Background:
Timely and proper intraocular pressure (IOP) management is vital to the prevention of visual impairment in children with primary congenital glaucoma (PCG). Although various surgical interventions have been proposed, no well-founded evidence exists on their comparative efficacies. We aimed to compare the efficacies of surgical interventions for PCG.
Methods:
We searched relevant sources up to 4 April 2022. Randomized controlled trials (RCTs) entailing surgical interventions for PCG in children were identified. A network meta-analysis (NMA) was performed, comparing 13 surgical interventions: Conventional partial trabeculotomy ([CPT] control), 240-degree trabeculotomy, Illuminated microcatheter-assisted circumferential trabeculotomy (IMCT), Viscocanalostomy, Visco-circumferential-suture-trabeculotomy, Goniotomy, Laser goniotomy, Kahook dual blade ab-interno trabeculectomy, Trabeculectomy with mitomycin C, Trabeculectomy with modified scleral bed, Deep sclerectomy, Combined trabeculectomy-trabeculotomy with mitomycin C, and Baerveldt implant. The main outcomes were mean IOP reduction and surgical success rate at postoperative 6 months. The mean differences (MDs) or odds ratios (ORs) were analyzed by a random-effects model, and the efficacies were ranked by P-score. We appraised the RCTs using the Cochrane risk-of-bias (ROB) tool (PROSPERO: CRD42022313954).
Results:
Sixteen RCTs were eligible for NMA, including 710 eyes of 485 participants and 13 surgical interventions, which formed a network of 14 nodes comprising both single interventions and intervention combinations. IMCT was superior to CPT in both IOP reduction [MD (95% CI): −3.10 (−5.50 to −0.69)] and surgical success rate [OR (95% CI): 4.38 (1.61–11.96)]. The MD and OR comparing the other surgical interventions and intervention combinations with CPT were not statistically significant. The P-scores ranked IMCT as the most efficacious surgical intervention in terms of success rate (P-score =0.777). Overall, the trials had a low-to-moderate ROB.
Conclusion:
This NMA indicated that IMCT is more effective than CPT and might be the most efficacious of the 13 surgical interventions for management of PCG.
Keywords: primary congenital glaucoma, surgical intervention, efficacy, randomized clinical trial, network meta-analysis
Introduction
Highlights
The comparative efficacies of surgical interventions for primary congenital glaucoma remain inconclusive.
Illuminated microcatheter-assisted circumferential trabeculotomy was superior to conventional partial trabeculotomy in both intraocular pressure reduction and success rate.
Illuminated microcatheter-assisted circumferential trabeculotomy was ranked as the most efficacious intervention in terms of success rate.
Primary congenital glaucoma-surgical interventions can be ranked by efficacy, and such ranking may be used to facilitate clinical decision making.
Primary congenital glaucoma (PCG) is an optic neuropathy with high intraocular pressure (IOP) characterized by anomalous development of the anterior chamber angle1. PCG accounts for up to 18% of all cases of childhood blindness2–6. This disease’s blinding and progressive nature7 necessitates management that is both timely and proper.
The definitive PCG management approach is surgery, mostly because, for pediatric patients, treatment with medication is poorly tolerated over the long term and less effective than for adults1,8. As PCG’s principal pathology is in the anterior chamber angle, two procedures are generally used: goniotomy or trabeculotomy. Both of these address the issue of an angle anomaly and increase aqueous outflow by directly connecting Schlemm’s canal to the anterior chamber8. Other types of surgical interventions, such as filtering surgery, glaucoma drainage devices, and cyclodestructive procedures, have also been proposed8, with the result that there is considerable heterogeneity in PCG management, even among experts.
Various randomized controlled trials (RCTs) therefore have been done to compare surgical interventions’ efficacies for PCG in pediatric patients9–24. Determining their comparative efficacies remains difficult, however, due specifically to the lack of head-to-head comparisons. Moreover, the current accumulated evidence is limited to pairwise comparisons between specific interventions25 and lacks comprehensive all-interventions comparisons. Consequently, there is still no well-founded evidence supporting a given intervention’s outstanding efficacy for PCG management.
Network meta-analysis (NMA), as an extension of traditional meta-analysis, enables intervention comparison based on not only direct evidence but also indirect evidence (i.e. from interventions that are not directly compared)26. Furthermore, intervention hierarchies can be obtained using valid methods of statistical inference27. Thus motivated, we performed an NMA on RCTs to assess the comparative efficacies of surgical interventions for PCG in pediatric patients.
Methods
The protocol of this systematic review was prospectively registered at PROSPERO (CRD42022313954) https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=313954 and has been published28. This NMA has been reported in accordance with and is fully compliant with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), Supplemental Digital Content 1, http://links.lww.com/JS9/A163, Supplemental Digital Content 2, http://links.lww.com/JS9/A164 202029, and AMSTAR 2, Supplemental Digital Content 3, http://links.lww.com/JS9/A165 (Assessing the methodological quality of systematic reviews) Guidelines30.
Eligibility criteria for present review
RCTs that had compared the efficacies of surgical interventions for PCG in pediatric patients were included. There were no restrictions on any surgical intervention types. Editorials, case reports as well as comments, abstracts, and letters were excluded. Studies that had only compared different application methods (e.g. exposure time and concentration) of the same adjunctive substance such as antimetabolite [e.g. mitomycin C (MMC), 5-fluorouracil] and bevacizumab [e.g. IOP-lowering effect of ‘trabeculectomy (TLE) with MMC application for 2 min’ vs. ‘TLE with MMC application for 4 min’] were also excluded; the reason for the exclusion was that the main purpose of this study was to compare the IOP-lowering effect of the surgical technique itself. In addition, the detailed application methods of adjuncts differed among studies.
Search methods for identification of studies
We systematically searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, PubMed, and EMBASE from inception to 4 April 2022. Our search strategies were developed in collaboration with an academic librarian expert in systematic review, and are based on established terminology such as MESH and EMBASE search terms. The following keywords were included: Congenital, Glaucoma, Surgery, and Children. We also screened the WHO International Clinical Trials Registry Platform, clinicaltrials.gov, and references from published papers to identify additional relevant studies. No language-based restrictions were imposed on our electronic searches. The complete search strategies are available in Supplementary Appendix 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A166.
Study selection
To identify pertinent articles, the titles and abstracts of those retrieved were exported to Endnote (version X9; Thomson Reuters), wherein duplicates were removed. The remaining titles and abstracts were assessed by two investigators (D.K./Y.J.L.) independently for eligibility, and for the eligible ones, the relevant full-text articles were retrieved. Then, the same two investigators independently assessed those articles for final eligibility. Eligibility classification discrepancies were resolved through discussion, consensus, or, when needed, third-party (Y.K.K.) adjudication.
Data collection and risk-of-bias assessment
For each of the trials, two individuals (D.K./A.H.) independently extracted data and then entered it (electronic format) into Microsoft Access 2016 (Microsoft Corporation). Any conflicting data entries were identified using an algorithm. The trial characteristics of interest were: (1) study ID (name of first author, year of publication), (2) country of study, (3) length of follow-up, (4) inclusion of participants with history of surgery, (5) surgical interventions, (6) number of eyes (participants), (7) baseline mean age, (8) baseline mean IOP, (9) postoperative 6-month and 1-year mean IOP reduction, and (10) surgical success rate.
Trial quality was evaluated using a revised tool for assessment of risk-of-bias (ROB) in randomized trials (RoB 2)27. The five domains of bias evaluated were as follows: randomization process, adherence to assigned interventions, missing outcome data, outcome measurement bias, and reported-results bias. Each domain was rated as having a low ROB, ‘some concerns’ or a high ROB. Any domain’s worst ROB was used to determine the overall ROB. In the evaluation, we referred to the Cochrane Database of Systematic Reviews25 for previously published articles’ contents that had been confirmed through communication with the authors. ROB was assessed by two investigators (Y.J.L./Y.K.K.) independently, with any discrepancies being resolved via discussion.
Definitions used in categorization of surgical interventions
To improve interpretability and, thereby, support decision making, we grouped the surgical intervention arms into the 13 categories that follow: (1) Conventional partial trabeculotomy ([CPT] control), (2) 240-degree trabeculotomy (240° trabeculotomy), (3) Illuminated microcatheter-assisted circumferential trabeculotomy (IMCT), (4) Viscocanalostomy (VC), (5) Visco-circumferential-suture-trabeculotomy (VCST), (6) Goniotomy, (7) Neodymium-YAG laser goniotomy (laser goniotomy), (8) Kahook dual blade ab-interno trabeculectomy (KDB trabeculectomy), (9) Trabeculectomy with mitomycin C (TM), (10) Trabeculectomy with modified scleral bed (TmS), (11) Deep sclerectomy (DS), (12) Combined trabeculectomy-trabeculotomy with mitomycin C (CTTM), and (13) Baerveldt implant (Table 1). Each surgical intervention is described in detail in Supplementary Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A166.
Table 1.
Classifications, types, and designated terms of surgical interventions.
| Classification | Type | Designated term |
|---|---|---|
| Angle surgery | Conventional partial trabeculotomy | CPT |
| 204-degree trabeculotomy | 240° trabeculotomy | |
| Illuminated microcatheter-assisted circumferential trabeculotomy | IMCT | |
| Viscocanalostomy | VC | |
| Visco-circumferential-suture-trabeculotomy | VCST | |
| Goniotomy | Goniotomy | |
| Neodymium-YAG laser goniotomy | Laser goniotomy | |
| Kahook dual blade ab-interno trabeculectomy | KDB trabeculectomy | |
| Filtering surgery | Trabeculectomy with mitomycin C | TM |
| Trabeculectomy with modified scleral bed | TmS | |
| Deep sclerectomy | DS | |
| Combined surgery | Combined trabeculectomy-trabeculotomy with mitomycin C | CTTM |
| Glaucoma drainage device | Baerveldt implant | Baerveldt implant |
Outcomes
The primary outcome measure was the amount of mean IOP reduction at 6 months postoperatively; in a large number of RCTs on PCG surgical interventions, the postoperative observation period was 6 months (as indicated in Table 2), and so the standard for a primary outcome was set at 6 months for this study. When comparing interventions A and B, stated IOP values representing intervention A/B difference were compared; a negative mean difference (MD), therefore, indicated the superiority of intervention A (i.e. a lower IOP). Results of intention-to-treat analyses were extracted preferentially. If postoperative 6-month data were not available, we adopted the data that was closest in terms of time point.
Table 2.
Characteristics of studies included in meta-analysis.
| References | Country | Follow-up duration (months) | Inclusion of participants with history of surgery | Surgical interventions (study arm) | Number of eyes (participants) randomized | Baseline mean age (months) | Baseline mean IOP (mmHg) | Postoperative 6-month mean IOP reduction (mmHg) | Postoperative 1-year mean IOP reduction (mmHg) | Surgical success rate (%) a |
|---|---|---|---|---|---|---|---|---|---|---|
| Elwehidy et al. 9 | Egypt | 36 | No | VCST | 84 (49) | 4.8 (2.1) | 29.1 (3.3) | 17.9 (3.0) | 17.7 (3.0) | 94.6 |
| CPT+VC | 4.9 (1.7) | 29.9 (3.2) | 18.0 (2.8) | 17.1 (2.8) | 87.8 | |||||
| Elhilali et al. 10 | Egypt | 12 | No | KDB trabeculectomy | 42 (29) | 9.1 (9.6) | 24.4 (6.8) | 10.1 (6.0) | 12.6 (6.0) | 57.1 b |
| Goniotomy | 6.3 (3.9) | 23.1 (3.7) | 9.9 (3.3) | 10.3 (3.3) | 57.1 b | |||||
| Elwehidy et al. 11 | Egypt | 60 | No | CPT+VC | 154 (92) | 5.0 (2.3) | 26.5 (2.9) | 15.0 (2.5) | 14.4 (2.5) | 89.7 |
| CPT | 5.0 (2.9) | 27.9 (3.1) | 16.2 (2.7) | 15.2 (2.7) | 85.5 | |||||
| Bor’i et al. 12 | Egypt | 14 (13–22) c | No | TmS | 50 (25) | 2.5 (0.5) | 31.6 (4.9) | 18.6 (4.3) | 16.1 (4.3) | 84.0 |
| TM | 2.5 (0.5) | 32.1 (4.0) | 17.1 (3.5) | 15.0 (3.6) | 88.0 | |||||
| Wagdy13 | Egypt | 12 | Yes | 240° trabeculotomy | 30 (30) | 14.1 (2.3) | 28.2 (1.7) | 14.0 (1.9) | 14.3 (1.8) | 93.3 d |
| CTTM | 14.2 (3.1) | 28.1 (3.5) | 13.5 (3.1) | 13.5 (3.1) | 86.7 d | |||||
| Rolim-de-Moura et al. 14 | Brazil | 12 | Yes | Baerveldt implant | 13 (13) | 40.8 (31.7) | 22.8 (5.9) | 10.6 (6.4) | 10.6 (5.2) | 100.0 |
| CTTM | 28.6 (17.7) | 23.7 (7.3) | 8.6 (8.0) | 8.1 (6.7) | 100.0 | |||||
| El Sayed et al. 15 | Egypt | 24 | No | IMCT | 62 (62) | 5.6 (4.8) | 25.1 (6.4) | 13.3 (5.7) | 13.2 (5.5) | 89.3 |
| CPT | 4.4 (3.8) | 22.3 (5.2) | 7.9 (5.1) | 9.5 (4.8) | 56.3 | |||||
| Shakrawal et al. 16 | India | 12 | No | IMCT | 40 (31) | 6.5 (3.9) | 24.7 (3.9) | 14.7 (3.5) | 15.2 (3.4) | 90.0 |
| CPT | 10.2 (5.4) | 24.6 (3.3) | 12.2 (2.9) | 12.9 (2.9) | 70.0 | |||||
| Khalil et al. 17 | Egypt | 36 | No | CPT | 28 (28) | 6.5 (3.9) | 24.1 (1.9) | 12.7 (4.2) | 14.1 (1.8) | 85.7 d |
| CTTM | 5.6 (4.0) | 24.1 (1.8) | 11.6 (4.5) | 13.6 (1.8) | 85.7 d | |||||
| Temkar et al. 18 | India | 12 | No | IMCT | 60 (30) | 6.6 (5.7) | 21.8 (9.8) | 10.4 (8.9) | 10.2 (8.6) | 93.3 |
| CTTM | 6.6 (5.7) | 21.7 (8.9) | 10.7 (8.0) | 10.1 (7.8) | 93.3 | |||||
| ElSheikha et al. 19 | Egypt | 6 | Yes | CPT+VC | 41 (31) | 6.8 (6.5) | 23.5 (5.4) | 6.1 (6.4) | NA | 66.7 |
| CPT | 6.9 (5.7) | 24.3 (4.4) | 6.4 (6.4) | NA | 60.0 | |||||
| Bayoumi20 | Egypt | 12 | No | CTTM | 20 (20) | 4.7 (2.0) | 16.7 (4.3) | 10.9 (4.0) | 11.8 (3.7) | 100 b |
| CTTM+DS | 7.0 (3.8) | 16.4 (8.4) | 10.9 (7.4) | 10.8 (7.3) | 100 b | |||||
| Reddy et al. 21 | India | 6 | No | CTTM | 32 (18) | <24.0 | 24.9 (6.8) | 9.0 (5.9) | NA | 75.0 |
| TM | <24.0 | 27.3 (4.6) | 12.3 (5.0) | NA | 81.3 | |||||
| Noureddin et al. 22 | Lebanon | 12 | No | CPT | 16 (8) | 3.4 (4.1) | 34 (2.6) | 20.5 (4.2) | 18.4 (3.8) e | NA |
| VC | 3.4 (4.1) | 32.3 (4.1) | 17.2 (5.8) | 19.4 (4.0) e | NA | |||||
| Senft et al. 23 | Saudi Arabia | 9.5 (2-15) c | Yes | Goniotomy | 20 (10) | 5.7 (3.9) | 28.4 (4.6) | 4.8 (7.8) f | NA | 40.0b |
| Laser goniotomy | 5.7 (3.9) | 29.5 (11.0) | 6.4 (10.1) f | NA | 40.0 b | |||||
| Anderson24 | USA | 34 | No | Goniotomy | 18 (9) | <9.0 | NA | NA g | NA | 66.6 b |
| CPT | <9.0 | NA | NA g | NA | 66.6 b |
Data on age, IOP, and surgical success rate were rounded to one decimal place, if applicable.
CPT, conventional partial trabeculotomy; CTTM, combined trabeculectomy-trabeculotomy with mitomycin C; DS, deep sclerectomy; IMCT, illuminated microcatheter-assisted circumferential trabeculotomy; IOP, intraocular pressure; KDB, Kahook dual blade; NA, not available; TM, trabeculectomy with mitomycin C; TmS, trabeculectomy with modified scleral bed; VC, viscocanalostomy; VCST, visco-circumferential-suture-trabeculotomy.
Adopted data for the closest point in time to postoperative 6 months in cases where their data are not available.
No distinction between complete (absolute) and qualified success.
Average follow-up duration (range).
Complete (absolute) surgical success rate.
Adopted postoperative 16-month IOP data due to lack of 1-year data.
Adopted IOP data following surgical intervention (measurement period not specified).
Adopted average IOP value for all of the other included CPT studies’ postoperative 6-month data due to lack of data (applied identical values for both interventions, considering their identical surgical success rates).
The secondary outcome measure was the surgical success rate at 6 months postoperatively, as defined based on each study’s definition (e.g. proportion of eyes showing IOP equal to or less than a given value, without any signs of glaucoma progression or serious visual complications). All of the definitions can be found in Supplementary Table 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A166. We applied qualified success rates to the analysis; in cases where such data were not available, we used the complete (absolute) values instead. Also, if no distinction between complete (absolute) and qualified success was provided, we applied the reported surgical success rate.
Data synthesis
We compared the effects of the competing surgical interventions on the primary outcome (i.e. postoperative 6-month mean IOP reduction) according to the MD with 95% CIs. As for the secondary outcome (i.e. postoperative 6-month surgical success rate), the odds ratio (OR) was calculated by dividing intervention group 1’s success proportion by that of intervention group 2. To combine direct evidence with indirect evidence, an NMA was performed with the R software package ‘netmeta’ (version 4.0.4; The R Foundation), which applies a frequentist method based on a graph-theoretical approach according to electrical network theory31,32. The ‘netmeta’ function takes within-study correlation into account by reweighting, using the Laplacian matrix and its pseudoinverse, all of each multiarm study’s comparisons based on back-calculation of variances33. Because the included studies were small in number and heterogeneous, we applied random-effects models34.
Data analysis
We ranked the interventions by P-score, which is the most frequent analog of the surface under the cumulative ranking curve (SUCRA)32. The P-score, as valued between 0 and 1, is the probability that a certain treatment is among the best ones27,35.
We assessed the cross-study heterogeneity of effect estimates and the study heterogeneity effects on the pooled effect estimate using Q statistics and I 2 statistics, respectively36,37. Inconsistency (i.e. nonagreement of direct with indirect intervention effects)38 was evaluated using Separating Indirect from Direct Evidence (SIDE; a.k.a. node-splitting)39. We assessed NMA-estimate confidence by a semiautomated web application [Confidence in Network Meta-analysis (CINeMA); Institute of Social and Preventive Medicine]40,41. A comparison-adjusted funnel plot with an accompanying Egger’s test for asymmetry was used to assess cross-study bias (i.e. publication bias) in NMA42. Statistical significance was recorded for cases where the two-sided α level was less than 0.05.
For the purposes of a sensitivity analysis, we repeated an NMA (1) for the primary outcome by excluding studies that had included patients with a history of previous surgery and (2) for the mean IOP reduction at 1-year postoperatively.
Results
Search results
Our systematic search uncovered 1186 articles, of which 1162 were unique reports; after excluding reports based on scrutiny of titles and abstracts, 58 full-text articles were retrieved. Upon full evaluation of these citations, 16 RCTs, comprising a total of 710 eyes from 485 participants, were deemed to have met the NMA inclusion criteria. Figure 1 is a flowchart of the process of selection for inclusion in our study. The excluded studies along with the rationales for their exclusion are provided in Supplementary Appendix 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A166.
Figure 1.
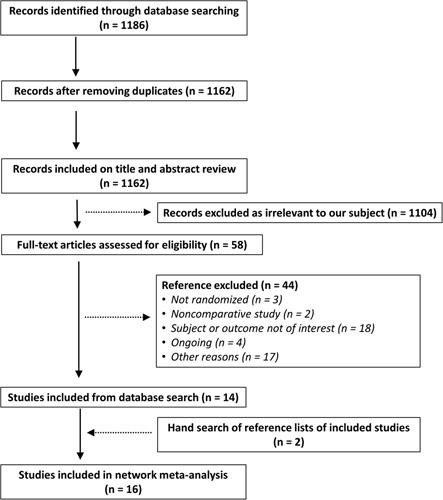
Flow diagram of study selection process for inclusion in network meta-analysis.
Study characteristics
The characteristics of the 16 RCTs included in the NMAs are provided in Table 2. Study duration (i.e. follow-up duration) ranged from 6 to 60 months, and the baseline mean IOP ranged from 16.4 to 34.0 mmHg. Nine studies had been conducted in Egypt9–13,15,17,19,20, three in India16,18,21, and one each in Brazil14, Lebanon22, Saudi Arabia23, and the USA24. Twelve studies9–12,15–18,20–22,24 included only patients lacking any surgical history, whereas four13,14,19,23 also included such patients. A schematic of the ROB assessment across all of the studies included in our analysis is provided in Supplementary Appendix 3, Supplemental Digital Content 4, http://links.lww.com/JS9/A166. Overall, the trials were determined to have a low-to-moderate ROB.
Primary outcome: intraocular pressure reduction
The total of 13 surgical interventions formed a 14-node network of both single interventions and intervention combinations (Fig. 2). As shown in Fig. 3A, IMCT effected a greater IOP reduction than did CPT, when combined in the NMA (MD: −3.10; 95% CI: −5.50 to −0.69, P-score=0.752; Supplementary Appendix 4, Supplemental Digital Content 4, http://links.lww.com/JS9/A166). However, the amounts of IOP reduction of the other 10 single surgical interventions and those of the two surgical intervention combinations were not significantly different from that of CPT, wherein TmS showed the lowest MD, followed by TM, Baerveldt implant, Laser goniotomy, 240° trabeculotomy, CTTM+DS, CTTM, KDB trabeculectomy, Goniotomy, CPT+VC, VCST, and VC (Fig. 3A). For illustration of the head-to-head comparisons, a net league table is provided in the form of Supplementary Figure 1A, Supplemental Digital Content 4, http://links.lww.com/JS9/A166.
Figure 2.
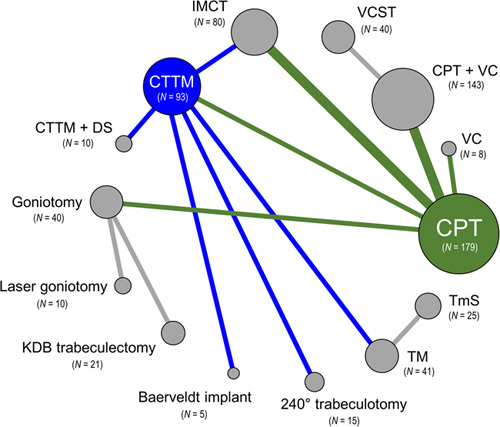
Network plot of primary outcome. Surgical interventions, with direct comparisons, are linked by lines, with the width of the lines being proportional to the number of trials comparing each pair of interventions. The size of each node is proportional to the number of eyes of participants (i.e. sample size) randomly assigned to each intervention. Green lines indicate direct comparisons with conventional partial trabeculotomy (CPT); blue indicates direct comparisons with combined trabeculectomy-trabeculotomy with mitomycin C (CTTM); gray indicates direct comparisons between other interventions. DS, deep sclerectomy; IMCT, illuminated microcatheter‐assisted circumferential trabeculotomy; KDB, Kahook dual blade; TM, trabeculectomy with mitomycin C; TmS, trabeculectomy with modified scleral bed; VC, viscocanalostomy; VCST, visco-circumferential-suture-trabeculotomy.
Figure 3.
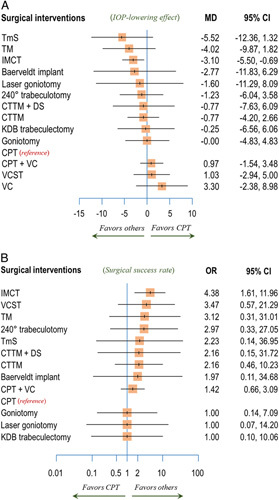
Forest plots of primary and secondary outcomes. (A) Mean intraocular pressure (IOP) reduction at postoperative 6 months. (B) Surgical success rate. Each surgical intervention was compared with conventional partial trabeculotomy (CPT), which was the reference group. CTTM, combined trabeculectomy-trabeculotomy with mitomycin C; DS, deep sclerectomy; IMCT, illuminated microcatheter‐assisted circumferential trabeculotomy; KDB, Kahook dual blade; MD, mean difference; OR, odds ratio; TM, trabeculectomy with mitomycin C; TmS, trabeculectomy with modified scleral bed; VC, viscocanalostomy; VCST, visco-circumferential-suture-trabeculotomy.
Secondary outcome: surgical success rate
Fifteen studies9–21,23,24 had reported success rates, entailing 13 intervention nodes (Supplementary Fig. 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A166). Complete (absolute) success rates were used in two studies13,17 in which qualified success rate data were not available. Also, four studies10,20,23,24 reported success rates without any distinction between complete (absolute) and qualified success. Of the present comparison’s interventions, IMCT showed a significantly higher success rate than that for CPT when combined in the NMA (OR: 4.38; 95% CI: 1.61–11.96; Fig. 3B). However, comparisons of others’ success rates with that of CPT were not statistically significant, with ORs ranging from 1.42 (CPT+VC) to 3.47 (VCST) (Fig. 3B). A net league table representative of the head-to-head comparison is shown in Supplementary Figure 1B, Supplemental Digital Content 4, http://links.lww.com/JS9/A166. According to the P-scores, IMCT (P-score =0.777) was the most efficacious surgical intervention as well (Supplementary Appendix 4, Supplemental Digital Content 4, http://links.lww.com/JS9/A166).
Examination of network model and validity of results
Our network model revealed moderate heterogeneity across studies for primary outcome (I 2=50.6%). Within-design heterogeneity was not significant (P=0.208), though between-design inconsistency was borderline-significant (P=0.087). When a full design-by-treatment random-effects model was assumed, the Q value was low (Q=2.41), and the between-design inconsistency ceased to be significant (P=0.120; Supplementary Table 3, Supplemental Digital Content 4, http://links.lww.com/JS9/A166). The SIDE analysis showed no disagreement (inconsistency) between the direct estimates and the indirect ones (all Ps≥0.05; Supplementary Table 4, Supplemental Digital Content 4, http://links.lww.com/JS9/A166). The comparison-adjusted funnel plot, which evaluated the risk of publication bias incurred in the NMA (Supplementary Fig. 3, Supplemental Digital Content 4, http://links.lww.com/JS9/A166), showed a relatively even distribution, which is to say, no bias to either side. This was corroborated by the Egger’s test, which indicated no significance (P=0.34). These findings, overall, indicated a low probability of small-study effects in the present network model. As for the primary outcome (i.e. IOP reduction), we examined the overall evidence certainty within the all-comparison network, and found it to be widely distributed from very low to moderate (Supplementary Appendix 5, Supplemental Digital Content 4, http://links.lww.com/JS9/A166).
Sensitivity analyses
In our analysis of mean IOP reduction at postoperative 6 months, which excluded studies that had included patients with a surgery history (Fig. 4A), IMCT showed a greater IOP reduction than did CPT, when combined in the NMA (MD: −3.05; 95% CI: −5.99 to −0.12; Fig. 4B, P-score =0.761; Supplementary Appendix 4, Supplemental Digital Content 4, http://links.lww.com/JS9/A166). The amounts of mean IOP reduction of the others, however, were not significantly different from that of CPT, with MDs ranging from −5.60 (TmS) to −0.25 (KDB trabeculectomy) (Fig. 4B). For illustration of head-to-head comparisons, a net league table is provided in the form of Supplementary Figure 4A, Supplemental Digital Content 4, http://links.lww.com/JS9/A166.
Figure 4.
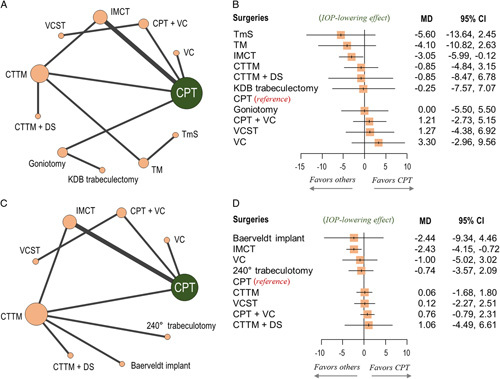
Network plots and forest plots for sensitivity analyses. (A and B) Mean intraocular pressure (IOP) reduction at postoperative 6 months, excluding studies that had included patients with history of previous surgery. (C and D) Mean IOP reduction at postoperative 1 year. In the forest plots, each surgical intervention was compared with conventional partial trabeculotomy (CPT), which was the reference group. CTTM, combined trabeculectomy-trabeculotomy with mitomycin C; DS, deep sclerectomy; IMCT, illuminated microcatheter‐assisted circumferential trabeculotomy; KDB, Kahook dual blade; MD, mean difference; TM, trabeculectomy with mitomycin C; TmS, trabeculectomy with modified scleral bed; VC, viscocanalostomy; VCST, visco-circumferential-suture-trabeculotomy.
A total of 12 studies9–18,20,22 with nine intervention nodes were included in our analysis of mean IOP reduction at postoperative 1 year (Fig. 4C). The IMCT effected a greater IOP reduction than did CPT (MD: −2.43; 95% CI: −4.15 to −0.72), while the others showed no significant differences with that of CPT, with MDs ranging from −2.44 (Baerveldt implant) to −0.74 (240° trabeculotomy) (Fig. 4D). A net league table is provided in the form of Supplementary Figure 4B, Supplemental Digital Content 4, http://links.lww.com/JS9/A166. According to the P-scores, IMCT (P-score = 0.864) was the most efficacious intervention (Supplementary Appendix 4, Supplemental Digital Content 4, http://links.lww.com/JS9/A166).
Discussion
This NMA, including 16 RCTs, represents a comprehensive synthesis of data on the comparative efficacies of different types of surgical interventions for PCG. We found that for pediatric patients with PCG, IMCT is more effective than CPT and that it might be the most efficacious among the total of 13 surgical interventions in terms of both IOP reduction and surgical success rate.
In a traditional pairwise meta-analysis of surgical interventions for PCG, Gagrani et al.25 likewise showed that mean IOP may be lower with IMCT than with CPT at 6 and 12 months. The evidence on the comparative efficacies of the other surgical interventions, however, was limited due to either a complete lack of studies or an insufficient number. A total of only seven studies, comprising three different pairwise comparisons, were included in their analysis, since the comparisons were limited to specific, direct-evidence-based ones. Going beyond this limitation, our NMA enabled both direct and indirect comparisons between interventions, exploiting all available evidence across the network while preserving within-trial randomization.
The results of our NMA revealed that IMCT is more efficacious than CPT in both IOP reduction and surgical success rate, though the reasons have yet to be elucidated. There are two possible explanations. First, a greater extent of angle opening in IMCT than in CPT could lead to a larger amount of aqueous drainage, resulting in greater IOP reduction and a correspondingly higher surgical success rate. Whereas CPT opens the angle partially, usually 100–120°, IMCT effects 360° of circumferential angle opening8. Second, during IMCT, the illuminated microcatheter tip is continuously visible transsclerally throughout the Schlemm’s canal passage. The visibility of the tip might minimize the risk of misdirection and false passage43, thereby contributing to better surgical outcomes.
In the built-up hierarchies of the interventions, IMCT was identified as the most efficacious surgical intervention with regard to surgical success rate, as it was in the sensitivity analyses on mean IOP reduction at 1-year postoperatively. In most of the head-to-head comparisons between IMCT and the others (other than CPT), however, the differences did not reach statistical significance. Therefore, given the evidence gathered to date, the superiority of IMCT over other interventions (other than CPT) is not yet clear and needs further investigation.
Several study limitations merit further discussion. First, the relatively small sample size in each trial might have incurred a small-study effect, which refers to the phenomenon that smaller trials show different, often larger treatment effects than larger ones44. The wide CI in studies with small sample sizes also should be taken into account when interpreting the results. Second, subgroup analyses by characteristics such as ethnicity, geographic location, disease severity, or age of onset were not feasible due to the inaccessibility of individual patient data or an insufficient number of trials. It has been reported that PCG incidence varies greatly with race, ethnicity, and level of consanguinity in the community45. Moreover, the prognosis of children with PCG has been known to differ according to age of onset46,47. Further studies examining other confounding factors are required in order to fully evaluate the comparative efficacies of surgical interventions for PCG. Third, the cross-trial definitions of surgical success rate and follow-up period were inconsistent, and these differences might limit the interstudy comparability. We had attempted to perform subgroup analyses according to the surgical success criteria. However, the number of studies in each subgroup included was relatively small for meaningful analyses. Outcome measures for future research should be standardized so as to improve the comparability of studies. Fourth and finally, studies investigating the effect of adjunct usage itself (e.g. IOP-lowering effect of ‘TLE with MMC’ vs. ‘TLE without MMC’) in surgical intervention could not be included in the final analysis because their study design or participants were not compliant with the current study’s eligibility criteria.
Notwithstanding these limitations, our study is of value, especially in light of the difficulties inherent in performing RCTs on PCG (due to its rarity), the reported incidence of which is known to range from 1 : 10 000 to 1 : 20 000 live births45,48–50 and to be higher in consanguineous populations (1 : 1250 in Slovakian gypsies50, 1 : 2500 in Saudi Arabia51, and 1 : 3300 in Andhra Pradesh, India52). Currently, among practitioners, there is no consensus on the surgical approach to PCG, which uncertainty may hinder their decision making and performance with respect to the optimal treatment modality for each patient. We believe that our NMA could form the basis for the establishment of evidence-based guidelines for the management of PCG.
The findings of the current study indicate several directions for future research. First, larger, multiethnic, and multicountry RCTs with long-term follow-ups are required to further accumulate evidence on the efficacy of surgical interventions for PCG. Second, utilization of a standardized reporting system for surgical outcomes is needed to improve comparability between studies. Third, studies comparing the complications of surgical interventions would also provide valuable information for practitioners on patient management, particularly regarding safety issues. Fourth and finally, investigations gathering evidence on the cost-effectiveness of surgical interventions or the quality of life of patients or caregivers would be worthwhile.
Conclusions
This NMA of RCTs indicated that IMCT is more effective than CPT and, moreover, that it might be the most efficacious of the 13 surgical interventions for PCG. These findings would provide comprehensive evidence for the determination of optimal treatment strategies for PCG in clinical practice.
Ethical approval
Not applicable.
Sources of funding
This study was supported by a National Research Foundation of Korea (NRF) grant (no. NRF-2022R1F1A1064186). The funder had no role in the initiation or design of the study, collection of samples, analysis, interpretation of data, paper writing, or submission for publication. The study and researchers are independent of the funder.
Author contribution
Y.K.K.: concept and design, and obtained funding. Y.J.L.: drafting of the manuscript. Y.J.L., A.H., Y.K.K.: critical revision of the manuscript for important intellectual content. S.R.S., Y.K.K.: statistical analysis. A.H., Y.K.K.: administrative, technical, or material support and supervision. Acquisition, analysis, or interpretation of data done by all authors.
Conflicts of interest disclosure
None declared.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique identifying number or registration ID: CRD42022313954.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022313954.
Guarantor
Young Kook Kim and Ahnul Ha.
Data statement
All data generated or analyzed during this study are included in this article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Ahnul Ha and Young Kook Kim contributed equally to this study as co-corresponding authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 31 March 2023
Contributor Information
Yun Jeong Lee, Email: lyj0603@naver.com.
Ahnul Ha, Email: zzammy486@gmail.com;zzammy486@snu.ac.kr.
Donghwee Kang, Email: tallman67@naver.com.
Sung Ryul Shim, Email: sungryul.shim@gmail.com.
Jin Wook Jeoung, Email: neuroprotect@gmail.com.
Ki Ho Park, Email: kihopark@snu.ac.kr.
Young Kook Kim, Email: md092@naver.com;eyedry@snu.ac.kr.
References
- 1. Stamper RL, Lieberman MF, Drake MV. Stamper RL, Lieberman MF, Drake MV. Developmental and childhood glaucoma. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas, 8th ed. Mosby Elsevier; 2009:294–329. [Google Scholar]
- 2. Franks W, Taylor D. Congenital glaucoma — a preventable cause of blindness. Arch Dis Child 1989;64:649–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert CE, Canovas R, Kocksch de Canovas R, et al. Causes of blindness and severe visual impairment in children in Chile. Dev Med Child Neurol 1994;36:326–333. [DOI] [PubMed] [Google Scholar]
- 4. Haddad MA, Sei M, Sampaio MW, et al. Causes of visual impairment in children: a study of 3,210 cases. J Pediatr Ophthalmol Strabismus 2007;44:232–240. [DOI] [PubMed] [Google Scholar]
- 5. Sitorus RS, Abidin MS, Prihartono J. Causes and temporal trends of childhood blindness in Indonesia: study at schools for the blind in Java. Br J Ophthalmol 2007;91:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorairaj SK, Bandrakalli P, Shetty C, et al. Childhood blindness in a rural population of southern India: prevalence and etiology. Ophthalmic Epidemiol 2008;15:176–182. [DOI] [PubMed] [Google Scholar]
- 7. Shields MB, Allingham RR, Damji KF, et al. Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G. Congenital glaucoma. Shields’ Textbook of Glaucoma, 5th ed. Lippincott Williams & Wilkins; 2005:235–252. [Google Scholar]
- 8. Shields MB, Allingham RR, Damji KF, et al. Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G. Medical and surgical treatment for childhood glaucomas. Shields’ Textbook of Glaucoma, 5th ed. Lippincott Williams & Wilkins; 2005:623–643. [Google Scholar]
- 9. Elwehidy AS, Bayoumi NHL, Abd Elfattah D, et al. Surgical outcomes of visco-circumferential-suture-trabeculotomy versus rigid probe trabeculotomy in primary congenital glaucoma: a 3-year randomized controlled study. J Glaucoma 2022;31:48–53. [DOI] [PubMed] [Google Scholar]
- 10. Elhilali HM, El Sayed YM, Elhusseiny AM, et al. Kahook dual blade ab-interno trabeculectomy compared with conventional goniotomy in the treatment of primary congenital glaucoma: 1-year results. J Glaucoma 2021;30:526–531. [DOI] [PubMed] [Google Scholar]
- 11. Elwehidy AS, Hagras SM, Bayoumi N, et al. Five-year results of viscotrabeculotomy versus conventional trabeculotomy in primary congenital glaucoma: a randomized controlled study. Eur J Ophthalmol 2021;31:786–795. [DOI] [PubMed] [Google Scholar]
- 12. Bor’i A, Al-Mosallamy SM, Elsayed TG, et al. C-sparing novel technique for subscleral trabeculectomy in primary congenital glaucoma. J Ophthalmol 2020;2020:2017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wagdy FM. Ab externo 240-degree trabeculotomy versus trabeculotomy-trabeculectomy in primary congenital glaucoma. Int Ophthalmol 2020;40:2699–2706. [DOI] [PubMed] [Google Scholar]
- 14. Rolim-de-Moura C, Esporcatte BLB, Netto CF, et al. Baerveldt implant versus trabeculectomy as the first filtering surgery for uncontrolled primary congenital glaucoma: a randomized clinical trial. Arq Bras Oftalmol 2020;83:215–224. [DOI] [PubMed] [Google Scholar]
- 15. El Sayed Y, Gawdat G. Two-year results of microcatheter-assisted trabeculotomy in paediatric glaucoma: a randomized controlled study. Acta Ophthalmol 2017;95:e713–e719. [DOI] [PubMed] [Google Scholar]
- 16. Shakrawal J, Bali S, Sidhu T, et al. Randomized trial on illuminated-microcatheter circumferential trabeculotomy versus conventional trabeculotomy in congenital glaucoma. Am J Ophthalmol 2017;180:158–164. [DOI] [PubMed] [Google Scholar]
- 17. Khalil DH, Abdelhakim MA. Primary trabeculotomy compared to combined trabeculectomy-trabeculotomy in congenital glaucoma: 3-year study. Acta Ophthalmol 2016;94:e550–e554. [DOI] [PubMed] [Google Scholar]
- 18. Temkar S, Gupta S, Sihota R, et al. Illuminated microcatheter circumferential trabeculotomy versus combined trabeculotomy-trabeculectomy for primary congenital glaucoma: a randomized controlled trial. Am J Ophthalmol 2015;159:490–497. [DOI] [PubMed] [Google Scholar]
- 19. ElSheikha OZ, Abdelhakim MASE, Elhilali HM, et al. Is viscotrabeculotomy superior to conventional trabeculotomy in the management of Egyptian infants with congenital glaucoma? Acta Ophthalmol 2015;93:e366–e371. [DOI] [PubMed] [Google Scholar]
- 20. Bayoumi NH. Deep sclerectomy in pediatric glaucoma filtering surgery. Eye (Lond) 2012;26:1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy B, Dada T, Sihota R, et al. Comparative evaluation of trabeculotomy-trabeculectomy with mitomycin c versus trabeculectomy with mitomycin C for primary congenital glaucoma. J Curr Glaucoma Pract 2011;5:15–19. [Google Scholar]
- 22. Noureddin BN, El-Haibi CP, Cheikha A, et al. Viscocanalostomy versus trabeculotomy ab externo in primary congenital glaucoma: 1-year follow-up of a prospective controlled pilot study. Br J Ophthalmol 2006;90:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Senft SH, Tomey KF, Traverso CE. Neodymium-YAG laser goniotomy vs surgical goniotomy. A preliminary study in paired eyes. Arch Ophthalmol 1989;107:1773–1776. [DOI] [PubMed] [Google Scholar]
- 24. Anderson DR. Discussion of paper by Quigley HA.Ophthalmology 1982;89:226–256. [Google Scholar]
- 25. Gagrani M, Garg I, Ghate D. Surgical interventions for primary congenital glaucoma. Cochrane Database Syst Rev 2020;8:CD008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lumley T. Network meta‐analysis for indirect treatment comparisons. Stat Med 2002;21:2313–2324. [DOI] [PubMed] [Google Scholar]
- 27. Salanti G, Ades A, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–171. [DOI] [PubMed] [Google Scholar]
- 28. Lee YJ, Kang D, Lee JE, et al. Protocol for systematic review and network meta-analysis of comparative effectiveness of surgical interventions for primary congenital glaucoma. BMJ Open 2022;12:e064264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 30. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rücker G. Network meta‐analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312–324. [DOI] [PubMed] [Google Scholar]
- 32. Shim SR, Kim S-J, Lee J, et al. Network meta-analysis: application and practice using R software. Epidemiol Health 2019;41:e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rücker G, Schwarzer G, Krahn U, et al. Package ‘netmeta’. Network Meta-Analysis using Frequentist Methods. R package version (Version 08-0), 2015.
- 34. Borenstein M, Hedges LV, Higgins JP, et al. Introduction to Meta-Analysis. John Wiley & Sons; 2021. [Google Scholar]
- 35. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cochran WG. The comparison of percentages in matched samples. Biometrika 1950;37:256–266. [PubMed] [Google Scholar]
- 38. Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159:130–137. [DOI] [PubMed] [Google Scholar]
- 39. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–944. [DOI] [PubMed] [Google Scholar]
- 40. Nikolakopoulou A, Higgins JP, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papakonstantinou T, Nikolakopoulou A, Higgins JP, et al. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta‐analysis. Campbell Syst Rev 2020;16:e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Res Synth Methods 2012;3:161–176. [DOI] [PubMed] [Google Scholar]
- 43. Sarkisian SR, Jr. An illuminated microcatheter for 360-degree trabeculotomy [corrected] in congenital glaucoma: a retrospective case series. J AAPOS 2010;14:412–416. [DOI] [PubMed] [Google Scholar]
- 44. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–1129. [DOI] [PubMed] [Google Scholar]
- 45. Papadopoulos M, Cable N, Rahi J, et al. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci 2007;48:4100–4106. [DOI] [PubMed] [Google Scholar]
- 46. Haas J. Principles and problems of therapy in congenital glaucoma. Invest Ophthalmol 1968;7:140–146. [PubMed] [Google Scholar]
- 47. deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma). Surv Ophthalmol 1983;28:1–19. [DOI] [PubMed] [Google Scholar]
- 48. Miller SJ. Genetic aspects of glaucoma. Trans Ophthalmol Soc U K 1966;86:425–434. [PubMed] [Google Scholar]
- 49. Francois J. Congenital glaucoma and its inheritance. Ophthalmologica 1980;181:61–73. [DOI] [PubMed] [Google Scholar]
- 50. Gencik A, Gencikova A, Ferak V. Population genetical aspects of primary congenital glaucoma. I. Incidence, prevalence, gene frequency, and age of onset. Hum Genet 1982;61:193–197. [DOI] [PubMed] [Google Scholar]
- 51. Jaafar MS. Reinecke RD. Care of the infantile glaucoma patient. Ophthalmology Annual. Raven Press; 1988:15–37. [Google Scholar]
- 52. Dandona L, Williams JD, Williams BC, et al. Population-based assessment of childhood blindness in southern India. Arch Ophthalmol 1998;116:545–546. [PubMed] [Google Scholar]


