Abstract
A functional RNA replication promoter for the paramyxovirus simian virus 5 (SV5) requires two essential and discontinuous elements: 19 bases at the 3′ terminus (conserved region I) and an 18-base internal region (conserved region II [CRII]) that is contained within the coding region of the L protein gene. A reverse-genetics system was used to determine the sequence requirements for the internal CRII element to function in RNA replication. A series of copyback defective interfering (DI) RNA analogs were constructed to contain point mutations in the 18 nucleotides composing CRII, and their relative replication levels were analyzed. The results indicated that SV5 DI RNA replication was reduced by substitutions for two CG dinucleotides, which in the nucleocapsid template are in the first two positions of the first two hexamers of CRII nucleotides. Substitutions for other bases within CRII did not reduce RNA synthesis. Thus, two consecutive 5′-CGNNNN-3′ hexamers form an important sequence in the SV5 CRII promoter element. The position of the CG dinucleotide within the SV5 leader and antitrailer promoters was highly conserved among other members of the Rubulavirus genus, but this motif differed significantly in both sequence and position from that previously identified for Sendai virus. The possible roles of the CRII internal promoter element in paramyxovirus RNA replication are discussed.
cis-acting sequences within a viral genome are important signals for directing the functions of a viral polymerase. In the case of the rhabdoviruses and paramyxoviruses, the relationship between viral RNA sequences and the polymerase functions that they modulate is particularly complex, because the viral polymerase recognizes the cis-acting signals in the viral RNA only when they are in the context of the nucleocapsid. The leader RNA at the 3′ terminus of the genome and the antitrailer at the 3′ terminus of the antigenome contain signals directing paramyxovirus replication (3, 11, 12), but features of these regions that are important for promoter activity are not completely understood.
The paramyxoviruses are a diverse family of nonsegmented minus-sense RNA viruses that includes Sendai virus (SeV), measles virus (MeV), and respiratory syncytial virus (RSV). In the family Paramxyoviridae, these viruses are prototypes of the respiroviruses, morbilliviruses, and pneumoviruses, respectively. Simian virus 5 (SV5) is a prototype of the Rubulavirus genus that also includes mumps virus (MuV), simian virus 41 (SV41), and human parainfluenza virus type 2 (HPIV2). While many general aspects of viral RNA replication are shared among these virus groups, significant differences in their requirements for cis-acting sequences have become evident through sequence analysis and the use of reverse genetics (reviewed in references 3, 4, and 11).
Our previous results have shown that a functional SV5 antigenomic promoter requires two discontinuous elements, conserved region I (CRI), comprising the 3′-terminal 19 nucleotides, and conserved region II (CRII), located 73 to 90 bases from the 3′ end of the antigenome (20). The CRII element is located in the 3′ coding region of the L protein gene (1, 20). SV5 RNA replication is very sensitive to changes in the spacing between CRI and CRII (20). Our experimental results have indicated that CRI and CRII are separated by an RNA segment which functions, at least in part, as a sequence-independent spacer region (20). In the work described here, we have analyzed the sequence-specific requirements for the internal promoter element CRII.
We have previously developed an experimental system whereby cDNA-derived SV5 polymerase components are used to direct in vivo replication of copyback defective interfering (DI) RNA analogs (20). The copyback DI RNA analog DI113-GL-90 encodes a 966-base antigenome with 113 5′-terminal and 90 3′-terminal SV5-specific bases flanking the Green Lantern (GL) open reading frame which serves as a nonviral reporter sequence (in the minus sense [Fig. 1A]). In pDI113-GL-90, bases 91 to 96 from the 3′ end of the genome were previously modified to a StuI site (20). To introduce nucleotide substitutions into CRII, the plasmid bearing DI113-GL-90 was used as a template in a PCR with Pwo polymerase (Boehringer Mannheim, Indianapolis, Ind.) along with oligonucleotide primers specific for the SP6 promoter in pGem and a mutagenic primer as described previously (24). The resulting PCR products were digested with StuI and SphI and ligated into the corresponding sites of pDI113-GL-90, which are located at bases 91 to 96 and in the multiple cloning site 3′ to CRI. The sequence of PCR-derived DNA segments was confirmed by nucleotide sequence analysis. DNA plasmids encoding the altered copyback DI RNAs were flanked on the 3′ and 5′ ends by the hepatitis delta virus ribozyme (28) and the T7 polymerase promoter (20, 25, 26), respectively.
FIG. 1.
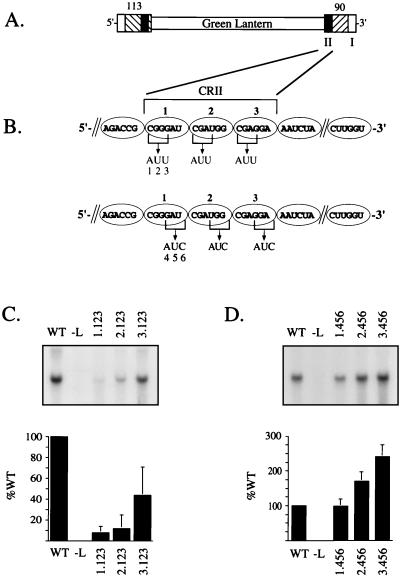
Mutational analysis of the SV5 internal promoter element CRII. (A) The DI113-GL-90 copyback DI RNA analog is shown schematically, with the crosshatched boxes representing terminal bases from the SV5 antigenome. The locations of CRI (I) and CRII (II) and the numbers of SV5 terminal bases are indicated. (B) An expanded listing of CRII sequences (bases 90 to 73 from the 3′ end of the DI RNA analog) is shown. Each oval represents one NP monomer binding 6 nucleotides. The positions of nucleotides within CRII are designated according to the NP monomer (1, 2, and 3) and their positions within the NP-bound hexamer (1 through 6, in a 5′-to-3′ direction). Nucleotide changes that replace the CGR in the 1.123, 2.123, and 3.123 positions with AUU and those that replace the 1.456, 2.456, and 3.456 positions with AUC are indicated. (C and D) Results from replication of SV5 GL DI RNA analogs containing alterations in the first three (C) or last three (D) positions within each CRII hexamer. vTF7.3-infected A549 cells were cotransfected with the SV5 L, P, and NP plasmids along with DNA encoding the indicated SV5 GL DI RNA. Total intracellular RNA was analyzed by Northern blotting with a 32P-labeled minus-sense GL-specific riboprobe. Lane −L represents a sample from cells in which the L plasmid was replaced with pGem control DNA. Relative replication levels are expressed as a percentage (plus standard deviation) of the level determined for the DI113-GL-90 DI RNA (WT) analyzed in parallel from three independent experiments. Note that the scales of the y axis differ for panels C and D.
A549 cells in 3.5-cm-diameter dishes were infected (multiplicity of infection, ∼5) for 1 h with vTF7.3 (8) and then cotransfected with DNA encoding the SV5 DI RNA analogs (1.0 μg), along with pGEM3-L (2.0 μg), pGEM2-P (0.2 μg), and pUC19-NP (2.0 μg), by using a cationic liposome reagent (31). Total intracellular RNA was analyzed by Northern blotting with a 32P-labeled minus-sense GL-specific riboprobe that was of the same polarity as the GL sequence within the vacT7 pol-derived RNA analogs, in order to monitor conversion of the SV5 GL RNA to the complementary strand by the SV5 polymerase (20). Blots were quantitated with the AMBIS 4000 image acquisition system and software (AMBIS, San Diego, Calif.).
For SeV, structural analysis based on electron micrograph images has indicated that each NP molecule binds to six nucleotides along the encapsidated viral RNA (7). When the 3′-terminal 90 bases from the SV5 antigenome were grouped as hexamers of nucleotides, the residues in CRII formed a repeated C-G-purine (CGR) motif (Fig. 1). This motif occurred in the first three positions of each of the three consecutive CRII hexamers (5′-CGGGAU CGAUGG CGAGAA-3′ [Fig. 1]). For nomenclature purposes, the position of a nucleotide within CRII is designated by a number indicating the NP-bound hexamer followed by the position within each hexamer (e.g., the CGG motif within the sequence 5′-CGGGAU-3′ is designated 1.123).
To determine if the CGR motif was important for SV5 RNA replication, three SV5 GL minigenomes were constructed such that the three CGR motifs were individually altered to AUU (Fig. 1B). As shown in Fig. 1C, SV5 GL minigenomes containing substitutions for the CGR motifs of the first and second hexamers (1.123 and 2.123, respectively) directed ∼10-fold-lower RNA replication than the wild-type (WT) genome, while substitutions in the corresponding position of the third hexamer (3.123) reduced replication by only ∼2-fold. To determine if the primary sequence in positions 4, 5, and 6 of the hexamers was important for efficient replication, a second set of minigenomes was prepared in which the last three (nonconserved) nucleotides of each hexamer (positions 4 to 6) were changed to AUC. As shown in Fig. 1D, the replication of a minigenome containing the GAU-to-AUC mutation in the first hexamer (1.456) was equivalent to that of the WT genome. Surprisingly, DI RNA analogs with alterations in the second (2.456) and third (3.456) hexamers gave replication levels that were actually increased ∼2-fold over that seen with the WT genome. These results indicate that the CGR motif found in the first three positions of the CRII hexamers is important for efficient SV5 RNA replication, while substitutions in the remaining sequences (positions 4, 5, and 6) do not have a negative influence on RNA synthesis.
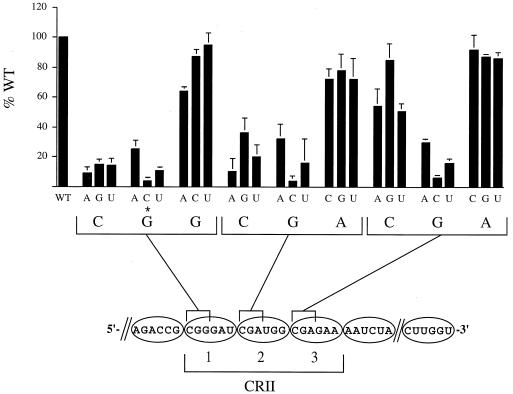
To determine the contribution to replication of each individual nucleotide within the three CGR motifs, 27 SV5 GL minigenomes were constructed such that the bases in each of the first three positions of the CRII hexamers were converted to the three alternative nucleotides (Fig. 2). RNA replication was analyzed for each of the individual SV5 GL minigenomes as described in the legend to Fig. 1, and the results were expressed as a percentage of the WT replication analyzed in parallel. On the basis of the results shown in Fig. 2, three main conclusions can be drawn from this analysis. First, RNA replication was most sensitive to substitutions for the G residue normally found in the second position of each hexamer. For all three of the CRII hexamers, substitutions of a C for this conserved G residue reduced replication >20-fold. Second, replacing the C in the first position of CRII hexamers 1 and 2 resulted in an ∼3- to 10-fold reduction in RNA replication. By contrast, replication was reduced only twofold or less by substitutions for the C residue of the first position of the third hexamer, indicating that this residue was less sensitive to sequence changes. The initial hypothesis was that a purine was required in the third position of the hexamers. However, substitutions in the third position had little effect on RNA replication, and there was no apparent preference for purine or pyrimidine in these positions. These data indicate that the sequence-specific requirements for the SV5 CRII promoter element consist of a CG sequence motif that is in the first two positions of three consecutive hexamers of bases. It is noteworthy that of the 18 nucleotides comprising CRII, the second nucleotide position (G residue) within the three hexamers of nucleotides is the only one strictly conserved among rubulaviruses and between the SV5 leader and trailer regions (20).
FIG. 2.
Saturation mutagenesis of the CGR motif in CRII. SV5 GL DI RNA analogs harboring individual point mutations within the repeated CGR motif were analyzed by Northern blotting as described in the legend to Fig. 1. A schematic representation of CRII is shown below the graph, with the positions of the three CGR motifs indicated as 1, 2, and 3 in the bracket. An asterisk indicates the G-to-C mutation in the second position of the first hexamer that creates a CG motif with an altered position within the NP hexamer. Relative replication levels are the mean of three experiments and are expressed as a percentage (plus standard deviation) of the level determined for the DI113-GL-90 (WT) antigenome analyzed in parallel.
The results shown in Fig. 2 also indicate that spacing between the CG repeats is important for replication. This is evident in the case of the point mutant containing a C in place of the G in the second position of the first hexamer (Fig. 2). This substitution recreates a CG dinucleotide which is now in the second and third positions of the hexamer instead of the normal first and second. It is not known if this reduced replication reflects a requirement for spacing between the individual CG repeats or for a particular position of the motif with respect to NP binding.
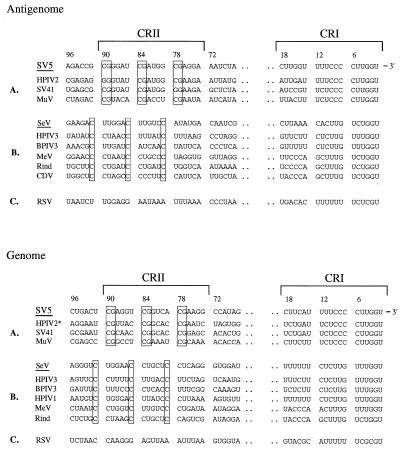
A sequence alignment was generated for the CRI and CRII elements of the Rubulavirus, Morbillivirus, Respirovirus, and Pneumovirus genus members (Fig. 3). For the rubulaviruses, the CG dinucleotide repeat in the first two positions of three consecutive hexamers was highly conserved between bases 73 and 90 of both the antigenomic and the genomic RNAs (Fig. 3, panels A). For the SV41 antigenomic promoter, a G is found in the first position of the third CRII hexamer, consistent with our mutational analysis (Fig. 2), showing that for SV5 this is the least disruptive of the substitutions for the first C residue. For HPIV2, a G is found in place of the C which normally occupies the first position of the first rubulavirus CRII hexamer. This suggests that the stringency of the requirement for the 5′-CGNNNN-3′ motif may vary even among closely related rubulaviruses. In addition, for SV5, HPIV2, and SV41, there is a G residue (base 95 [Fig. 3]) in the second position of the hexamer located immediately 5′ to CRII (bases 91 to 96). It is unclear if this G residue also contributes to the activity of the SV5 antigenomic promoter.
FIG. 3.
Sequence alignment of the Paramyxoviridae antigenomic and genomic CRII and CRI. The antigenome and genome CRII and CRI sequences are listed (5′ to 3′) for members of the Rubulavirus genus (SV5, HPIV2, SV41, and MuV), Respirovirus genus (SeV, HPIV3, BPIV3, and HPIV1), Morbillivirus genus (MeV, rinderpest virus [Rind], and canine distemper virus [CDV]), and Pneumovirus genus (RSV). Boxes indicate the position of the SV5 CG dinucleotides and the SeV C residues that have been shown experimentally to be important for RNA replication. Sequences are from the work of Tapparel et al. (36) or from the work of the following groups or individuals: for SV5, Parks et al. (23); for HPIV2, Kawano et al. (9) and Yuasa et al. (37); for SV41, Ogawa et al. (21); for MuV, Okazaki et al. (22); for MeV, Blumberg et al. (1); for CDV, Sidhu et al. (34, 35); for SeV, HPIV3, and BPIV3, Tapparel et al. (36); for HPIV1, Matsuoka and Ray (15) and Rochat et al. (30); and for RSV, Collins (3) and Mink et al. (16). ∗, genomic sequences were shifted one nucleotide toward the 5′ terminus to optimize the alignment.
For SeV copyback DI genomes, a previous analysis showed that RNA replication required a (5′-GNNNNN-3′)3 motif (shown as the nascent RNA strand) located 79 to 96 bases from the 5′ termini of the antigenomic and genomic RNAs (36). For ease of comparison with our results for SV5, the SeV motif is referred to as 5′-NNNNNC-3′, corresponding to the sequence of the template strand. As shown in Fig. 3 (panels B), the CG motif that we have shown to be important for SV5 replication was not found in the corresponding position for SeV and the related respiroviruses or morbilliviruses. Instead, a conserved C residue spaced every sixth nucleotide was evident, and the importance of this motif for SeV RNA replication has been reported (36). Alignment of these same regions from the prototype Pneumovirus RSV showed no identity with the SV5- or SeV-like motifs (Fig. 3, panels C). Thus, the requirements in the CRII element for SV5 and SeV RNA replication differ in several regards, including the sequence (CG for SV5 and C for SeV), the position of essential bases within an NP hexamer (the first and second bases for SV5 and the sixth for SeV), and the distance of the critical CRII bases from the 3′ end of the viral RNA (bases 73 to 90 for SV5 and 79 to 96 for SeV). Furthermore, SeV RNA replication shows an absolute requirement for all three of the conserved C residues in CRII (36), whereas SV5 RNA synthesis appears to be somewhat less sensitive to point mutations in the CG motif.
Two factors that can influence the level of RNA synthesized from the paramyxovirus antigenomic promoter have been described previously: the total number of nucleotides in the viral RNA and the primary nucleotide sequence at the termini of the viral RNA. The length of a paramyxovirus RNA can be a major factor determining the level of RNA replication, with genome replication being most efficient when the total number of nucleotides is an even multiple of six (2). This rule of six is thought to reflect a requirement that the 3′-end nucleotides of the template be precisely encapsidated (2) or that critical cis-acting sequences (e.g., CRII) can be recognized only when they are in the correct positions within their encapsidating NP monomers (phasing [11, 27]). The degree to which replication of a particular paramyxovirus genome adheres to the rule-of-six requirement differs among viruses. For SeV the rule of six is an apparent strict requisite (2), while there is no particular replicative advantage to RSV genome analogs having any of the integer genome lengths tested (32). SV5 and HPIV3 are unique in this regard, since RNA replication is clearly most efficient for 6N-length genomes, but the stringency of the rule of six for these viruses is intermediate between the stringencies found previously for SeV and RSV (6, 19). Thus, the presence of the SV5- or the SeV-like CRII motifs does not correlate with the degree to which these viruses adhere to the rule of six (e.g., SeV and HPIV3 [Fig. 3]).
A second major factor directing the efficiency of paramyxovirus RNA replication is the primary sequence at the termini of the viral RNA. A previous analysis has shown that the antigenomic promoter for the paramyxovirus SV5 contains two essential sequence-specific elements (CRI and CRII) separated by an RNA segment which functions in part as a sequence-independent spacer region (20). Our mutational analysis has shown that the SV5 internal promoter element CRII consists of a relatively simple CG motif located in the first two positions of three sequential hexamers of nucleotides. The importance of CRII bases 73 to 96 to rubulavirus, respirovirus, and morbillivirus RNA replication is apparent by the structure of all naturally occurring defective genomes of these viruses, in which at least 100 consecutive terminal bases are retained (13, 17, 19, 29, 33). We recently reported an apparent exception to the need for conserving CRII sequences in the case of a naturally occurring SV5 copyback DI RNA genome (19). DI698 is a 698-base copyback DI RNA which contains only 80 consecutive 3′-terminal bases, since the crossover junction (linkage between polymerase fall-off and reentry sites) occurred in the middle of CRII. Inspection of the DI698 crossover junction revealed that the fusion of L protein gene sequences had created a pseudo-CRII region (5′-UGGCGCGAUAUCGAUGG/CGAGGAAAUCUA-3′, in which the backslash is the crossover junction). Spacing requirements between the individual SV5 CG repeats have not been thoroughly investigated, and it is possible that this pseudo-CRII element can function in directing RNA replication.
We have previously identified a third factor that can dramatically influence the activity of the paramyxovirus antigenomic promoter: the relative spacing of CRI and CRII (20). Therefore, three features of the viral RNA template that can significantly influence paramyxovirus RNA synthesis are the overall number of nucleotides in a viral RNA (rule of six), the primary nucleotide sequence of two regions of the viral RNA (CRI and CRII), and the proper spacing of CRI and CRII.
The essential internal CRII motif has been proposed to function as a nucleation site for RNA encapsidation (36) or as a site for viral polymerase binding (20). During paramyxovirus replication, encapsidation of the nascent chain by NP is tightly coupled to RNA synthesis (12, 18), and it is possible that CRII functions as a nucleation site on the nascent RNA to initiate NP encapsidation. While formally possible, theoretical considerations are inconsistent with this proposed function. First, the current model for the switch from RNA transcription to replication proposes that NP binding to the nascent chain prevents the viral polymerase from terminating at the leader-NP junction (12), a site located ∼55 bases from the 3′ end of the genome. Presumably, this decision would be made prior to the synthesizing of nascent RNA that contains CRII by polymerase, since this region is located 73 to 96 bases from the 3′ end of the genome (within the NP gene). Second, SV5 and SeV RNA replication are very sensitive to changes in the length of the RNA segment located between CRI and CRII (20, 36). Based on this experimental result, it is difficult to envision a mechanism whereby encapsidation of the RNA chain depends so strongly on the proper spacing between CRI and CRII.
We favor the alternative hypothesis that CRII and CRI may function on the nucleocapsid template to create a binding site for polymerase-associated proteins (20). Previous structural analyses of the SeV nucleocapsid showed that each turn of the left-handed helical nucleocapsid consisted of 13 NP molecules on average, with each NP binding 6 nucleotides for a total of 78 nucleotides per turn of the nucleocapsid (7). Assuming that the SV5 nucleocapsid has similar properties, we have proposed a hypothetical model which predicts that the CRI and CRII are aligned to the same face of the helical nucleocapsid (20). This model is consistent with our previous experimental results showing a strict requirement for the proper spacing between CRI and CRII (20).
It has been proposed that the paramyxovirus P protein functions as a homotrimer (5) and that six P proteins (two homotrimers) are associated with each L protein on the nucleocapsid. It is intriguing to speculate that the three CG dinucleotides within the SV5 CRII element represent P protein binding sites. Given the repeated pyrimidine motif that is also found within the three hexamers of the SV5 3′-terminal CRI (5′-CUUGGU UUUCCC CUUGGU-3′, with repeats underlined [Fig. 3]), it is possible that a second P trimer may also bind to CRI. Thus, six P molecules may bind as two homotrimers to CRI and CRII, which map to the same face of the helical nucleocapsid (20). Lin et al. (14) have shown that the SV5 P and V proteins bind to RNA via a region spanning amino acids 74 to 81, which are rich in basic residues. In the case of the rhabdovirus vesicular stomatitis virus, previous work has indicated that the P (NS) protein makes sequence-specific contacts within the leader region of the genome (10), and it is possible that the same holds true for the SV5 P protein. The proposed models for the function of CRII (and CRI) in encapsidation and polymerase binding are not mutually exclusive. Given the propensity for viruses to employ multifunctional proteins, it is conceivable that the internal promoter segment CRII has dual functions in polymerase binding and in encapsidation of the nascent RNA.
Acknowledgments
We thank John Rassa and Doug Lyles for constructive criticism.
This work was supported by NIH grant AI42023. Oligonucleotide synthesis was performed in the DNA Synthesis Core Laboratory of the Cancer Center of Wake Forest University, supported in part by NIH grant CA-12197.
REFERENCES
- 1.Blumberg B M, Chan J, Udem S A. Function of paramyxovirus 3′ and 5′ end sequences. In theory and practice. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 235–247. [Google Scholar]
- 2.Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins P. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 4.Conzelmann K-K. Genetic manipulation of non-segmented negative-strand RNA viruses. J Gen Virol. 1996;77:381–389. doi: 10.1099/0022-1317-77-3-381. [DOI] [PubMed] [Google Scholar]
- 5.Curran J, Boeck R, Lin-Marq N, Lupas A, Kolakofsky D. Paramyxovirus phosphoproteins form homotrimers as determined by an epitope dilution assay, via predicted coiled coils. Virology. 1995;214:139–149. doi: 10.1006/viro.1995.9946. [DOI] [PubMed] [Google Scholar]
- 6.Durbin A P, Siew J W, Murphy B R, Collins P L. Minimum protein requirements for transcription and RNA replication of a minigenome of human parainfluenza virus type 3 and evaluation of the rule of six. Virology. 1997;234:74–83. doi: 10.1006/viro.1997.8633. [DOI] [PubMed] [Google Scholar]
- 7.Egelman E H, Wu S-S, Amrein M, Portner A, Murti G. The Sendai virus nucleocapsid exists in at least four different helical states. J Virol. 1989;63:2233–2243. doi: 10.1128/jvi.63.5.2233-2243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano M, Okamoto K, Bando H, Kondo K, Tsurudome M, Komada H, Nishio M, Ito Y. Characterizations of the human parainfluenza type 2 virus gene encoding the L protein and the intergenic sequences. Nucleic Acids Res. 1991;19:2739–2746. doi: 10.1093/nar/19.10.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keene J D, Thornton B J, Emerson S U. Sequence-specific contacts between the RNA polymerase of vesicular stomatitis virus and the leader RNA gene. Proc Natl Acad Sci USA. 1981;78:6191–6195. doi: 10.1073/pnas.78.10.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1177–1204. [Google Scholar]
- 13.Leppert M, Kort L, Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977;12:539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- 14.Lin G Y, Paterson R G, Lamb R A. The RNA binding region of the paramyxovirus SV5 V and P proteins. Virology. 1997;238:460–469. doi: 10.1006/viro.1997.8866. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka Y, Ray R. Sequence analysis and expression of the human parainfluenza type 1 virus nucleoprotein gene. Virology. 1991;181:403–407. doi: 10.1016/0042-6822(91)90514-c. [DOI] [PubMed] [Google Scholar]
- 16.Mink M, Stec D, Collins P. Nucleotide sequences of the 3′ leader and 5′ trailer regions of human respiratory syncytial virus genomic RNA. Virology. 1991;185:615–624. doi: 10.1016/0042-6822(91)90532-g. [DOI] [PubMed] [Google Scholar]
- 17.Mottet G, Roux L. Budding efficiency of Sendai virus nucleocapsids: influence of size and ends of the RNA. Virus Res. 1989;14:175–188. doi: 10.1016/0168-1702(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 18.Moyer S A, Horikami S M. The role of viral and host cell proteins in paramyxovirus transcription and replication. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 249–274. [Google Scholar]
- 19.Murphy S K, Parks G D. Genome nucleotide lengths that are divisible by six are not essential but enhance replication of defective interfering RNAs of the paramyxovirus simian virus 5. Virology. 1997;232:145–157. doi: 10.1006/viro.1997.8530. [DOI] [PubMed] [Google Scholar]
- 20.Murphy S K, Ito Y, Parks G D. A functional antigenomic promoter for the paramyxovirus simian virus 5 requires proper spacing between an essential internal segment and the 3′ terminus. J Virol. 1998;72:10–19. doi: 10.1128/jvi.72.1.10-19.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa M, Noriko M, Tsurudome M, Kawano M, Matsumura H, Kusagawa S, Komada H, Nishio M, Ito Y. Nucleotide sequence analysis of the simian virus 41 gene encoding the large (L) protein and construction of a phylogenetic tree for the L proteins of paramyxoviruses. J Gen Virol. 1992;73:2743–2750. doi: 10.1099/0022-1317-73-10-2743. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki K, Tanabayashi K, Takeuchi K, Hishiyama M, Okazaki K, Yamada A. Molecular cloning and sequence analysis of the mumps virus gene encoding the L protein and the trailer sequence. Virology. 1992;188:926–930. doi: 10.1016/0042-6822(92)90555-4. [DOI] [PubMed] [Google Scholar]
- 23.Parks G D, Ward C D, Lamb R A. Molecular cloning of the NP and L genes of simian virus 5: identification of highly conserved domains in paramyxovirus NP and L proteins. Virus Res. 1992;22:259–279. doi: 10.1016/0168-1702(92)90057-g. [DOI] [PubMed] [Google Scholar]
- 24.Parks G D. Mapping of a region of the paramyxovirus L protein required for the formation of a stable complex with the viral phosphoprotein P. J Virol. 1994;68:4862–4872. doi: 10.1128/jvi.68.8.4862-4872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattnaik A, Ball A, Legrone A, Wertz G. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992;69:1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- 26.Pattnaik A K, Wertz G W. Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs. J Virol. 1990;64:2948–2957. doi: 10.1128/jvi.64.6.2948-2957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelet T, Delenda C, Gubbay O, Garcin D, Kolakofsky D. Partial characterization of a Sendai virus replication promoter and the rule of six. Virology. 1996;224:405–414. doi: 10.1006/viro.1996.0547. [DOI] [PubMed] [Google Scholar]
- 28.Perrotta A T, Been M D. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature (London) 1991;350:434–436. doi: 10.1038/350434a0. [DOI] [PubMed] [Google Scholar]
- 29.Re G. Deletion mutants of paramyxoviruses. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 275–298. [Google Scholar]
- 30.Rochat S, Komada H, Kolakofsky D. Loss of V protein expression in human parainfluenza virus type 1 is not a recent event. Virus Res. 1992;24:137–144. doi: 10.1016/0168-1702(92)90002-q. [DOI] [PubMed] [Google Scholar]
- 31.Rose J K, Buonocore L, Whitt M A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Bio Techniques. 1991;10:520–525. [PubMed] [Google Scholar]
- 32.Samal S K, Collins P L. RNA replication by a respiratory syncytial virus RNA analog does not obey the rule of six and retains a nonviral trinucleotide extension at the leader end. J Virol. 1996;70:5075–5082. doi: 10.1128/jvi.70.8.5075-5082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidhu M, Crowley J, Lowenthal A, Karcher D, Menonna J, Cook S, Udem S, Dowling P. Defective measles virus in human subacute sclerosing panencephalitis brain. Virology. 1994;202:631–641. doi: 10.1006/viro.1994.1384. [DOI] [PubMed] [Google Scholar]
- 34.Sidhu M S, Menonna J P, Cook S D, Dowling P C, Udem S A. Canine distemper virus L gene: sequence and comparison with related viruses. Virology. 1993;193:50–65. doi: 10.1006/viro.1993.1102. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu M S, Husar W, Cook S D, Dowling P C, Udem S A. Canine distemper terminal and intergenic non-protein coding nucleotide sequences: completion of the entire CDV genome sequence. Virology. 1993;193:66–72. doi: 10.1006/viro.1993.1103. [DOI] [PubMed] [Google Scholar]
- 36.Tapparel C, Maurice D, Roux L. The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: a motif (GNNNNN)3 is essential for replication. J Virol. 1998;72:3117–3128. doi: 10.1128/jvi.72.4.3117-3128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuasa T, Bando H, Kawano M, Tsurudome M, Nishio M, Kondo K, Komada H, Ito Y. Sequence analysis of the 3′ genome end and NP gene of human parainfluenza type 2 virus: sequence variation of the gene-starting signal and the conserved 3′ end. Virology. 1990;179:777–784. doi: 10.1016/0042-6822(90)90145-h. [DOI] [PubMed] [Google Scholar]