the treatment of lymphedema: a systematic review of the literature and meta-analysis. Lymphology 2020;53:172–194. [PubMed] [Google Scholar]
- 175. Scaglioni MF, Fontein DBY, Arvanitakis M, et al. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017;37:947–953. [DOI] [PubMed] [Google Scholar]
- 176. Kleinhans E, Rg B, Dietbert Hahn, et al. Evaluation of transport kinetics in lymphoscintigraphy: follow-up study in patients with transplanted lymphatic vessels. Eur J Nucl Med 1985;10:349–52. [DOI] [PubMed] [Google Scholar]
- 177. Maccauro M, Villa G, Manzara A, et al. Linfogammagrafía para la evaluación de trastornos del flujo linfático de las extremidades: informe de estándares de procedimientos técnicos de un panel de expertos de medicina nuclear italiano. Rev Esp Med Nucl E Imagen Mol 2019;38:335–340. [DOI] [PubMed] [Google Scholar]
- 178. Villa G, Cc C, R M, et al. Procedural recommendations for lymphoscintigraphy in the diagnosis of peripheral lymphedema: the Genoa Protocol. Nucl Med Mol Imaging 2019;53:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sykehuset Telemark. Supermicrosurgery for Breast Cancer Survivors With Lymphedema. -Prospective Randomized Clinical and Patient-Reported Outcomes. clinicaltrials.gov; 2020.
- 180. M.D. Anderson Cancer Center. Prophylactic Lymphovenous Bypass Procedure Following Axillary Lymphadenectomy in Patients With Inflammatory Breast Cancer: A Prospective, Randomized Study. clinicaltrials.gov; 2020.
- 181. Universitaire Ziekenhuizen Leuven. Comparison of Reconstructive Lymphatic Surgery Versus No Surgery, Additional to Decongestive Lymphatic Therapy (Usual Care), for the Treatment of Iymphoedema, Through a Multicentre Randomised Controlled Trial. clinicaltrials.gov; 2021.
- 182. Memorial Sloan Kettering Cancer Center. A Prospective Study Comparing Quality of Life in Patients With Lymphedema Who Undergo Surgical Treatment Versus Non-Surgical Management. clinicaltrials.gov; 2021.


