Dear Editor,
Within the 2 years of the pandemic, the collaborative efforts between the industry, academia, funders, regulatory bodies, and government agencies have provided us with a range of antiviral therapies and vaccines. These success lessons have helped us to learn how to develop a ‘rapid’ vaccine or therapeutics with the help of collaborative efforts using modern technologies. Previously, several researchers have urged collaborative efforts to contain the coronavirus disease 2019 (COVID-19) pandemic, from drug discovery to vaccine development1,2. Now the time has come to develop for pharma and computer industry partnerships to develop artificial intelligence (AI)-enabled clinical trial technologies or platforms.
We believe that the fastest way to contain the pandemic would be through AI-enabled system that could identify, report, and mitigate with new drugs and vaccines faster. This system integrates mathematical modeling (viral transmission via faster algorithms), biological science (molecular variations of virus leading to vaccine), and bioinformatics (evolution of the virus aiding in drug screening).
The AI-based infectious disease surveillance unit had identified COVID-19 in Wuhan based on first report from hospital. The Canadian AI model developed by BlueDot (https://bluedot.global/products/) predicted that COVID-19 would become pandemic and issued early warnings on 31 December 2019. Likewise, the AI-based chest computed tomography for COVID-19 was developed by Shanghai Public Health Clinical Center was successfully used in January 2020 to screen patients in Wuhan.
The AI technology is used in drug discovery and Pfizer has developed IBM Watson that adopts machine learning (ML) process to power quick searching of drug molecules. Similarly, Genentech uses GNS Healthcare platform for cancer search3. Clinical trials consume about 1.5–2 billion USD during 10–15 years and many molecules often fail. So, the AI adopts the Eroom’s Law into Moore’s Law for drug discovery and development to increase success rate by means of cohort composition designs plus recruitment and monitoring of patients4.
Suitable patient enrollment is necessary for cohort composition design. If trials use medical imaging techniques through AI, it will move towards patient selection by using ‘omic data’ along with electronic medical record (EMR). However, developing an efficient platform to run clinical trials through ‘omic data’ alongside EMR is a tough task. So, the high-tech companies try to implement amalgamation of ‘omic data’ along with EMR for cohort composition and the AI uses features namely population heterogeneity, prognostic enrichment, and predictive enrichment5.
Patient dropout is a concern in clinical trials as the average dropout rate goes beyond 30%. But, AI can easily predict whether or not patients can dropout, thereby reducing delays in trials. ML algorithms and deep learning (DL) interface-authorized system can further assist in proper feedback loop and algorithms6. Patient monitoring can be performed through ML and DL to aid in visual object recognition, speech recognition, medical images processing, etc. There are three broad implementation of clinical trial designing that include trial automation, the ‘go,’ ‘no go’ decision, and insights into business decisions on new drug clinical trials. When a trial fails, the system will identify options where the drug can fit.
The AI models include general algorithms, pattern recognition, ML, edge-detection algorithms, and DL algorithms. Other significant algorithms from drug discovery to developments are the recurrent neural network, feedforward neural network, convolutional neural network, random forest, k-nearest neighbors, etc.7. These algorithms are used for AI-enabled clinical trial platforms or technology development. Data mining can act as middleware connected to large datasets that can be linked to cloud computing that can be accessed by log-on procedure through a mobile processor or personal computer. Platforms for coding environments and custom-developed hardware are available from IBM’s TrueNorth chip, Intel’s Movidius processor, and Qualcomm’s Snapdragon chip series. By using conventional mobile processors, mobile platform can be used as a platform for typical AI coding such as Apple Watch platform.
Several scientists provided guidelines for the interventions of the clinical trial protocols using AI. This guideline is called the SPIRIT-AI extension. It includes 15 new items which are significant for clinical trial protocols and AI interventions. It will help promote completeness and transparency of AI interventions and clinical trials. However, AI is a quickly evolving area. Therefore, it will be necessary to update SPIRIT-AI from time to time as the new technology and newer applications will develop8,9.
Over 500 drugs have been registered for clinical trials since start of the pandemic and most are drug repurposing on existing drugs for new therapeutic purposes10–12. Companies are currently spending billions of dollars and enormous time to produce drugs for COVID-19. Drugs such as molnupiravir, remdesivir, nirmatrelvir, nirmatrelvir and ritonavir, regdanvimab, and sotrovimab, bamlanivimab+etesevimab (antibodies cocktails), casirivimab+imdevimab (antibodies cocktails) tixagevimab+cilgavimab (antibodies cocktails), tocilizumab, ivermectin, and dexamethasone are some examples (Fig. 1). Also, the humanized-monoclonal antibodies (tocilizumab, sarilumab) are in trial for cytokine storm syndrome. Alongside trials, vaccines were also registered13–15. When it comes to drug repurposing, AI can search faster COVID-19-related drugs and literature an example is LitCovid16. Also, it can aid in database yielding search results faster through microprocessors as shown in Figure 1. It can assist physicians with classification of symptoms, grouping of patients in cohort design, recruitment, monitoring, geographical distribution, chest imaging, and other variables17. However, to handle the Big Data Challenges in Life Sciences Research, IBM Watson might be a powerful platform. It can connect millions of text pages significantly faster18.
Figure 1.
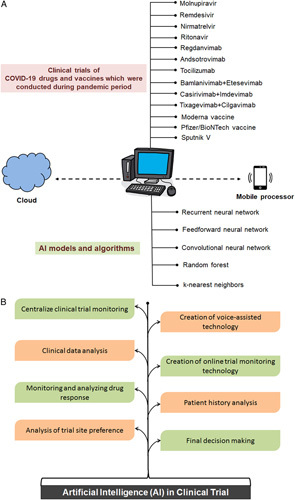
Schematic diagram showing the AI-enabled model and AI-enabled system with potential to improve diverse areas of clinical trial. (a) AI-enabled model against COVID-19 drugs. (b) The AI-enabled system with potential to improve diverse areas of clinical trial. AI, artificial intelligence; COVID-19, coronavirus disease 2019.
It has been noted that at least three clinical trials were performed using an AI-based platform. In an Australian cancer hospital, Alexander and colleagues developed a system for AI-based clinical trials for matching a cohort of lung cancer patients. The trial cohort included 102 lung cancer patients. The AI-based clinical trial allows reliable and efficient screening of cancer patients with 95.7% accuracy19. Yao et al.20 used AI algorithm-based ECG monitoring during a randomized clinical trial. Here, an AI-based system was used to identify the patients’ low ejection fraction of the heart. Similarly, to assess dosing compliance in the Phase II Clinical Trial, Bain et al.21 used an AI-based platform on mobile device.
Over 73 000 clinical trials have been registered (https://clinicaltrials.gov/) and they need to be monitored by drug companies and regulatory authorities. But, large number of clinical trials has not been placed in any online platforms, which is surprising. Monitoring clinical trials is essential and AI can solve easily this issue. During the pandemic, many countries implemented lockdowns, and drug company employees worked from home. In this difficult situation, there is no choice except to increase the implementation of AI to fast-forward clinical trials. In any future pandemic, AI-based technologies might be a significant choice in clinical trials.
Due to lack of data on one side and too much data on the other, the AI has not made any major impact against the COVID-19. So, better dataset and manpower are needed to develop the AI-based technology with better potential to develop efficient systems of early warning, tracking, predicting, managing data, diagnosing, forecasting, treating patients, conducting clinical trials, developing drugs and vaccines. It can also prioritize patients for faster treatment and monitoring as the system could quickly predict mortality, risk factors, interventions, community-level control, and prevention strategies leading to efficient disease epidemic and pandemic control strategies in near future.
Ethical approval
None.
Sources of funding
None.
Authors’ contribution
C.C.: conceptualization, data curation, investigation, writing – original draft, writing – review and editing. M.B.: validation and figure development. K.D. validation and reviewing. G.A.: validation; editing-reviewing. All authors critically reviewed and approved the final version of the manuscript.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
None.
Guarantor
Chiranjib Chakraborty.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Acknowledgements
The authors are thankful to their respective institutes and universities.
Footnotes
This manuscript has been peer reviewed.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Chiranjib Chakraborty, Email: drchiranjib@yahoo.com.
Manojit Bhattacharya, Email: mbhattacharya09@gmail.com.
Kuldeep Dhama, Email: kdhama@rediffmail.com.
Govindasamy Agoramoorthy, Email: agoram@tajen.edu.tw.
References
- 1. Chakraborty C, Sharma AR, Sharma AR, Sharma G, Bhattacharya M, Saha RP, Lee SS. Ext ensive partnership, collaboration, and teamwork is required to stop the COVID-19 outbreak. Arch Med Res 2020;51:728–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lexchin J. Are academia-pharma partnerships essential for novel drug discovery in the time of the COVID-19 pandemic? Expert Opin Drug Discov 2021;16:475–479. [DOI] [PubMed] [Google Scholar]
- 3. Fleming N. How artificial intelligence is changing drug discovery. Nature 2018;557:S55–S57. [DOI] [PubMed] [Google Scholar]
- 4. Ringel MS, Scannell JW, Baedeker M, Schulze U. Breaking Eroom’s Law. Nat Rev Drug Discov 2020;19:833–834. [DOI] [PubMed] [Google Scholar]
- 5. Harrer S, Shah P, Antony B, Hu J. Artificial intelligence for clinical trial design. Trends Pharmacol Sci 2019;40:577–591. [DOI] [PubMed] [Google Scholar]
- 6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521:436–444. [DOI] [PubMed] [Google Scholar]
- 7. Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today 2021;26:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rivera SC, Liu X, Chan AW, Denniston AK, Calvert MJ. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. BMJ 2020;370:m3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz Rivera S, Liu X, Chan AW, Denniston AK, Calvert MJ. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Lancet Digit Health 2020;2:e549–e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng MP, Lee TC, Tan DHS, Murthy S. Generating randomized trial evidence to optimize treatment in the COVID-19 pandemic. CMAJ 2020;192:E405–E407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saha RP, Sharma AR, Singh MK, Samanta S, Bhakta S, Mandal S, et al. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front Pharmacol 2020;11:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee SS. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: lessons learned from major clinical studies. Front Pharmacol 2021;12:704205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chakraborty C, Sharma AR, Bhattacharya M, Lee SS. From COVID-19 to cancer mRNA vaccines: moving from bench to clinic in the vaccine landscape. Front Immunol 2021;12:679344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Saha RP, Lee SS. Ongoing clinical trials of vaccines to fight against COVID-19 pandemic. Immune Netw 2021;21:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee SS. Asian-origin approved COVID-19 vaccines and current status of COVID-19 vaccination program in asia: a critical analysis. Vaccines (Basel) 2021;9:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Q, Allot A, Lu Z. LitCovid: an open database of COVID-19 literature. Nucleic Acids Res 2021;49(D1):D1534–D1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ting DSW, Carin L, Dzau V, Wong TY. Digital technology and COVID-19. Nat Med 2020;26:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Elenee Argentinis JD, Weber G. IBM Watson: how cognitive computing can be applied to big data challenges in life sciences research. Clin Ther 2016;38:688–701. [DOI] [PubMed] [Google Scholar]
- 19. Alexander M, Solomon B, Ball DL, Sheerin M, Sheerin M, Dankwa-Mullan I, Preininger AM, et al. Evaluation of an artificial intelligence clinical trial matching system in Australian lung cancer patients. JAMIA Open 2020;3:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao X, Rushlow DR, Inselman JW, McCoy RG, Thacher TD, Behnken EM, et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med 2021;27:815–819. [DOI] [PubMed] [Google Scholar]
- 21. Bain EE, Shafner L, Walling DP, Othman AA, Chuang-Stein C, Hinkle J, Hanina A. Use of a novel artificial intelligence platform on mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR Mhealth Uhealth 2017;5:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]


