Background and Aim:
Treatment strategy for hepatocellular carcinoma (HCC) and Vp4 [main trunk] portal vein tumor thrombosis (PVTT) remains limited due to posttreatment liver failure. We aimed to assess the efficacy of irradiation stent placement with 125I plus transcatheter arterial chemoembolization (TACE) (ISP-TACE) compared to sorafenib plus TACE (Sora-TACE) in these patients.
Methods:
In this multicenter randomized controlled trial, participants with HCC and Vp4 PVTT without extrahepatic metastases were enrolled from November 2018 to July 2021 at 16 medical centers. The primary endpoint was overall survival (OS). The secondary endpoints were hepatic function, time to symptomatic progression, patency of portal vein, disease control rate, and treatment safety.
Results:
Of 105 randomized participants, 51 were assigned to the ISP-TACE group, and 54 were assigned to the Sora-TACE group. The median OS was 9.9 months versus 6.3 months (95% CI: 0.27–0.82; P=0.01). Incidence of acute hepatic decompensation was 16% (8 of 51) versus 33% (18 of 54) (P=0.036). The time to symptomatic progression was 6.6 months versus 4.2 months (95% CI: 0.38–0.93; P=0.037). The median stent patency was 7.2 months (interquartile range, 4.7–9.3) in the ISP-TACE group. The disease control rate was 86% (44 of 51) versus 67% (36 of 54) (P=0.018). Incidences of adverse events at least grade 3 were comparable between the safety populations of the two groups: 16 of 49 (33%) versus 18 of 50 (36%) (P=0.73).
Conclusion:
Irradiation stent placement plus TACE showed superior results compared with sorafenib plus TACE in prolonging OS in patients with HCC and Vp4 PVTT.
Keywords: hepatocellular carcinoma, irradiation stent, portal vein tumor thrombosis, transcatheter arterial chemoembolization
Introduction
Highlights
Irradiation stent placement plus transcatheter arterial chemoembolization is superior to sorafenib plus transcatheter arterial chemoembolization in prolonging overall in patients with hepatocellular carcinoma and Vp4 type PVTT (portal vein tumor thrombosis).
Prior irradiation stent placement decreased the occurrence rate of acute liver failure related to transcatheter arterial chemoembolization.
The use of irradiation stent is safe without increasing the incidence of treatment-related complications.
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide1. The majority of patients with HCC at diagnosis are in advanced disease stages, and 10–40% have macroscopic portal vein tumor thrombosis (PVTT)2. PVTT is a bottleneck in getting good long-term survival outcomes in the treatment of HCC due to its impact on overall hepatic blood flow and local tumor progression, especially when PVTT has invaded the main portal trunk.
Systemic therapy is the recommended treatment across the guidelines for patients with HCC with main PVTT3,4. Recently, atezolizumab plus bevacizumab have been shown by the milestone IMbrave150 trial to be a good first-line treatment for patients with unresectable HCC, including patients with PVTT5.
Transcatheter arterial chemoembolization (TACE) combined with sorafenib has widely been used in the past decade to treat patients with advanced stages of HCC, including patients with PVTT or extrahepatic metastases6–8. The TACTICS (transarterial chemoembolization in combination with sorafenib for intermediate-stage hepatocellular carcinoma treatment: a randomized controlled trial) trial, which combined sorafenib with TACE, showed significant improvement in progression-free survival (PFS) in patients with unresectable HCC6. In the IMbrave150 trial, over 40% of patients in the control group received at least one session of TACE prior to sorafenib9, supporting that in selected patients with advanced stages of HCC, TACE combined with sorafenib can be used. This treatment combination has also been advocated in the recent consensus guidelines from China and Korea7,10.
Stent placement in the main portal trunk can immediately restore portal venous blood flow, improve liver function, and decrease the incidence of variceal hemorrhage11. However, stent restenosis has been shown to occur in 50% of patients within 3 months due to tumor infiltration and tissue proliferation12. To prevent stent restenosis, integration of endovascular brachytherapy with iodine-125 (125I) seeds with stent placement has been investigated, with or without sequential TACE13–15. More recently, an irradiation stent system has been developed to provide a homogeneous and persistent radiation distribution to PVTT16. The combination of irradiation stent with TACE has been shown to be a safe and effective alternative therapy for HCC with PVTT17.
This randomized study aims to test the hypothesis that irradiation stent placement plus TACE (ISP-TACE) is superior to sorafenib plus TACE (Sora-TACE) in prolonging overall survival (OS) of patients with HCC and Vp4 [main trunk] PVTT.
Materials and methods
This randomized controlled trial (ClinicalTrials.gov Identifier: NCT03730675) was approved by the ethics committee of each participating center and complied with the Good Clinical Practice guidelines, the Declaration of Helsinki, and all applicable local laws. Each participant gave written informed consent prior to enrollment. The work has been reported in line with the Consolidated Standards of Reporting Trials (CONSORT, Supplemental Digital Content 1, http://links.lww.com/JS9/A284; Supplemental Digital Content 2, http://links.lww.com/JS9/A285) Guidelines. This trial had been registered at ClinicalTrials.gov, identifier: NCT03730675.
Study design and participants
This open-label randomized comparative trial was conducted at 16 medical centers in China. The main inclusion criteria were participants with age 18 years or above; histologically confirmed or clinically diagnosed HCC18; histologically confirmed or imaging-based diagnosed PVTT which had extended to the main portal vein; at least the right or left portal vein not being completely obstructed; measurable intrahepatic disease according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST); Child–Pugh class A or B.
The main exclusion criteria included patients with the presence of extrahepatic metastases; history or concurrence of other malignancies; greater than 70% tumor involvement of hepatic parenchyma; prior systemic or locoregional therapy, including surgery, radiation therapy, hepatic arterial embolization, TACE, hepatic arterial infusion, radiofrequency ablation, percutaneous ethanol injection, and cryoablation; presence of clinically relevant ascites as classified as a Child–Pugh score of ascites of 3.
Randomization and masking
The study statistician computer-generated stratified block randomization sequences with a block size of 4 before recruitment, using the software of SAS version 9.4 (SAS Institute Inc.). Randomization was performed centrally immediately after admission by staff members in the trial office. Participants were parallelly assigned to receive either irradiation stent placement plus TACE (the ISP-TACE group) or sorafenib plus TACE (the Sora-TACE group). Neither the participants nor the investigators were masked to the randomization results because of the operation and radiation protection necessity in the ISP-TACE group.
Procedures
In the ISP-TACE group, stent placement was performed under fluoroscopic and ultrasound guidance. The dosage of 125I seeds was determined by the extent of tumor thrombosis based on the treatment planning system (Qilin Co., Ltd). A patent second-order portal vein branch was punctured under ultrasound guidance. Portography was performed to show the location and extent of the thrombosis. After a Super Stiff Guidewire was advanced into the superior mesenteric vein, a 10-French (Fr) sheath was introduced. An outer irradiation stent (loaded with 125I seeds) was then inserted across the PVTT, followed immediately by a self-expandable metallic stent inserted through the same guidewire and sheath to overlap within the outer irradiation stent (Fig. 1). Radiation safety and management procedures were based on the recommendations of the International Commission on Radiological Protection.
Figure 1.
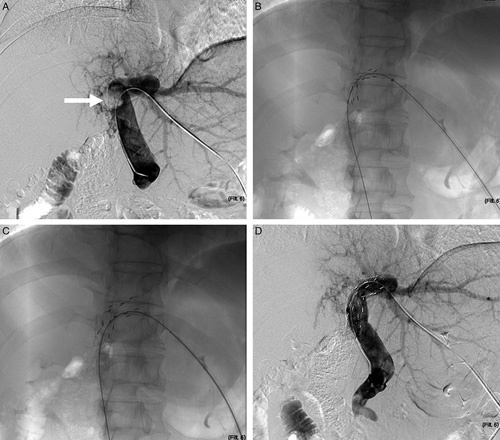
Representative images of irradiation stent placement for portal vein tumor thrombosis (PVTT). Representative images of a 50-year-old male patient who underwent irradiation portal vein stent placement for PVTT. (A) Portography showed a filling defect at the right branch of the portal vein and proximal main portal trunk (Vp4). (B) The self-expandable 125I radioactive seed-loaded stent was first inserted under fluoroscopic guidance. (C) A self-expandable nitinol stent was introduced and overlapped the outer stent within. (D) Portography showed immediate recanalization of the portal vein.
After stent placement, a daily dose of subcutaneous low-molecular-weight heparin was administrated for 1 week, followed by oral warfarin after hospital discharge to maintain an International Normalized Ratio from 1.8 to 2.5. The anticoagulant therapy was paused 12 h before TACE, and resumed the day after, and continued for 6 months unless there was any high risk of bleeding as clinically judged by the investigators or in the presence of thrombocytopenia. Portal vein recanalization was evaluated on treatment days 3–5 using color Doppler ultrasound16. Portal vein recanalization was defined as an increase of at least 50% in portal venous flow volume (PVFV) on color Doppler ultrasound. The estimated PVFV was calculated using the equation: PVFV (ml/min)=cross-sectional area (cm2)×mean portal blood velocity (cm/s)×60 s. If portal vein recanalization was achieved, conventional TACE was performed by using a mixture of Lipiodol (Guerbet LLC), chemotherapeutics (doxorubicin and cisplatin), and 500–700 µm gelatin sponge particles (Varian Medical System). The volume of Lipiodol injected was generally determined by the size and vascularity of the tumor, with common usage of 5–20 ml. All tumor-feeding arteries were embolized to achieve the devascularization of the tumor. The endpoint of TACE was the presence of flow stasis of the tumor-feeding arteries under fluoroscopic monitoring. Subsequent sequential TACE was performed on demand, if there were recurrences, residual tumors, or newly detected lesions on imaging follow-up. For participants with tumors assessed as complete response and participants with deteriorated liver function to Child–Pugh class C, PS score at least 3, serum total bilirubin (TB) greater than 5 mg/dl, and/or extrahepatic metastasis, TACE was discontinued.
In both the Sora-TACE and the ISP-TACE groups, the TACE regimen was the same. In the ISP-TACE group, sorafenib was not given, while in the Sora-TACE group, participants received 400 mg of sorafenib (Bayer) orally twice daily. Sorafenib was paused for 3 days before TACE and sorafenib was resumed 4–7 days after TACE. If drug-related adverse reactions occurred, the drug was discontinued, or the dosage was reduced (first to 200 mg twice daily, then to 200 mg once daily) by the assessing investigator.
Outcomes
The primary endpoint was OS, which was measured from the date of randomization to the date of death from any cause, or to the date of the last follow-up. The secondary endpoints were hepatic function, time to symptomatic progression, patency of portal vein, disease control rate (DCR), and treatment safety. Liver function at baseline and 3 months posttreatment was compared, including levels of serum TB, albumin, aspartate transaminase (AST), and serum alanine transaminase (ALT). Because anticoagulant therapy was given to patients after stent placement in the ISP-TACE group, prothrombin time was not used to evaluate liver function. Post-TACE liver failure was defined as the presence of any of the following: new onset hepatic encephalopathy, increasing or newly developed ascites, or an increase in the serum TB concentration of 0.9 mg/dl, or more 2 weeks posttreatment19. Time to symptomatic progression was measured from the date of randomization to the date of the first documented event of symptomatic progression [Eastern Cooperative Oncology Group (ECOG) PS score deteriorated to 4 or death from the baseline score (0, 1, or 2)]20. The DCR rate referred to the percentages of study participants who experienced complete response, partial response, or stable disease of intrahepatic lesions (PVTT being considered a nontargetable lesion). In the ISP-TACE group, patency of the portal vein was defined as the time between stent placement and first stent restenosis, which was defined as a reduction of at least 50% in portal venous flow volume on color Doppler ultrasound21. Treatment adverse events were evaluated by the type, incidence, and severity as graded by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.02.
Statistical analysis
Previous studies demonstrated that in patients with HCC with main portal vein invasion, the median OS after stent placement with 125I seeds combined with TACE was ∼8.4–12.5 months16,22,23, while that after sorafenib combined with TACE was ∼6.0–7.8 months24–26. Assuming the median OS to be 10.0 months in the ISP-TACE group versus 7.0 months in the Sora-TACE group, and considering a two-sided α-level test of 0.05, power 80%, and a drop-out rate of 5%, the sample size was estimated to be 308 participants by using the PASS11 software (NCSS, LLC).
The current study used a group sequential design method that included three planned interim analyses for OS. The Pocock design method was employed in the interim analysis to control the total type I errors of the trial. In the interim analysis, only the possibility of terminating the trial due to validity was considered. The first and second interim analyses were carried out when the number of enrolled participants reached 1/3 (103) and 2/3 (206) of the total sample (308). At the interim analysis, if P is 0.022 or less, the trial was considered to reach validity and then terminated. If P is greater than 0.022, it will continue until the end of the study.
Variables were expressed as numbers (percentages), medians with interquartile range (IQR), or range. The endpoints, including OS, liver function, DCR, time to symptomatic progression, and patency of portal vein, were assessed in the full analysis set, which was defined as all patients who were enrolled and randomized in the study using the intention-to-treat principle. Liver function and DCR for each group were compared using the Pearson χ 2 or Fisher exact test (χ 2 test or Fisher exact test). Between-group comparisons in OS and time to symptomatic progression were made using the log-rank test. The Cox proportional hazard model was used to estimate hazard ratios (HRs) for OS. Treatment safety was assessed in participants who received at least one study treatment and had safety evaluation data afterward.
A P value 0.022 or less was considered to indicate statistical significance on the OS between groups. For comparisons on other endpoints, a significant level of 0.05 was applied. All statistical analyses were performed using R software (R version 4.1.1)
Role of the funding source
The study was supported by the Jiangsu Provincial Special Program of Medical Science (BE2019750, BK20190350), the National Natural Science Foundation of China (81827805, 82001935), and the National Key Research and Development Program (2018YFA0704100, 2018YFA0704104). The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication. L.-G.L., W.-Y.L., and G.-J.T. had full access to all data in the study and took final responsibility for manuscript submission.
Results
After analyses of data by the Trial Management Committee on 15 July 2021, the Committee decided that the differences in OS between groups were so significant that it was unethical to continue with the study. The results of this interim analysis are shown below.
Participant characteristics
Between November 2018 and July 2021, of 152 participants who were screened, 105 were enrolled and randomized either to the ISP-TACE group (n=51) or the Sora-TACE group (n=54) (Fig. 2). Of the randomized participants, 105 were treated with the study protocol treatments entered the full analysis set; Supplemental Digital Content 3, http://links.lww.com/JS9/A286. The safety population of the ISP-TACE group comprised participants who received irradiation stent placement and at least one TACE session (n=49). The safety population of the Sora-TACE group comprised participants who received at least one dose of sorafenib and one TACE session (n=50). The baseline characteristics were generally balanced (Table 1).
Figure 2.
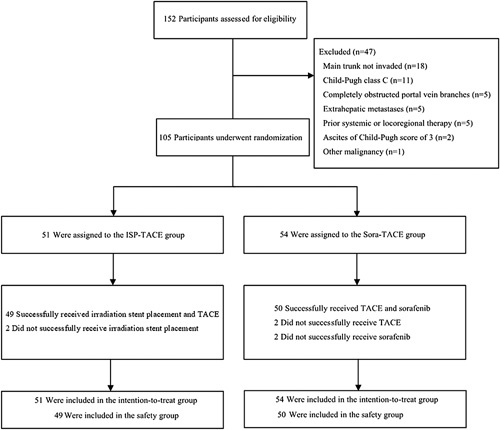
Trial profile. ISP-TACE, irradiation stent placement plus transcatheter arterial chemoembolization; TACE, transcatheter arterial chemoembolization.
Table 1.
Baseline characteristics.
| Characteristic | ISP-TACE group (n=51) | Sora-TACE group (n=54) | P |
|---|---|---|---|
| Age (years) | |||
| <65 | 41 (80) | 40 (74) | 0.59 |
| ≥65 | 10 (20) | 14 (26) | |
| Gender | |||
| Male | 49 (96) | 48 (89) | 0.47 |
| Female | 2 (4) | 6 (11) | |
| Child–Pugh class | |||
| A | 24 (47) | 26 (48) | 0.13 |
| B | 27 (53) | 28 (52) | |
| AFP (ng/ml) | |||
| <200 | 25 (49) | 32 (59) | 0.14 |
| ≥200 | 26 (51) | 22 (41) | |
| Serum total bilirubin (μmol/l, IQR) | 1.2 (0.9–2.0) | 1.2(0.8–1.9) | 0.12 |
| Tumor diameter (cm) | |||
| <5 | 16 (31) | 21 (39) | 0.21 |
| ≥5 | 35 (69) | 33 (61) | |
| BMI (kg/m2, IQR) | 22 (20–25) | 22 (20–25) | 0.88 |
| ECOG performance status score | |||
| 0 | 12 (24) | 17 (31) | 0.08 |
| 1 | 27 (53) | 23 (43) | |
| 2 | 12 (23) | 14 (26) | |
| HCC morphology | |||
| Mass type | 33 (64) | 40 (74) | 0.57 |
| Nodular type | 7 (14) | 5 (9) | |
| Diffuse type | 11 (22) | 9 (17) | |
| Number of intrahepatic tumors | |||
| 1 | 22 (43) | 18 (33) | 0.16 |
| 2 | 10 (20) | 15 (28) | |
| 3 | 4 (8) | 5 (9) | |
| >3 | 15 (29) | 16 (30) | |
| PVTT extension | |||
| Main trunk | 4 (8) | 4 (7) | 0.70 |
| Main trunk+unilateral branch | 28 (55) | 28 (52) | |
| Main trunk+bilateral branches | 19 (37) | 22 (41) | |
| Etiology of HCC | |||
| HBV | 39 (76) | 47 (87) | 0.29 |
| HBC | 3 (6) | 2 (44) | |
| Alcoholic hepatitis related | 0 | 1 (2) | |
| Unknown | 9 (17) | 4 (7) | |
Note: percentages may not total 100 because of rounding.
Data are n (%) unless otherwise indicated.
AFP, alpha fetoprotein; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IQR, interquartile range; ISP-TACE, irradiation stent placement plus transcatheter arterial chemoembolization; PVTT, portal vein tumor thrombosis. Sora-TACE, sorafenib plus transcatheter arterial chemoembolization.
The mean number of TACE cycles per participant was 2.6 in the ISP-TACE group (range, 1–6) versus 1.5 (range, 0–5) in the Sora-TACE group (P<0.001). In the ISP-TACE group, 49 irradiation stents were successfully placed in 49 participants (one stent per participant), whereas the stent failed to pass through the tumor occlusion in the remaining two participants. The median activity of 125I seeds used in the ISP-TACE group was 12 millicuries (mCi; range, 6–16 mCi). The median calculated radiation dose at the dose prescription point was 66 gray (Gy; range, 30–90 Gy). In the Sora-TACE group, the median duration treated with sorafenib was 105 days (range, 7–420 days) and the median actual daily dose of sorafenib was 386 mg (range, 285–800 mg).
Primary endpoint
As of the date of clinical data cutoff (15 July 2021), the median duration of follow-up was 10.7 months (IQR, 8.9–14.2) for the ISP-TACE group, versus 7.0 months (IQR, 5.3–8.5) for the Sora-TACE group. A total of 36 (67%) of 51 participants in the ISP-TACE group and 38 (75%) of 54 in the Sora-TACE group were demised (HR=0.64; 95% CI: 0.27–0.82; P=0.010). The median OS was 9.9 months (95% CI: 7.6–12.2) in the ISP-TACE group compared with 6.3 months (95% CI: 5.4–7.2) in the Sora-TACE group (P=0.016). The estimated rates of survival at 3, 6, and 12 months in the ISP-TACE group were 84% (95% CI: 74.9–94.9), 72.5% (95% CI: 61.1–85.8), and 21.6% (95% CI: 13.2–41.4) versus 82% (95% CI: 71.0–92.3), 41% (95% CI: 34.6–64.2), and 9% (95% CI: 2.3–48.9) in the Sora-TACE group, respectively (Fig. 3). The most common cause of death in the ISP-TACE group was a liver failure (45%), in contrast to liver failure (59%) in the Sora-TACE group.
Figure 3.
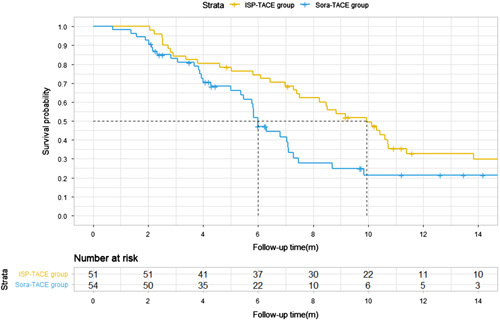
Kaplan–Meier estimate of overall survival (OS). Kaplan–Meier curves show the median OS as the primary endpoint of this study was 9.9 months (95% CI: 7.6–12.2) in the ISP-TACE group compared with 6.3 months (95% CI: 5.4–7.2) in the Sora-TACE group (P=0.016). The survival rates at 3, 6, and 12 months in the ISP-TACE group versus the Sora-TACE group were 84, 73, and 22% versus 81, 41, and 9%, respectively (P=0.016). ISP-TACE group, irradiation stent placement plus transcatheter arterial chemoembolization group; Sora-TACE group, sorafenib plus transcatheter arterial chemoembolization group; TACE, transcatheter arterial chemoembolization.
Post hoc subgroup analysis was done for the proportion of participants who had the risk of death. Forest plot revealed that the HR value of OS benefit between the ISP-TACE group and the Sora-TACE group was 0.56, indicating that ISP-TACE could actually reduce the risk of death by 44% compared to Sora-TACE. OS for participants in all the subgroups benefited from ISP-TACE treatment (Fig. 4).
Figure 4.
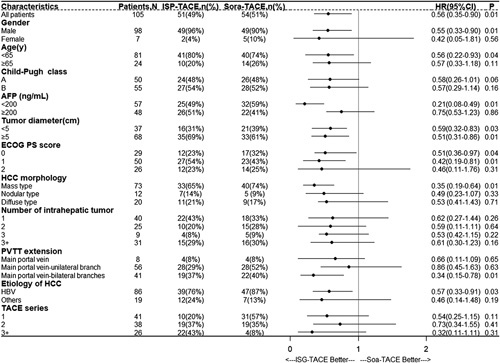
Overall survival of select subgroups according to baseline prognostic factors. The forest plot shows that the risk of death for participants in the ISP-TACE group was reduced by 44% when compared with the Sora-TACE group. OS for participants in all the subgroups benefited from ISP-TACE treatment. AFP, alpha fetoprotein; ECOG PS, Eastern Cooperative Oncology Group performance status; HCC, hepatocellular carcinoma; ISP-TACE, irradiation stent placement plus transcatheter arterial chemoembolization; PVTT, portal vein tumor thrombosis; Sora-TACE, sorafenib plus transcatheter arterial chemoembolization; TACE, transcatheter arterial chemoembolization.
Secondary endpoints
For the ISP-TACE group, there were no significant differences in the levels of TB and albumin 3 months posttreatment compared to baseline values (P>0.05). In contrast, the levels of AST and ALT decreased significantly (P<0.001). For the Sora-TACE group, there were significant differences in the levels of albumin, AST, and ALT 3 months posttreatment compared to baseline values (P<0.05). There was no significant difference in the level of TB 3 months posttreatment compared to the baseline value (P>0.05) (Table 2). Post-TACE liver failure was recorded in 3 (6%) participants in the ISP-TACE group and 11 (20%) in the Sora-TACE group (P=0.035). For the three participants in the ISP-TACE group, liver function returned to its pretreatment level within 1 month. Of the 11 participants in the Sora-TACE group, 2 (12%) participants eventually developed irreversible liver failure.
Table 2.
Comparison of liver function at baseline and 3 months posttreatment.
| Baseline | 3-month value | P | |
|---|---|---|---|
| TB (mg/dl) | TB (mg/dl) | ||
| ISP-TACE group | 1.2 (0.9–2.0) | 1.3 (0.9–1.7) | 0.99 |
| Sora-TACE group | 1.2 (0.8–1.9) | 1.5 (1.3–2.0) | 0.07 |
| Albumin (g/l) | Albumin (g/l) | ||
| ISP-TACE group | 37.6 (32.0–42.3) | 36.0 (30.9–38.8) | 0.25 |
| Sora-TACE group | 36.8 (32.8–40.2) | 35.5 (31.5–37.8) | 0.03 |
| AST (U/l) | AST (U/l) | ||
| ISP-TACE group | 59.2 (50.6–67.8) | 40.4 (34.5–47.1) | <0.001 |
| Sora-TACE group | 60.0 (43.6–94.5) | 72.5 (63.5–82.2) | <0.001 |
| ALT (U/l) | ALT (U/l) | ||
| ISP-TACE group | 45.1(38.5–51.7) | 33.3(28.1–38.3) | <0.001 |
| Sora-TACE group | 39.6(34.4–44.8) | 46.2(40.1–52.3) | <0.001 |
ALT, alanine transaminase; AST, aspartate transaminase; ISP-TACE, irradiation stent placement plus transcatheter arterial chemoembolization; Sora-TACE, sorafenib plus transcatheter arterial chemoembolization; TB, total bilirubin.
A total of 40 (78%) of 51 participants in the ISP-TACE group and 45 (83%) of 54 in the Sora-TACE group had symptomatic progression. The time to symptomatic progression was 6.6 months (95% CI: 5.1–8.1) in the ISP-TACE group versus 4.2 months (95% CI: 3.6–4.8) in the Sora-TACE group (HR=0.60; 95% CI: 0.38–0.93; P=0.037). The 6-month symptomatic progression was 40% (95% CI: 24.9–53.6) in the ISP-TACE group versus 75.4% (95% CI: 59.4–92.3) in the Sora-TACE group (Fig. 5).
Figure 5.
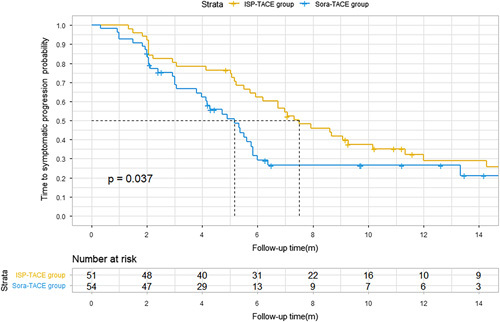
Kaplan–Meier estimate of time to symptomatic progression. Kaplan–Meier curves show the median time to symptomatic progression was 6.6 months (95% CI: 5.1–8.1) in the ISP-TACE group and 4.2 months (95% CI: 3.6–4.8) in the Sora-TACE group (P=0.04). ISP-TACE group, irradiation stent placement plus transcatheter arterial chemoembolization group; Sora-TACE group, sorafenib plus transcatheter arterial chemoembolization group; TACE, transcatheter arterial chemoembolization.
The median stent patency in the ISP-TACE group was 7.2 months (IQR, 4.7–9.3). The estimated rates of stent patency at 6 months and 12 months were 89% (95% CI: 72.4–98.7) and 45% (95% CI: 23.1–63.5), respectively.
A complete response or partial response was observed in 29 participants in the ISP-TACE group versus 19 in the Sora-TACE group. The DCR was 86% (44 of 51) in the ISP-TACE group versus 67% (36 of 54) in the Sora-TACE group (P=0.018) (Table 3).
Table 3.
Intrahepatic disease response assessed using mRECIST criteria.
| Response | ISP-TACE group | Sora-TACE group | P |
|---|---|---|---|
| CR | 11 (22) | 3 (6) | |
| PR | 18 (35) | 16 (30) | |
| SD | 15 (29) | 17 (31) | |
| PD | 7 (14) | 18 (33) | |
| DCR (CR+PR+SD) | 44 (86) | 36 (67) | 0.018 |
Data are n (%) unless otherwise indicated.
CR, complete response; DCR, disease control rate; ISP-TACE, irradiation stent placement plus transcatheter arterial chemoembolization; mRECIST, modified Response Evaluation Criteria in Solid Tumors; PD, progressive disease; PR, partial response; SD, stable disease; Sora-TACE, sorafenib plus transcatheter arterial chemoembolization.
Adverse events of any causes which occurred in at least 10% of the participants in the safety population are listed in Table 3. Adverse events of special interest, including radiation-induced liver disease (RILD) and migration of radioactive seeds, were not observed in the ISP-TACE group. Adverse events at least grade 3 were detected in 16 (33%) of the 49 treated participants in the ISP-TACE group, and in 18 (36%) of 50 in the Sora-TACE group (P=0.726). The most common grade 3 or higher adverse event were abdominal pain (10%) in the ISP-TACE group, elevated AST level (6%), and hand–foot skin reaction (6%) in the Sora-TACE group. There was no treatment-related death within 4 weeks of treatment in either of the two groups (Table 4).
Table 4.
Adverse events of any causes that occurred in ≥10% of participants in the safety population.
| ISP-TACE group (n=49) | Sora-TACE group (n=50) | ||||||
|---|---|---|---|---|---|---|---|
| Adverse event | Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | P |
| Higher levels of AST | 41 (84) | 2 (4) | 0 | 44 (88) | 2 (4) | 1 (2) | 0.28 |
| Higher levels of ALT | 38 (78) | 2 (4) | 0 | 42 (84) | 2 (4) | 0 | 0.38 |
| Hyperbilirubinemia | 13 (27) | 0 | 0 | 15 (30) | 1 (2) | 0 | 0.55 |
| Anemia | 34 (70) | 3 (6) | 0 | 33 (66) | 2 (4) | 0 | 0.54 |
| Thrombocytopenia | 32 (65) | 2 (4) | 0 | 34 (68) | 2 (4) | 0 | 0.78 |
| Neutropenia | 9 (18) | 0 | 0 | 7 (14) | 0 | 0 | 0.56 |
| Leukopenia | 15 (31) | 0 | 0 | 19 (38) | 1 (2) | 0 | 0.33 |
| Fatigue | 5 (10) | 0 | 0 | 12 (24) | 0 | 0 | 0.07 |
| Abdominal pain | 14 (29) | 4 (8) | 1 (2) | 6 (12) | 1 (2) | 0 | 0.01 |
| Diarrhea | 1 (2) | 0 | 0 | 5 (10) | 0 | 0 | 0.09 |
| Nausea/vomiting | 18 (37) | 1 (2) | 0 | 21 (42) | 1 (2) | 0 | 0.82 |
| Rash | 2 (4) | 0 | 0 | 7 (14) | 0 | 0 | 0.08 |
| Hand–foot skin reaction | 0 | 0 | 0 | 23 (46) | 3 (6) | 0 | / |
| Pruritus | 2 (4) | 0 | 0 | 18 (36) | 1 (2) | 0 | 0.01 |
| Alopecia | 1 (1) | 0 | 0 | 12 (24) | 0 | 0 | 0.01 |
| Constipation | 3 (6) | 0 | 0 | 5 (10) | 0 | 0 | 0.48 |
| Hypertension | 2 (4) | 0 | 0 | 6 (12) | 1 (2) | 0 | 0.08 |
| Fever | 21 (43) | 0 | 0 | 20 (40) | 0 | 0 | 0.77 |
| Malaise | 6 (12) | 0 | 0 | 11 (22) | 0 | 0 | 0.20 |
| Anorexia | 5 (10) | 0 | 0 | 7 (14) | 0 | 0 | 0.57 |
| Weight loss | 2 (4) | 0 | 0 | 5 (10) | 0 | 0 | 0.25 |
| Hoarseness | 0 | 0 | 0 | 5 (10) | 0 | 0 | / |
| Hemorrhage | 5 (10) | 1 (2) | 0 | 3 (6) | 0 | 0 | 0.28 |
Data are noted in n (%). Adverse events are presented according to the Medical Dictionary for Regulatory Affairs system organ class.
ALT, alanine aminotransaminase; AST, aspartate aminotransferase; ISP-TACE, irradiation stent placement plus transcatheter arterial chemoembolization; Sora-TACE, sorafenib plus transcatheter arterial chemoembolization.
Discussion
The present trial focused on a specific group of patients with HCC and Vp4 [main trunk] PVTT without extrahepatic metastases. The results showed that irradiation stent placement plus TACE significantly prolonged OS in patients with HCC and Vp4 [main trunk] PVTT compared with those treated with sorafenib plus TACE. Besides, our study showed that the time to symptomatic progression in the ISP-TACE group was significantly longer than that in the Sora-TACE group, the rate of post-TACE liver failure was significantly lower in the ISP-TACE group than that in the Sora-TACE group, and the DCR was better in the ISP-TACE group than that in the Sora-TACE group. Comparable grade 3 or 4 adverse events were reported between the two groups.
This regimen in managing Vp4 [main trunk] PVTT was designed to improve liver function by recanalizing the main portal vein to locally treat PVTT with 125I seeds and to treat liver tumors using TACE. Although many studies have shown the treatment safety of TACE in patients with HCC and PVTT27,28, there are still concerns about inducing liver failure after concurrent interruption of blood flow to both the hepatic artery and portal vein. In addition, one study showed that the lower the degree of liver damage (new or increased ascites, elevated serum bilirubin, decreased serum albumin, etc.) after TACE, the better the survival benefit29. In our study, the liver function, including the levels of TB, albumin, AST, and ALT, were improved or restored despite the continued TACE in the ISP-TACE group of patients. In contrast, the levels of albumin, AST, and ALT in the Sora-TACE group remained worse compared with baseline values. Moreover, irradiation stent placement decreased the rate of post-TACE liver failure. Our results indicated an association of portal vein recanalization with improvement in OS, owing to the decreased liver damage after TACE treatment. The placement of an irradiation stent prior to TACE could serve as a useful bridge for the subsequent treatment of TACE.
Radiotherapy has been recommended for HCC with PVTT in the East and the West guidelines, with or without TACE8,10. A recent randomized controlled study revealed that the median OS of TACE combined with external radiotherapy was 12.8 months, which was significantly higher than the 10.0 months treated with sorafenib30. Excessive external radiation may lead to RILD, and a safe threshold of radiation dose is only 30 Gy31. As radiation tolerance of liver parenchyma is reduced in the presence of liver failure31, dosage delivered to tumors using external radiation is often inadequate. Selective internal radiation therapy (SIRT) with 90Y microspheres has been regarded as a viable treatment option for HCC with PVTT. The SARAH (SorAfenib versus Radioembolisation in Advanced Hepatocellular carcinoma) trial revealed that the study participants in the SIRT group obtained a modest OS of 8 months, which was still inferior to the sorafenib group of 9.9 months32. Our study showed that in the ISP-TACE group, a conformal and a high dose of endovascular brachytherapy could be delivered by inserting a radioactive 125I seed-loaded stent. Although direct evaluation of the PVTT response was not possible due to the change in the shape of the PVTT after stent expansion, the long-term patency of the portal vein indicated that radioactive 125I seeds could kill or inhibit extension of PVTT and prevented stent restenosis caused by PVTT growth, which was beneficial to patient’s ability to tolerate continued therapy.
For patients with advanced HCC, intrahepatic tumor progression and liver failure are the two most common causes of death, even for patients with extrahepatic metastases33. To achieve improved long-term survival outcomes, controlling intrahepatic tumor growth using locoregional therapies is necessary. Improved tumor response of intrahepatic tumors to TACE was observed in the ISP-TACE group than in the Sora-TACE group. Such a phenomenon can be attributed to the more sessions of TACE being performed in the ISP-TACE group (average, 2.6 vs. 1.5), with improvements in the functional reserve of these participants from prior stent placement. Post hoc analysis revealed that OS for the participants in all the subgroups benefited from ISP-TACE treatment, despite some patients having characteristics associated with poor prognosis-including older age (≥65 years), greater ECOG PS of score 2, worse liver function (Child–Pugh B), a more heavy burden of intrahepatic tumor (diameter ≥5 cm, number ≥3, or morphology of diffuse type) or more extensive spread of PVTT.
Radiation safety of 125I seed stent has been demonstrated in previous studies in treating esophageal cancer, malignant biliary obstruction, and airway obstruction34–36 and in the portal vein to treat PVTT16. The estimated radiation dosage of 125I seeds of portal vein irradiation stent in our study was 66 Gy, which is comparable to the 57 Gy of the targeted radiation dose of stereotactic body radiation therapy37. The 125I seed has a very low dose rate with an incipient rate of 7 cGy/h and has a half-value layer of 17 mm. The local radiation effect outside of the target decreases rapidly with the inverse square law to the surrounding hilar structures. In our study, RILD was not observed in the ISP-TACE group, indicating the safe and effective design of the irradiation stent. Abdominal pain was a special adverse event observed during stent placement. Nevertheless, most of the abdominal pain was in grade 1 or 2. The pain was related to the process of portal vein puncture and stent insertion and was relieved when the operation was completed.
Our study had limitations. First, it was conducted mainly on hepatitis B virus-related HCC. Second, all the centers involved came from China, which all have huge experience in treating HCC with PVTT. Thus, our results may not be repeated in small centers with less experience.
In summary, the findings suggested irradiation stent placement plus TACE was superior to sorafenib plus TACE in prolonging OS in patients with HCC and Vp4 [main trunk] PVTT. Whether these results can be extrapolated to patients with other etiologies of HCC, need to be further validated.
Ethical approval
This randomized controlled trial was approved by the ethics committee of participating center. The relevant Judgement’s reference number was 2018ZDSYLL113-P01.
Sources of funding
The study was supported by the Jiangsu Provincial Special Program of Medical Science (BE2019750, BK20190350), the National Natural Science Foundation of China (81827805, 82001935), and the National Key Research and Development Program (2018YFA0704100, 2018YFA0704104). The funders of the study had no role in the study protocol design, data analysis and interpretation, or writing of the report.
Author contribution
J.L.: conceptualization, data curation, investigation, methodology, software, validation, visualization, writing – original draft, and writing – review and editing; J.-H.G.: conceptualization, data curation, investigation, methodology, resources, validation, and visualization; J.-S.J. and Y.-L.L.: conceptualization, investigation, resources, validation, and visualization; W.-F.L.: conceptualization, investigation, methodology, resources, validation, and visualization; H.-D.Z.: conceptualization, data curation, investigation, methodology, validation, visualization, writing – original draft, and writing – review and editing; J.-H.S., W.-X.R., F.-J.Z., W.-D.W., H.-B.S., G.-S.C., H.-L.L., K.G., P.Y., and G.-W.Y.: investigation and resources; G.-Y.Z.: conceptualization and methodology; F.-Z.W.: investigation, methodology, and software; W.-J.W., D.L., and S.-Q.C.: investigation, methodology, and software; J.M.: data curation, formal analysis, and methodology; Y.Z.: formal analysis, methodology, software, validation, visualization, and writing – review and editing; R.L.: formal analysis, methodology, software, validation, and visualization; L.-G.L. and W.Y.L.: conceptualization, data curation, investigation, methodology, resources, software, validation, visualization, writing – original draft, and writing – review and editing; G.-J.T.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, software, validation, visualization, writing – original draft, and writing – review and editing.
Conflicts of interest disclosure
The authors declare there are no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Irradiation Stent Placement Plus TACE for HCC and PVTT.
Unique identifying number or registration ID: NCT03730675.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.clinicaltrials.gov/ct2/show/NCT03730675?cond=irradiation+stent&draw=2&rank=1
Guarantor
Li-Gong Lu, Wan Yee Lau, and Gao-Jun Teng.
Data availability statement
All relevant data are available within the manuscript and its supplementary material files. Further inquiries can be directed to the corresponding author (Gao-Jun Teng).
Supplementary Material
Footnotes
J.L., J.H.G., J.S.J., Y.L.L., W.F.L., and H.D.Z. contributed equally to this article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 12 April 2023
Contributor Information
Jian Lu, Email: lujian43307131@126.com.
Jin-He Guo, Email: jinheguo@sina.com.
Jian-Song Ji, Email: jjstcty@sina.com.
Yu-Liang Li, Email: lyl.pro@163.com.
Wei-Fu Lv, Email: lwf99@126.com.
Hai-Dong Zhu, Email: zhuhaidong9509@163.com.
Jun-Hui Sun, Email: sunjh121@163.com.
Wei-Xin Ren, Email: rwx1031@163.com.
Fu-Jun Zhang, Email: zhangfj@sysucc.org.cn.
Wei-Dong Wang, Email: wdoc@sina.com.
Hai-Bo Shao, Email: haiboshao@aliyun.com.
Guang-Shao Cao, Email: yajuncao98@126.com.
Hai-Liang Li, Email: lihailiang0589@sina.com.
Kun Gao, Email: gaokun2000cn@aliyun.com.
Po Yang, Email: 13704810561@163.com.
Guo-Wen Yin, Email: jsnjygw@163.com.
Guang-Yu Zhu, Email: njzgy@sina.com.
Fa-Zong Wu, Email: 13567621225@139.com.
Wu-Jie Wang, Email: 48202178@qq.com.
Dong Lu, Email: hyjh2004@126.com.
Sheng-Qun Chen, Email: chenshengqun2007@163.com.
Jie Min, Email: minjie93@163.com.
Yang Zhao, Email: yzhao@njmu.edu.cn.
Rui Li, Email: qmjk_xsj@163.com.
Li-Gong Lu, Email: lu_ligong@163.com.
Wan Yee Lau, Email: josephlau@cuhk.edu.hk.
Gao-Jun Teng, Email: gjteng@vip.sina.com.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Ogren M, Bergqvist D, Bjorck M, et al. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol 2006;12:2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver. Electronic address easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 4. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–50. [DOI] [PubMed] [Google Scholar]
- 5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Department of Medical Administration, National Health and Health Commission of the People’s Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi 2020;28:112–128. [DOI] [PubMed] [Google Scholar]
- 8. Cheng S, Chen M, Cai J, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 edition). Liver Cancer 2020;9:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salem R, Li D, Sommer N, et al. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: results from the IMbrave150 trial. Cancer Med 2021;10:5437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korean Liver Cancer Association, National Cancer Center. 2018 Korean Liver Cancer Association – National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol 2019;20:1042–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan SL, Chong CC, Chan AW, et al. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol 2016;22:7289–7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamakado K, Nakatsuka A, Tanaka N, et al. Malignant portal venous obstructions treated by stent placement: significant factors affecting patency. J Vasc Interv Radiol 2001;12:1407–1415. [DOI] [PubMed] [Google Scholar]
- 13. Zhang ZH, Zhang W, Gu JY, et al. Treatment of hepatocellular carcinoma with tumor thrombus with the use of iodine-125 seed strand implantation and transarterial chemoembolization: a propensity-score analysis. J Vasc Interv Radiol 2018;29:1085–93. [DOI] [PubMed] [Google Scholar]
- 14. Lu J, Zhong BY, Zhu HD, et al. Embolotherapy of unresectable hepatocellular carcinoma: Eastern perspective. Chin Clin Oncol 2019;8:60. [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Teng M, Zhang H, et al. Advanced radionuclides in diagnosis and therapy for hepatocellular carcinoma. Chin Chem Lett 2022;33:13. [Google Scholar]
- 16. Lu J, Guo JH, Zhu HD, et al. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol 2017;28:786–94.e3. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Guo JH, Lu J, et al. I(125) irradiation stent for treatment of hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. Cancer Radiother 2021;25:340–349. [DOI] [PubMed] [Google Scholar]
- 18. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer 2018;7:235–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 1995;332:1256–1261. [DOI] [PubMed] [Google Scholar]
- 20. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 21. Niciforovic D, Till V, Hadnadev D, et al. Duplex Doppler sonography in portal hypertension. Med Pregl 2007;60:161–167. [DOI] [PubMed] [Google Scholar]
- 22. Luo JJ, Zhang ZH, Liu QX, et al. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int 2016;10:185–195. [DOI] [PubMed] [Google Scholar]
- 23. Luo J, Yan Z, Liu Q, et al. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol 2011;22:479–489. [DOI] [PubMed] [Google Scholar]
- 24. Zhang ZH, Liu QX, Zhang W, et al. Combined endovascular brachytherapy, sorafenib, and transarterial chemobolization therapy for hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol 2017;23:7735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist 2015;20:1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan T, Li XS, Xie QK, et al. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol 2014;69:e553–e561. [DOI] [PubMed] [Google Scholar]
- 27. Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011;258:627–634. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y, Cai G, Zhou L, et al. Transarterial chemoembolization in hepatocellular carcinoma with vascular invasion or extrahepatic metastasis: a systematic review. Asia-Pac J Clin Oncol 2013;9:357–364. [DOI] [PubMed] [Google Scholar]
- 29. Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461–469. [DOI] [PubMed] [Google Scholar]
- 30. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018;4:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys 2006;65:426–434. [DOI] [PubMed] [Google Scholar]
- 32. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624–36. [DOI] [PubMed] [Google Scholar]
- 33. Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 2007;13:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu HD, Guo JH, Mao AW, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol 2014;15:612–619. [DOI] [PubMed] [Google Scholar]
- 35. Zhu HD, Guo JH, Huang M, et al. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: a multicenter trial. J Hepatol 2018;68:970–977. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Lu J, Guo JH, et al. A novel tracheobronchial stent loaded with (125)I seeds in patients with malignant airway obstruction compared to a conventional stent: a prospective randomized controlled study. EBioMedicine 2018;33:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaub SK, Hartvigson PE, Lock MI, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: current trends and controversies. Technol Cancer Res Treat 2018;17:1533033818790217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available within the manuscript and its supplementary material files. Further inquiries can be directed to the corresponding author (Gao-Jun Teng).


