Background:
Laparoscopic D2 lymph node dissection (LND) for gastric cancer has a wide range and high difficulty. In the past, the quality of surgery was often judged by the time of the operation or the amount of blood loss, but the analysis based on surgical video was rarely reported. The purpose of this study was to analyze the relationship between the quality of laparoscopic D2 LND for gastric cancer and postoperative complications.
Methods:
The surgical video and clinicopathological data of 610 patients in two randomized controlled trials in our center from 2013 to 2016 were retrospectively analyzed. Klass-02-QC LND scale and general error score tool were used to quantitatively evaluate the intraoperative performance of D2 LND. Logistic regression was used to analyze the influencing factors of postoperative complications.
Results:
The overall incidence of complications (CD classification≥2) was 20.6%; the incidence of surgical complications was 6.9%. According to whether the LND score reached 44, patients were divided into a qualified group (73%) and a not-qualified group (27%). Event score (ES) by quartile was divided into grade 1 (21.7%), grade 2 (26%), grade 3 (28%), and grade 4 (24.3%) from low to high. Univariate logistic regression analysis showed that ES greater than or equal to 3, tumor size greater than or equal to 35 mm, and cTNM >II were independent risk factors for not-qualified LND. Male,tumor size greater than or equal to 35 mm and cTNM >II were independent risk factors for grade 4 ES. Not-qualified LND (OR=1.62, 95% CI: 1.16–3.89, P=0.021), grade 4 ES (OR=3.21, 95% CI: 1.52–3.90, P=0.035), and cTNM >II (OR=1.74, 95% CI: 1.39–7.33, P=0.041) were independent risk factors for postoperative surgical complications.
Conclusions:
The qualification of LND and intraoperative events based on surgical video are the independent influencing factors of postoperative complications of laparoscopic gastric cancer surgery. Specialist training and teaching based on surgical video may help to improve the surgical skills of specialists and improve the postoperative outcome of patients.
Keywords: clinical outcomes, D2 lymph node dissection, gastric cancer, quality of surgery
Introduction
Highlights
The quality of laparoscopic radical gastrectomy can be identified by surgical video.
Not-qualified lymph node dissection was associated with complications.
More intraoperative events can lead to postoperative complications.
Video of surgery is helpful for reproduction and teaching.
Distal subtotal gastrectomy plus D2 lymph node dissection (LND) is a standardization method for middle and lower 1/3 locally advanced gastric cancer1. The studies of Class-01 and Klass-02 show that compared with laparotomy, laparoscopic surgery has the advantages of rapid recovery and mild pain symptoms without increasing postoperative complications2,3. Therefore, laparoscopic surgery has gradually become the standard surgical method for locally advanced distal gastric cancer.
D2 LND is the basic requirement for laparoscopic radical gastric cancer surgery1, but because of its high difficulty, even experienced surgeons may have an incomplete LND due to various reasons. Although a consensus has been reached on the scope of LND in each country, there is a lack of unified evaluation criteria for the actual completion of D2 LND by surgeons4. The KLASS-02-QC scale is the evaluation standard of the Korean KLASS-02 research group on laparoscopic D2 LND for advanced gastric cancer5. We assume that the KLASS-02-QC scale is the closest to the qualified D2 LND scale, and use it to evaluate the qualified of D2 LDN in our center.
Due to the different conditions of the patients, even the same surgeon may have different surgical results. It is very important to identify the risk factors that may affect the surgical outcome. Generic error rating tool (GERT) can objectively and reliably evaluate the surgical effect of laparoscopic surgery6. After excluding the confounding factors of the surgeons, more intraoperative events usually represent higher surgical difficulty.
The purpose of this study is to analyze the relationship between the quality of laparoscopic D2 LND for gastric cancer and postoperative complications through these video tools.
Methods
The CLASS-01 trial is a prospective multicenter randomized controlled study (ClinicalTrials.gov.number:NCT01609309) to compare the oncological efficacy of laparoscopy and laparotomy in the treatment of locally advanced distal gastric cancer. In this study, 119 patients who underwent laparoscopic surgery at our center who participated in CLASS-01 were selected. All the patients were operated with a 2D laparoscopic system. The 2D/3D trial is a prospective clinical trial (ClinicalTrials.gov.number:NCT02327481) that compares the curative effect of 2D and 3D laparoscopic radical gastrectomy in our center. Seventy cases of 2D laparoscopic distal gastrectomy were selected (Fig. 1). The reason for including these two clinical trials at the same time is that they have similar inclusion and exclusion criteria, and the surgery was performed during the same period and by the same team. According to the LND score in KLASS-02-QC, the qualified of LND was judged, and the GERT was used to record the events during the operation. The video content only shows a selected part of the laparoscopic D2 LND. Our study has been reported in accordance with strengthening the reporting of cohort studies in surgery (STROCSS) standards7, Supplemental Digital Content 1, http://links.lww.com/JS9/A247.
Figure 1.
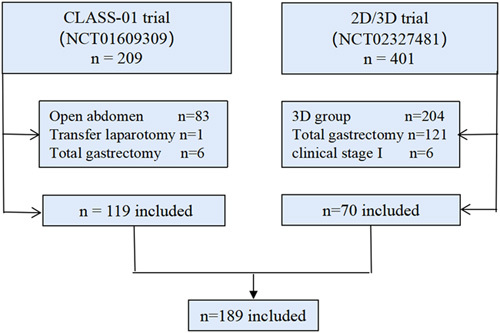
Study flowchart.
Data collection
The KLASS-02-QC LND score and GERT have extensive evidence in the literature to support their use5,8. The total score of LND was 22 points, which included the surgical procedures of excision of the omentum, inferior pylorus, superior pancreas, lesser curvature of gastric, and left gastroepiploic. The loss score is defined as insufficient LND, incomplete vascular naked, or damage to normal tissues (Fig. 2).Only when the scores of the two raters add up to 44 points (LND score=44) are defined as the qualified LND group, and the others are defined as the not-qualified group(<44 score). The scoring standard of each region is according to eTable 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A248. Multiple lymph node loss points in the same patient were recorded in the lymph node loss rate at each station. In the GERT instrument, there are seven different laparoscopic operations (for example, the use of grasping retractors, the use separation of energy devices, etc.) and four different event patterns (for example, excessive small force, a lack of visual field, etc.) (eTable 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A248). An event is defined as any damage to the tissue caused by a failure of the planned action (for example, the slippage of the grabbed tissue leads to a serosa tear) (eFigure 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A248). Event score (ES) is to record one point for each event, and the assessment process in this study was in accordance with the current conceptual Messick framework of validity9. The ES is divided into four grades: (0–25%), (25–50%), (50–75%), and (75–100%), from the lowest to highest quartile. The video of the operation is an unedited original video. The raters did not participate in the operation and scored the operation by watching the recorded video. Blind method was used to analyze the surgical results and clinicopathological factors of the patients, and the scoring process was carried out independently. When there is a difference in the scoring process, it is judged by a third surgical expert.
Figure 2.
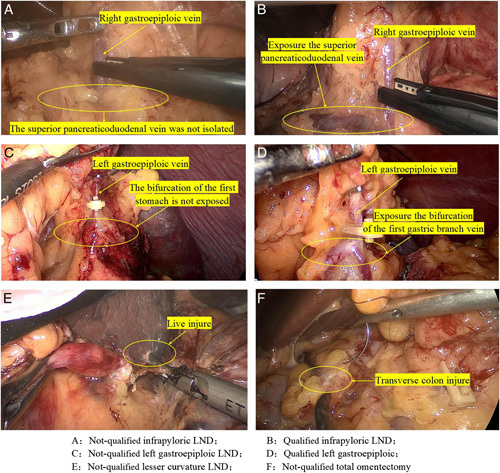
Some examples of qualified and not-qualified lymph node dissection.
Surgical procedure
All patients enrolled in the two studies were operated on by either of the two surgeons (C.-H.Z. or C.-M.H.), both with experience of more than 50 laparoscopic distal gastrectomys before the trials. To reduce bias in the results caused by the surgical variability, both studies stipulated that the surgery, including lymphadenectomy and digestive reconstruction, must be completely independently performed by one of the two surgeons. Lymph nodes were sorted and evaluated according to the standardization method of the ‘Japanese Classification of Gastric Cancer (ver.3)’10. The operation procedure was performed according to eTable 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A248.
Outcome measurements
Both NCT01609309 and NCT02327481 received a unified perioperative management and follow-up plan. Complications (Clavien–Dindo classification ≥2)11 within 30 days after surgery were evaluated, which were divided into surgical complications and systemic complications, including abdominal bleeding, gastrointestinal bleeding, lymphatic leakage, abdominal effusion, intestinal obstruction, incision infection, and abdominal infection; systemic complications included lower limb deep venous thrombosis, urinary complications, respiratory complications, and anesthesia-related complications (such as postoperative delirium)3. The time of LND was calculated from the resection of the greater omentum in the operation video to the dissection of the left gastroepiploic lymph node.
Statistical analysis
Normally distributed continuous data were presented as means with SD, non-normally distributed continuous data were presented as medians with interquartile ranges; categorical (binary, nominal, and ordinal) data were presented as frequencies with percentages, and the normality of data distribution was evaluated using the Shapiro–Wilk test. According to the different normality of the data, the student’s t-test or Mann–Whitney U test was used for the comparison between the two groups of continuous variable data, and the analysis of variance or Kruskal–Wallis test was used for multiple groups of data. The χ 2 correlation test was used to compare classified variables. When the sample size was less than 5, the Fischer accurate test was used. The Mantel–Haenszel χ 2-test was used to test the linear relationship among clinicopathological stages, qualified LNDs, and four groups of ESs. The intraclass correlation coefficient is used to check the consistency of the Klass-02-QC score and GERT score between the two raters12,13. The correlation between LND scores and other variables was calculated using the Spearman correlation test and their interactions were tested14. Variables with P less than 0.05 in univariate logical analysis were included in the multivariate regression model, and a backward selection strategy was adopted. A tumor size of 35 mm was obtained by taking the cut-off value of the area under the ROC curve.
Results
Basic clinicopathological data and video scores
In the NCT01609309 trial, 209 cases were involved in the center, 83 cases were excluded from laparotomy, one case was converted to laparotomy by laparotomy, and six cases underwent total gastrectomy (the tumor location was judged to be high during operation). There were 401 cases in the NCT02327481 trial, 204 cases were excluded from the 3D group, 121 cases were under total gastrectomy, and six cases were in the cT1 stage. Ultimately, 189 patients (119 cases and 70 cases from NCT01609309 and NCT02327481, respectively) were included.
A total of 213.5 h unedited video were included in the analysis. In the video score, the median score of LND was 44 (42–44) and the median score of events was 22 (14–36). According to whether the LND score reached 44, patients were divided into a qualified group (n = 138, 73%) and a not-qualified group (n = 51, 27%). There were differences in gender,ES,clinical stage, and tumor size between the two groups (Table 1). According to the ES, 41 cases were classified as grade 1 (21.7%), grade 2 in 49 cases (26%), grade 3 in 53 cases (28%), and grade 4 in 46 cases (24.3%). There were differences in sex, clinical stage among the four groups in the baseline data (eTable 5, Supplemental Digital Content 2, http://links.lww.com/JS9/A248). The kappa coefficient of consistency among raters is shown in eTable 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A248 and 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A248.
Table 1.
Comparison of clinicopathological data and short-term clinical outcome between qualified and not-qualified LND.
| Qualified n=138 | Not-qualified n=51 | ||
|---|---|---|---|
| Median (IQR),% | Median (IQR),% | P | |
| Age (years) | |||
| ≤65 | 69.4 | 64.7 | |
| >65 | 30.4 | 35.3 | 0.524 |
| Sex | |||
| Male | 65.4 | 58.8 | |
| Female | 34.6 | 41.2 | 0.426 |
| BMI | |||
| <25 | 83.3 | 80.4 | |
| ≥25 | 16.7 | 19.7 | 0.636 |
| ASA score | |||
| I | 28.3 | 19.6 | |
| II | 30.4 | 23.5 | |
| III–IV | 41.3 | 56.9 | 0.159 |
| ECOG score | |||
| 0 | 68.4 | 80 | |
| 1 | 24.8 | 10 | |
| 2 | 6.8 | 10 | 0.081 |
| Tumor size (mm) | |||
| <35 | 44.9 | 33.3 | |
| ≥35 | 55.1 | 66.7 | 0.013 |
| Clinical stages | |||
| II | 60.9 | 29.4 | |
| III | 39.1 | 70.6 | 0.012 |
| Event score grade | |||
| 1 | 29 | 2 | |
| 2 | 30.4 | 13.7 | |
| 3 | 31.9 | 17.6 | |
| 4 | 8.7 | 66.7 | 0.014 |
| Pathological data | |||
| Pathological stage | |||
| I | 14.5 | 2.0 | |
| II | 55.8 | 25.7 | |
| III | 29.7 | 72.5 | <0.001 |
| Total number of LN | 34 (27–43) | 36 (29–50) | 0.074 |
| Metastatic LN | 4 (1–5) | 14 (4–23) | <0.001 |
| Surgery time (min) | 67 (63–71) | 70 (66–76) | 0.002 |
| Estimated blood loss (ml) | 56 (30–58) | 80 (50–100) | <0.001 |
| Clinical outcome | |||
| Any 30 day complications | |||
| Yes | 17.4 | 29.4 | |
| No | 82.6 | 70.6 | 0.118 |
| Surgical Complications (%) | |||
| Yes | 4.3 | 13.7 | |
| No | 95.7 | 86.3 | 0.024 |
| Systemic complications | |||
| Yes | 13 | 15.7 | |
| No | 87 | 84.3 | 0.259 |
| Hospitalization time (days) | 10 (9–12) | 12 (10–14) | 0.003 |
The bold value represents the P value with statistical significance.
ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group; LN, Lymph node; LND, Lymph node dissection.
Comparison between Qualified group and Not-qualified LND group
Compared with the qualified group, the not-qualified group has a higher incidence of grade 4 events (66.7 vs. 8.7%,P=0.014), a higher proportion of patients with clinical stage III (70.6 vs. 39.1%,P=0.012), larger tumors (proportion of tumor size≥35 mm:66.7 vs. 55.1%,P=0.013). ES ≥grade 3 (OR=8.65, 95% CI: 2.50–49.83, P=0.016), tumor size greater than or equal to 35 mm(OR=1.54, 95% CI: 1.22–2.34, P=0.034), and cTNM >II (OR=4.54, 95% CI: 1.37–18.88, P=0.038) were independent risk factors for not-qualified LND (Table 2).
Table 2.
Predictors of not-qualified LND.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | ||||||
| <65 | ||||||
| ≥65 | 0.94 | 0.37–2.41 | 0.904 | |||
| Sex | ||||||
| Male | ||||||
| Female | 1.11 | 0.59–4.22 | 0.428 | |||
| ASA score | ||||||
| I | Ref | |||||
| II | 2.03 | 0.85–4.85 | 0.110 | |||
| III–IV | 1.30 | 1.49–7.23 | 0.213 | |||
| ECOG score | ||||||
| 0 | Ref | |||||
| 1 | 0.39 | 0.12–1.26 | 0.114 | |||
| 2 | 1.81 | 0.39–8.34 | 0.449 | |||
| Clinical stage | ||||||
| II | ||||||
| III | 10.14 | 2.75–37.41 | 0.001 | 4.54 | 1.37–18.88 | 0.038 |
| Event score grade | ||||||
| 1 | Ref | |||||
| 2 | 4.65 | 2.17–27.17 | 0.031 | 3.49 | 4.15–36.74 | 0.056 |
| 3 | 10.03 | 2.17–46.34 | 0.003 | 8.65 | 2.50–49.83 | 0.016 |
| 4 | 38.75 | 35.49–181.14 | <0.001 | 39.23 | 35.18–198.21 | <0.001 |
| BMI | ||||||
| <25 | ||||||
| ≥25 | 1.21 | 0.57–2.56 | 0.622 | |||
| Tumor size (mm) | ||||||
| <35 | ||||||
| ≥35 | 1.84 | 1.00–3.86 | 0.05 | 1.54 | 1.22–2.34 | 0.034 |
The bold value represents the P value with statistical significance.
The estimated blood loss and metastatic lymph nodes in the not-qualified group were higher than those in the qualified group (estimated blood loss: 80 vs. 56 ml, P<0.001; median of metastatic lymph nodes: 14 vs. 4, P<0.001). The time of LND and postoperative hospitalization time were longer than qualified group (operation time: 70 vs. 67 min; hospitalization time: 12 vs. 10 days) (Table 1). With regard to the laboratory examination, in the not-qualified group, the white blood cell count was higher on the first and third days (D1: 15.9 vs. 10.4×109/l, D3: 12.3 vs. 8.9×109/l, P<0.001), and the C-reactive protein level was higher on the third and fifth days after the operation (D3: 93.4 vs. 67.2 mg/l, P=0.006; D5: 63.3 vs. 41.8 mg/l, P=0.005). On the first, third, and fifth days, the abdominal drainage volume was more (187.4 vs. 104.6 ml, 127.7 vs. 71.1 ml, and 56.6 vs. 34.3 ml, respectively; P<0.001) (eFigure 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A248).
Influencing factors of ES and short-term outcome difference
Male sex (OR=4.31, 95% CI: 1.60–11.66, P=0.004) , tumor size ≥ 35 mm (OR=2.09, 95% CI:1.80–6.65,P=0.022) and pTNM >II (OR=6.85, 95% CI: 2.67–40.75, P=0.002) were independent risk factors for the development of a grade 4 ES (eTable 6, Supplemental Digital Content 2, http://links.lww.com/JS9/A248).
There were significant differences in the number of metastatic lymph nodes, tumor size, and estimated blood loss among the four groups (metastatic lymph nodes: 0 vs. 3 vs. 5 vs. 15; tumor size: 33 vs. 35 vs. 40 vs. 57.5 mm; estimated blood loss: 42 vs. 50 vs. 50 vs. 80 ml, P<0.001). The postoperative hospitalization time for grade 4 was longer than that for grade 1 (11 vs. 9 days, P=0.036). In the laboratory findings, compared with the grade 1 group, the grade 4 group had lower hemoglobin levels on the first day (107 vs. 116 g/l, P<0.001), higher white blood cell count on the first and third days (D1: 15.7 vs. 9.2×109/l, D3: 12.2 vs. 8.2×109/l, P<0.001), and a higher C-reactive protein on the third and fifth days (95.5% vs. 58.1 and 61.4 vs. 38.1 mg/l, respectively; P<0.001). Furthermore, the abdominal drainage volume on the first, third, and fifth days were more (191.1 vs. 78.3 ml, 126.3 vs. 57.6 ml, 58.7 v s25.9 ml, P<0.001) (eFigure 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A248).
Influencing factors of postoperative surgical complications
The overall complication rate was 20.6% (39/189) 30 days after the operation; the systematic complication rate was 13.7% (26/189) and the surgical complication rate was 6.9% (13/189). The specific complication types are shown in eTable 7, Supplemental Digital Content 2, http://links.lww.com/JS9/A248.
In the total population, the surgical complications in the qualified LND group were significantly lower than those in not-qualified LND group (13.7 vs. 4.3%, P=0.024). Multivariate logistic analyses showed that not-qualified LND (OR=1.62, 95% CI: 1.16–3.89, P=0.021), ES greater than 3 (OR=3.21, 95% CI: 1.52–3.90, P=0.035), and cTNM >II (OR=1.74, 95% CI: 1.39–7.33, P=0.041) were independent risk factors for surgical complications (Table 3). In order to further verify that not-qualified LND is an independent prognostic factor of postoperative complications, we analyzed the correlation between not-qualified LND and the influencing factors of postoperative complications. The results showed that not-qualified LND was weakly positively correlated with ES4 grade (Spearman’r=0.290) and cTNM (Spearman’r=0.280)15, but not with tumor size (Spearman’r=0.104) (efigure 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A248). Next, we tested the interaction between the LND score, ES score, and cTNM, and found that there was no interaction between them (LND score*ES grade, P=0.516; LND score*cTNM, P=0.513) (eTable 8, Supplemental Digital Content 2, http://links.lww.com/JS9/A248).
Table 3.
Predictors of postoperative surgical complications.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | ||||||
| <65 | ||||||
| ≥65 | 1.32 | 0.41–4.24 | 0.640 | |||
| Sex | ||||||
| Male | ||||||
| Female | 0.43 | 0.09–2.01 | 0.283 | |||
| BMI | ||||||
| <25 | ||||||
| ≥25 | 1.46 | 0.38–5.63 | 0.582 | |||
| ECOG score | ||||||
| 0 | Ref | |||||
| 1 | 1.97 | 0.33–11.7 | 0.459 | |||
| 2 | 1.68 | 0.56–3.24 | 0.329 | |||
| Tumor size (mm) | ||||||
| <35 | ||||||
| ≥35 | 2.68 | 1.67–8.23 | 0.031 | 2.09 | 0.93–7.61 | 0.215 |
| ASA score | ||||||
| I | Ref | |||||
| II | 0.90 | 0.17–4.69 | 0.902 | |||
| III–IV | 1.38 | 0.34–5.58 | 0.655 | |||
| Clinical stage | ||||||
| II | ||||||
| III | 1.45 | 1.15–9.84 | 0.035 | 1.74 | 1.39–7.33 | 0.041 |
| LND score | ||||||
| 44 full mark | ||||||
| <44 | 2.9 | 1.29–3.54 | 0.032 | 1.62 | 1.16–3.89 | 0.021 |
| Event score grade | ||||||
| 1 | Ref | Ref | ||||
| 2 | 1.34 | 0.55–2.34 | 0.385 | 1.68 | 0.22–2.11 | 0.500 |
| 3 | 2.18 | 0.45–3.15 | 0.292 | 2.18 | 0.41–1.81 | 0.371 |
| 4 | 3.56 | 2.88–4.76 | 0.028 | 3.21 | 1.52–3.90 | 0.035 |
The bold value represents the P value with statistical significance.
Common not-qualified LND and event types
The highest score loss rate of LND was the infrapyloric lymph node, which was 14.3% (27/189), followed by the left gastroepiploic lymph node in 11.1% (21/189) and hepatoduodenal lymph node 7.9% (15/189). In the infrapyloric LND, ‘ use of retractors and grasping’ was the most frequently occurring operational event type, accounting for 35.3% of the total events in this station. Common event types in other areas are shown in Figure 3.
Figure 3.
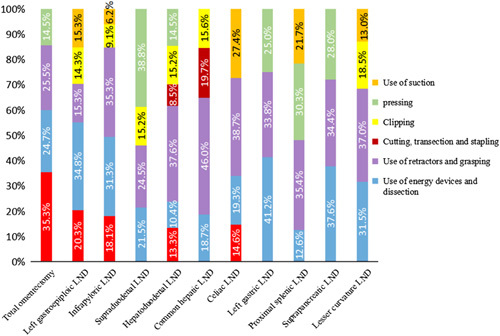
Ratio of lymph node event types in each region.
In order to explore the specific effects of tumor size and clinical stage on the difficulty of operation, we also compared the event differences between tumor larger than 35 mm and tumor less than 35 mm and different clinical stages of tumor. It was found that the larger the tumor and the later the stage, the more likely it was to have events in the operations such as ‘finding fascia’, ‘use of energy devices’, ‘cutting, transection and stapling’, and ‘grasping and dissection’.
Discussion
As far as we know, this is the first study to objectively evaluate the quality of laparoscopic D2 LND surgery for gastric cancer through surgical video. Our study found that intraoperative events were related to gender, tumor size, and clinical stage, while not-qualified LND was related to tumor size, clinical stage, and intraoperative events. Finally, high intraoperative events and not-qualified LND will lead to postoperative complications.
Laparoscopic surgery is a complicated process, and there are many factors that affect the quality of surgery16,17. Previous studies considered that the volume of the operation center and the experience of surgeons were important factors affecting the operation quality18–20. When the Korean KLASS research team used the Klass-02-QC LND scale to conduct a video evaluation to be recruited, it was found that even experienced surgeons still had 55.6% of LND not qualified5. Cai et al.21 found that high BMI , accompanying disease, and pTNM stage were the independent risk factors of postoperative complications in laparoscopic D2 radical gastrectomy. Targarona et al.22. found in laparoscopic rectal surgery, sex, BMI, lower pelvis diameter, and tumor size are independent predictors for conversion, operative time, and morbidity. These studies show that the surgical outcome is not only related to the operator’s experience, but also to the clinicopathological characteristics of the patients.
In this study, although all patients underwent radical surgery in accordance with standard operating procedures, 27% were still not-qualified according to the Klass-02-QC LND scale. Not-qualified group has a higher incidence of grade 4 events, a higher proportion of patients with clinical stage III, larger tumors. Multivariate regression showed that clinical stage, tumor size, and high ES were independent risk factors for not-qualified LND. A high clinical stage indicates a deeper infiltration level and more metastatic lymph nodes. Previous studies have shown that the anatomical manifestations of metastatic lymph nodes are enlarged lymph nodes, hard texture, and cluster fusion23. Enlarged lymph nodes can compress or wrap around blood vessels, destroying the local fascia structure, which complicates the anatomical structure and can lead to inaccurate location at the anatomical level or abnormal anatomy of blood vessels mistaken for enlarged lymph nodes, leading to intraoperative events.
In addition, according to the results of this study, a high event grade (grade=4) was associated with male, clinical stage, and tumor size. Previous studies reported that male was an independent predictor of the difficulty of laparoscopic surgery for colon cancer24. The reason may be that male have a higher body mass index and greater abdominal visceral fat deposition than female25, which leads to the difficulty of laparoscopic operation. Similarly, larger tumor size can hinder laparoscopic anatomy. Previous studies have shown that large tumor size are one of the common causes of conversion to laparotomy26,27. Local anatomy and pathological features will directly affect the effect of laparoscopic surgery for gastric cancer. This is mainly due to the technical difficulties of anatomy, although it has been initially judged before the operation and the operation is performed by the same surgical team.
Surgical video provides more direct evidence for assessing surgical quality28. In the past, the evaluation of the difficulty of an operation is usually reflected indirectly by the amount of blood loss or the time of operation29,30. Different from previous studies, our study is to evaluate the surgical video directly through specific video evaluation tools. According to the evaluation results, the risk factors affecting intraoperative events and not-qualified LND were identified, and the risk factors could be predicted before operation, such as sex, tumor size, and clinical stage (not pathological stage).
Compared with traditional open surgery, laparoscopic surgery is more able to preserve, reproduce, and teach the surgical process in the way of digital video. In this study, qualified and unqualified LND are not determined in advance. It is scored by the raters (the raters do not know the clinicopathological information of the patients) by watching unedited surgical videos, and then compare the differences between the two groups according to different scores to get the above results. The innovation of this study is to judge whether laparoscopic D2 LND is qualified and intraoperative events through the unedited operation video, and to present the specific types of errors in the operation, which will be helpful to the training or teaching of specialists in the future31.
Moreover, we also analyzed the most prone to incomplete LND in D2 LND and the most common events during operation (for example, the most prone to incomplete dissection was the infrapyloric lymph node, with an incidence of 14.3%. The most common event in the process of infrapyloric LND was the ‘use of retractors and grasping’, with an incidence of 35.3%. Therefore, the results of this study may help surgeons to know these adverse factors in advance, help to choose more effective surgical strategies.
This study has some limitations. First, it was a single-center study limited by the number and source of cases. The results may not be fully applicable to other centers, especially hospitals in western countries. Second, although the raters have received strict training in evaluation methods before, there is still subjectivity in evaluation. Third, there is no further analysis of the influence of these factors on the difficulty of digestive tract reconstruction, which will be the next research direction.
Conclusion
The qualification of LND and intraoperative events based on surgical video are the independent influencing factors of postoperative complications of laparoscopic gastric cancer surgery. Specialist training and teaching based on surgical video may help to improve the surgical skills of specialists and improve the postoperative outcome of patients.
Ethical approval
This study passed the Institutional Review Board (IRB) of the FJMUUH (IRB number: 2022KY186).
Sources of funding
Construction of ‘creating double highs’ of medical treatment in Fujian Province (Minwei Medical Administration (2021) No. 76). Fujian Research and Training Grants for Young and Middle–aged Leaders in Healthcare for Ping Li and Jun Lu.
Author contribution
J.L., J.B.H., and D.W.: conceived of the study, analyzed the data, and drafted the manuscript; H.C.M., Z.C.H., and L.P.: helped revise the manuscript critically for important intellectual content; X.J.W., W.J.B., J.X.L.: helped collect data and design the study. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Name of the registry: ClinicalTrials.gov
Unique Identifying number or registration ID: NCT05585073
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/ct2/show/NCT05585073
Guarantor
Ping Li.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgments
The authors are grateful to the patients and his family for their participation in this study.
Footnotes
Jun Lu, Jiao-Bao Huang, and Dong Wu contributed equally to this work.
Jun Lu and Jiao-Bao Huang are co-first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 7 April 2023
Contributor Information
Jun Lu, Email: 78379048@qq.com.
Jiao-Bao Huang, Email: m296394375@163.com.
Dong Wu, Email: 1979138986@qq.com.
Jian-Wei Xie, Email: 364531721@qq.com.
Jia-Bin Wang, Email: wjb2002wh@163.com.
Jian-Xian Lin, Email: linjian379@163.com.
Chao-Hui Zheng, Email: wwkzch@163.com.
Chang-Ming Huang, Email: hcmlr2002@163.com.
Ping Li, Email: pingli811002@163.com.
References
- 1. Association JGC. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2020;24(suppl 1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the class-01 randomized clinical trial. Jama 2019;321:1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open d2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:1350. [DOI] [PubMed] [Google Scholar]
- 4. Bencivenga M, Verlato G, Mengardo V, et al. Do all the European surgeons perform the same D2? The need of D2 audit in Europe. Updat Surg 2018;70:189–195. [DOI] [PubMed] [Google Scholar]
- 5. Han SU, Hur H, Lee HJ, et al. Surgeon quality control and standardization of D2 lymphadenectomy for gastric cancer: a prospective multicenter observational study (KLASS-02-QC). Ann Surg 2021;273:315–324. [DOI] [PubMed] [Google Scholar]
- 6. Bonrath EM, Zevin B, Dedy NJ, et al. Error rating tool to identify and analyse technical errors and events in laparoscopic surgery. Br J Surg 2013;100:1080–1088. [DOI] [PubMed] [Google Scholar]
- 7. Ra A, Ar B, Ec C, et al. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156–165. [DOI] [PubMed] [Google Scholar]
- 8. Fecso AB, Bhatti JA, Stotland PK, et al. Technical performance as a predictor of clinical outcomes in laparoscopic gastric cancer surgery. Ann Surg 2019;270:115–120. [DOI] [PubMed] [Google Scholar]
- 9. Ghaderi I, Manji F, Park YS, et al. Technical skills assessment toolbox a review using the unitary framework of validity. Ann Surg 2014;261:251–262. [DOI] [PubMed] [Google Scholar]
- 10. Japanese Gastric Canc Assoc. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–123. [DOI] [PubMed] [Google Scholar]
- 11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conover WJ. Statistical methods for rates and proportions. Technometrics 1981;16:326–327. [Google Scholar]
- 13. Lin IK. A concordance correlation-coefficient to evaluate reproducibility. Biometrics 1989;45:255–268. [PubMed] [Google Scholar]
- 14. Boehnke JanR. . Explanation in causal inference: methods for mediation and interaction. Q J Exp Psychol QJEP 2016;69:1243–1244. [DOI] [PubMed] [Google Scholar]
- 15. Hinkle D. Applied statistics for the behavioral sciences. Vol. 663. Houghton Mifflin College Division, 2003. [Google Scholar]
- 16. Stotland PK, Chia S, Cyriac J, et al. Safe implementation of laparoscopic gastrectomy in a community-based general surgery practice. Surg Endosc 2009;23:356. [DOI] [PubMed] [Google Scholar]
- 17. Li ZY, Shan F, Zhang LH, et al. Complications after radical gastrectomy following FOLFOX7 neoadjuvant chemotherapy for gastric cancer. World J Surg Oncol 2011;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duclos A, Peix JL, Colin C, et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ Online 2012;344:d8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rutegard M, Lagergren J, Rouvelas I, et al. Surgeon volume is a poor proxy for skill in esophageal cancer surgery. Ann Surg 2009;249:256–261. [DOI] [PubMed] [Google Scholar]
- 20. Markar SR, Wiggins T, Ni M, et al. Assessment of the quality of surgery within randomised controlled trials for the treatment of gastro-oesophageal cancer: a systematic review. Lancet Oncol 2015;16:E23–E31. [DOI] [PubMed] [Google Scholar]
- 21. Cai M, Zeng XY, Xiong Z, et al. Early postoperative complications and risk factors in laparoscopic D2 radical gastrectomy for gastric cancer. J Gastrointest Surg 2019;22:742–747. [DOI] [PubMed] [Google Scholar]
- 22. Targarona EM, Balague C, Pernas JC, et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg 2008;247:642. [DOI] [PubMed] [Google Scholar]
- 23. Park SR, Min JK, Ryu KW, et al. Prognostic value of preoperative clinical staging assessed by computed tomography in resectable gastric cancer patients: a viewpoint in the era of preoperative treatment. Ann Surg 2010;251:428–435. [DOI] [PubMed] [Google Scholar]
- 24. Akiyoshi T, Kuroyanagi H, Oya M, et al. Factors affecting difficulty of laparoscopic surgery for left-sided colon cancer. Surg Endosc 2010;24:2749–2754. [DOI] [PubMed] [Google Scholar]
- 25. Tsujinaka S, Konishi F, Kawamura YJ, et al. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum 2008;51:1757–1765. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu S, Noshiro H, Nagai E, et al. Laparoscopic gastric surgery in a Japanese institution: analysis of the initial 100 procedures. J Am Coll Surg 2003;197:372–378. [DOI] [PubMed] [Google Scholar]
- 27. Pugliese R, Maggioni D, Sansonna F, et al. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc 2007;21:21–27. [DOI] [PubMed] [Google Scholar]
- 28. Makary MA. The power of video recording. Jama 2013;309:1591. [DOI] [PubMed] [Google Scholar]
- 29. Alberici L, Paganini AM, Ricci C, et al. Development and validation of a preoperative ‘difficulty score’ for laparoscopic transabdominal adrenalectomy: a multicenter retrospective study. Surg Endosc 2022;36:3549–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pothet C, Drumez É, Joosten A, et al. Predicting intraoperative difficulty of open liver resections: the DIFF-scOR study, an analysis of 1393 consecutive hepatectomies from a French multicenter cohort. Ann Surg 2021;274:805–813. [DOI] [PubMed] [Google Scholar]
- 31. Grenda TR, Pradarelli JC, Dimick JB. Using surgical video to improve technique and skill. Ann Surg 2016;264:32. [DOI] [PMC free article] [PubMed] [Google Scholar]


