Background:
Surgical resection of pheochromocytomas and paragangliomas (PPGLs) is associated with a significant risk of intraoperative hemodynamic instability and cardiovascular complications. α-blockade remains the routine preoperative medical preparation despite controversies over the lack of evidence. We presented an updated meta-analysis to ulteriorly evaluate the potential efficacy of preoperative α-blockade versus no blockade for PPGL patients undergoing surgery.
Materials and methods:
Randomized and nonrandomized comparative studies assessing preoperative α-blockade for PPGL surgery in adults were identified through a systematic literature search via MEDLINE, Embase, Web of Science, and CENTRAL up to November 2022. Outcome data of intraoperative hemodynamic parameters and major postoperative events were extracted. Mean difference and risk ratio were synthesized as appropriate for each outcome to determine the cumulative effect size.
Results:
Fifteen nonrandomized studies involving 3542 patients were finally eligible. Intraoperatively, none of the analyzed hemodynamic parameters differed between patients with or without α-blockade: maximum and minimum systolic blood pressure, hypertensive and hypotensive hemodynamic instability episodes, and peak heart rate, subgroup analysis of normotensive PPGL patients yielded similar results with the overall effects. Postoperatively, α-blockade was associated with prolonged hypotension and vasopressor usage (risk ratio: 4.21, 95% CI: 1.17–15.18, P=0.03). ICU admission, length of stay, overall cardiovascular morbidity, and mortality were similar between the two groups.
Conclusions:
Preoperative α-blockade ensured neither more stable intraoperative hemodynamics nor better perioperative outcome over no blockade for PPGL surgery. However, large-volume randomized controlled trials are still warranted to ascertain these findings.
Keywords: α-blockade, meta-analysis, paraganglioma, pheochromocytoma
Introduction
Highlights
Eleven new studies were eligible for this updated meta-analysis.
Preoperative α-blockade demonstrated no beneficial efficacy over no blockade.
These findings were consistent in both normotensive and hypertensive patients.
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors deriving from chromaffin cells of the adrenal medulla and extra-adrenal ganglia, characterized by the episodic release of catecholamines that may arouse drastic blood pressure fluctuation and even life-threatening multiorgan dysfunction. Surgical removal remains the only curative option for localized PPGL. The procedure involves a significant risk of intraoperative hemodynamic instability (HDI) and cardiovascular complications like stroke, left ventricular failure, and myocardial infarction1,2. Historically, perioperative mortality reached as high as 20–45% in functional PPGL patients3,4.
According to relevant guidelines, systemic α-blockade with selective or nonselective blockers should be accomplished routinely before PPGL resections, even with biochemically silent and normotensive patients5–8. It was advocated to correct catecholamine-related vasoconstriction and hypovolemia by blocking α1-adrenoceptor. However, these recommendations were almost entirely based on observational findings and expert consensus rather than convincing evidence, with the absence of randomized controlled studies5,9.
Overall perioperative major morbidity and mortality of PPGL surgeries has fallen steadily to less than 1–3% contemporarily since the introduction of α-blockade pretreatment7,10,11. However, the extent of their correlations remains doubtful, considering the profound evolvement of diagnostic modalities, anesthetic monitoring, as well as surgical approaches. Recent studies indicated that despite optimal α-blockade, intraoperative hemodynamic fluctuations were still common but rarely associated with major cardiovascular events12,13. Prolonged α-blockade predicted more perioperative hypotension episodes14. Consequently, pheochromocytoma surgery omitting preoperative α-blockade and even hypotensors is safe and feasible for selected patients15,16. Hence the dogmatic α-blockade modality before PPGL surgery necessitates a comprehensive appraisal.
Herein, we conducted a systematic review and updated meta-analysis of the current literature to evaluate the efficacy of α-blockade pretreatment for PPGL patients undergoing surgical resections regarding intraoperative hemodynamics and postoperative events.
Materials and methods
The protocol was formulated a priori and registered in the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42022370263). This meta-analysis has been formulated following PRISMA standards and complies with the PRISMA 2020 statement17.
Search strategy
A comprehensive literature search of the electronic databases MEDLINE (via PubMed), Embase, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) was performed by two independent reviews up to 6 November 2022. The search strategy for MEDLINE consisted of the key search terms ‘pheochromocytoma,’ ‘paraganglioma,’ ‘surgery,’ ‘adrenalectomy,’ and their synonyms, as well as related Medical Subject Headings (MeSH) terms combined with ‘alpha-blockade’ and the associated medications (‘phenoxybenzamine,’ ‘doxazosin,’ ‘prazosin,’ ‘terazosin,’ et al.). No restriction on language and the publication date was predefined. The reference lists of relevant studies were manually screened to reduce omission.
Study eligibility
Inclusion: Randomized controlled trials (RCTs), nonrandomized prospective and retrospective studies comparing preoperative α-blockade with no blockade in histologically confirmed PPGL patients undergoing surgery concerning intraoperative hemodynamics and/or perioperative morbidity and mortality.
Exclusion: All other designs like reviews, meeting abstracts, comments, and editorials; Studies involving animals, pediatric participants, or surgeries other than PPGL resections; irrelevant outcomes or sufficient outcome data not retrievable.
The study screening and inclusion process was carried out by two reviewers independently. Any disagreement was resolved by consultation with a third reviewer.
Data extraction
A standardized form was formulated for data extraction. The following items of demographic and clinical details were recorded: country(s), center(s), study design, study period, number of participants, α-blockade regimen, sex ratio, approach, and site of surgery. The outcome measurements described below were extracted synchronously. If multiple comparisons of nonselective phenoxybenzamine and selective α-blockers were presented in a single study, combined patient data of all α-blocked procedures were calculated for analysis. Data extraction was conducted by two reviewers independently. Any discrepancy was resolved by consensus or consulting a third author. The valid data was recorded in an excel spreadsheet for analysis.
Outcome measures
The intraoperative outcome measures were intraoperative hypertensive HDI episode (defined as maximum systolic blood pressure>200 mmHg), hypotensive HDI episode (defined as minimum systolic blood pressure<80 mmHg or mean arterial pressure<60 mmHg), mean maximum systolic blood pressure, mean minimum systolic blood pressure, and mean highest heart rate. The postoperative outcome measures were postoperative vasopressor usage, cardiovascular complications, ICU admission, length of stay, and mortality.
Quality assessment
The Cochrane Collaboration tool was utilized for quality assessment of RCTs, including indexes of randomization sequencing, allocation concealment, blinding to personnel and participants, blinding of outcome assessment, incomplete outcome data, selective report of results, and other bias18. The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was employed to assess the quality of included nonobservational studies, involving bias associated with confounding, selection of participants into the study, classification of intervention, deviations from intended interventions, missing data, outcome measurement, and selection of the reported result19. Two independent reviewers assessed the quality of each study. In case of discrepancy, a third reviewer was consulted until consistency was achieved.
Statistical analysis
Meta-analyses were performed using the Review Manager version 5.3 (The Cochrane Collaboration) and Stata SE 15.1 (StataCorp LLC) software package. Risk ratio (RR) and 95% CI was pooled for dichotomous outcomes using the Mantel–Haenszel method. Continuous outcomes were pooled as mean difference (MD) with 95% CI with the inverse variance method. In studies where outcomes were reported as medians and quartiles, means and SDs were calculated using methods proposed by Luo et al.20 and Wan et al 21. The I 2 test was utilized to quantify the effects of heterogeneity among the studies (I 2<25% and >75% values correspond to low and high statistical heterogeneity, respectively). Subgroup analyses and sensitivity analyses were performed as appropriate to explore possible causes of heterogeneity among study results.
Result
The literature search and eligibility
The initial literature search yielded 1871 records. After duplicate removal, titles and abstracts screening was conducted for 545 articles, of which 26 relevant studies were retrieved for full-text analysis. Finally, four studies from the previous review and 11 new studies were eligible for the qualitative and quantitative analysis, involving 3824 procedures performed in 3542 participants (282 patients had multiple resections due to bilateral, multifocal, or relapsed tumors) from 12 countries22–36, of which 2636 received preoperative α-blockade and 906 without α-blockade. All studies were nonrandomized, comprising three prospective cohort studies26,28,29 and 12 retrospective studies22–25,27,30–36.
There were studies concerning the same author28,31 or the same affiliation22,24, which resulted in a possible overlap of total participants. However, they were not excluded from the quantitative analysis because different outcomes of interest were involved. Figure 1 shows the flow diagram of the literature search and inclusion process arranged according to the PRISMA 2020 statement.
Figure 1.
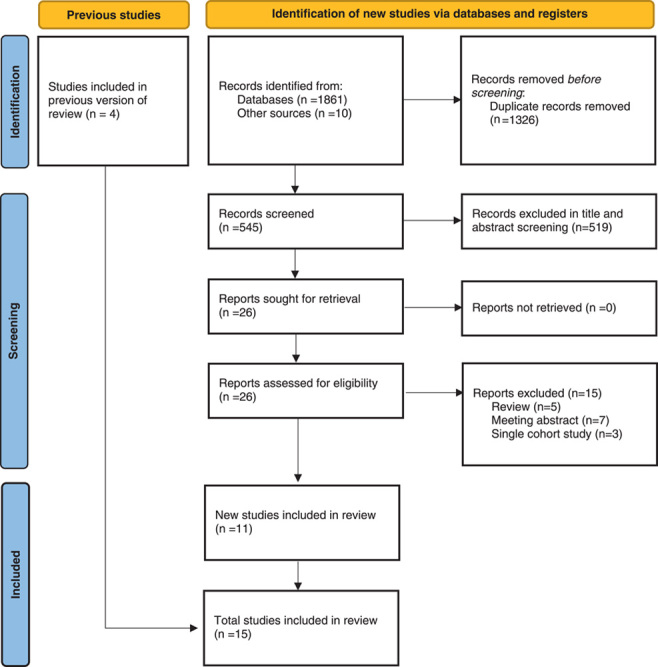
PRISMA flow diagram.
Description of studies
The main characteristics of eligible studies are presented in Table 1. There were four multi-institutional studies27,31,32,34 and one based on statistics retrieved from a national database36. The sex ratio is similar between groups. Nonselective phenoxybenzamine was used in the majority, followed by selective α-blockers of doxazosin, terazosin, and prazosin. The course of preoperative α-blockade was deemed adequate between 7 days to more than 3 weeks in most studies. Monotherapy or combinations with other hypotensive drugs were noticed in both groups of α-blockade and no blockade, as per targets of adequate preoperative medication management for the individuals, of which β-blockers and calcium channel blockers were mostly used. Three studies25,26,30 and one34 only recruited normotensive and biochemically silent PPGL patients, respectively.
Table 1.
Main characteristics of eligible studies
| Surgical procedures | α-blockade regimen | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Country(s) | Center(s) | Design | Study period | α-blockade | No blockade | Drug(s) | Course | Sex (male:female) | Open/laparoscopic | Adrenal/extra-adrenal |
| Boutros et al. 22 | USA | 1 | Retrospective | 1978–1988 | 34 | 29 | PBZ or PRA | 7 days | NA | Yes/no | Yes/yes |
| Steinsapir et al. 23 | USA | 1 | Retrospective | 1959–1996 | 6 | 7 | PBZ | ≥3 weeks | 6:7 | Yes/no | Yes/yes |
| Ulchaker et al. 24 | USA | 1 | Retrospective | 1977–1994 | 79 | 34 | PBZ or SAB | NA | 53:60 | Yes/no | Yes/yes |
| Agarwal et al. 25 | India | 1 | Retrospective | 1990–2003 | 7 | 2 | PRA | Mean: 9.5 days (9–15 days) | 1:8 | Yes/no | Yes/yes |
| Shao et al. 26 | China | 1 | Prospective | 2003–2011 | 38 | 21 | DOX | 14 days | 26:33 | Yes/yes | Yes/no |
| Brunaud et al. 27 | France | 3 | Retrospective | 2002–2012 | 41 | 4 | PBZ | ≥10 days | 16:29 | No/yes | Yes/no |
| Groeben et al. 28 | Germany | 1 | Retrospective | 2008–2016 | 110 | 166 | PBZ or DOX | NA | 153:150 | Yes/yes | Yes/yes |
| Liu et al. 29 | China | 1 | Prospective | 2007–2016 | 492 | 23 | PBZ or SAB | Mean: 13.5 days | 166:200 | Yes/no | Yes/no |
| Kong et al. 30 | China | 1 | 2008–2017 | 45 | 25 | PBZ or DOX | ≥7 days | NA | Yes/yes | Yes/yes | |
| Groeben et al. 31 | Australia, Germany, Ireland, Japan, The Netherlands, and USA | 21 | Prospective | 2000–2017 | 1517 | 343 | PBZ or SAB | NA | NA | Yes/yes | Yes/yes |
| Buscemi et al. 32 | Italy | 2 | Retrospective | 2000–2017 | 49 | 14 | PBZ or DOX | 2–7d before admission; 5–10 days after admission | 27:36 | No/yes | Yes/no |
| Furnica et al. 33 | Belgium | 1 | Retrospective | 1988–2018 | 29 | 51 | PRA | ≥14 days Median: 30 days |
39:41 | Yes/yes | Yes/no |
| Fountas et al. 34 | Greece | 2 | Retrospective | 2014–2021 | 5 | 5 | PBZ | Mean: 14 days | 5:5 | Yes/yes | Yes/no |
| Liu et al. 35 | China | 1 | Retrospective | 2000–2017 | 61 | 106 | PBZ or TER | ≥14 days | 79:88 | Yes/yes | No/yes |
| Kuo et al. 36 | USA | Nationwide database | Retrospective | 2008–2019 | 405 | 76 | PBZ or SAB | NA | 148:219 | Yes/yes | Yes/no |
DOX, doxazosin; NA, data not available; PBZ, phenoxybenzamine; PRA, prazosin; SAB, selective α-blockers; TER, terazosin.
Quality assessment
Quality assessment with the ROBINS-I tool was presented in Table 2. Only one study was deemed a low overall risk of bias, in which confounders like age, duration of surgery, tumor size, tumor etiology, and catecholamine production were adjusted by propensity score matching28. All the observational studies were judged to have a moderate to serious risk of bias for their intrinsic confounders associated with patient selection, intervention allocation, or insufficient information. Data integrity, outcome measurement, and selection of reported results were deemed adequate in most studies.
Table 2.
Risk of bias assessment with the ROBINS-I tool
| References | Bias due to confounding | Bias in selection of participants into the stud y | Bias in classification of intervention | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Boutros et al. 22 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | Moderate risk of bias |
| Steinsapir et al. 23 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | Serious risk of bias |
| Ulchaker et al. 24 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | Serious risk of bias |
| Agarwal et al. 25 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | Serious risk of bias |
| Shao et al. 26 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | Moderate Risk of bias |
| Brunaud et al. 27 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | Serious risk of bias |
| Groeben et al. 28 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low risk of bias |
| Liu et al. 29 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate risk of bias |
| Kong et al. 30 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | Moderate risk of bias |
| Groeben et al. 31 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | Moderate risk of bias |
| Buscemi et al. 32 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate risk of bias |
| Furnica et al. 33 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | Moderate risk of bias |
| Liu et al. 35 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | Serious risk of bias |
| Fountas et al. 34 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | Moderate risk of bias |
| Kuo et al. 36 | 3 | 2 | 3 | 3 | 3 | 1 | 2 | Serious risk of bias |
0=no information/unclear, 1=low risk, 2=moderate risk, 3=serious risk, 4=critical risk of bias.
ROBINS-I, Risk Of Bias In Non-randomized Studies of Interventions.
Intraoperative hemodynamic outcomes
Maximum systolic blood pressure
Nine studies reported the maximum intraoperative systolic blood pressure, involving 879 procedures with preoperative α-blockade and 388 without23–30,35. No difference was shown in the pooled result between groups: MD=0.98 (−5.06, 7.02) mmHg (P=0.75, I 2=40%). Subgroup analysis of three studies25,26,30 comprising only normotensive PPGL patients showed a similar result: MD=−2.84 (−13.95, 8.27) mmHg (P=0.54, I 2=0%) (Fig. 2).
Figure 2.
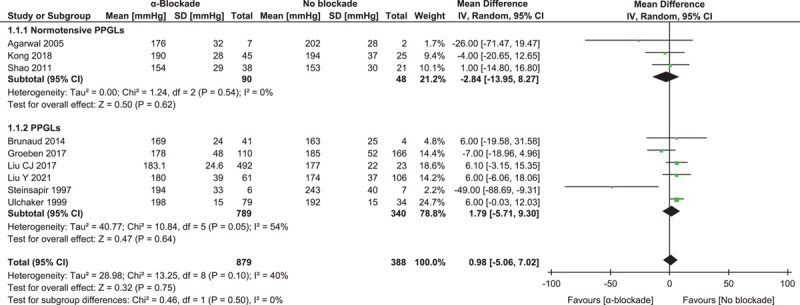
Forest plot of intraoperative maximum systolic blood pressure comparing α-blockade with no blockade. PPGL, pheochromocytoma and paraganglioma.
Minimum systolic blood pressure
Seven studies reported the minimum intraoperative systolic blood pressure, involving 763 procedures with preoperative α-blockade and 215 without24–27,29,30,35. The pooled result showed a lower value associated with α-blockade, but the difference was not statistically significant: MD=−4.53 (−11.86, 2.79) mmHg (P=0.23, I 2=85%). Subgroup analysis of two studies with normotensive PPGL patients26,30 showed a minimal difference: MD=−0.88 (−5.84, 4.07) mmHg (P=0.62, I 2=0%) (Fig. 3).
Figure 3.
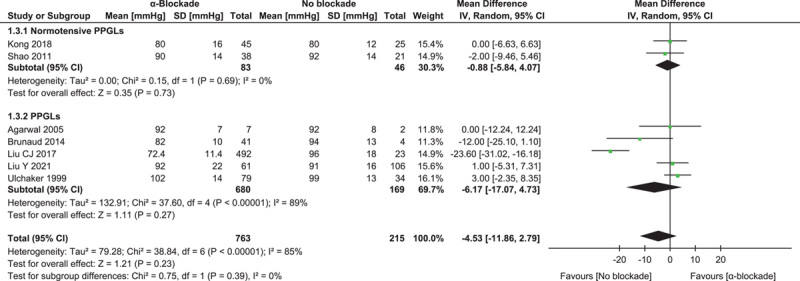
Forest plot of intraoperative minimum systolic blood pressure comparing α-blockade with no blockade. PPGL, pheochromocytoma and paraganglioma.
Hypertensive hemodynamic instability episode
Intraoperative hypertensive HDI, defined as at least one episode of systolic blood pressure above 200 mmHg, was reported in three studies of 356 procedures28,30,34. The pooled result showed no difference in hypertensive HDI incidence: RR=0.82 (0.50, 1.35) (P=0.44, I 2=0%) (Fig. 4).
Figure 4.
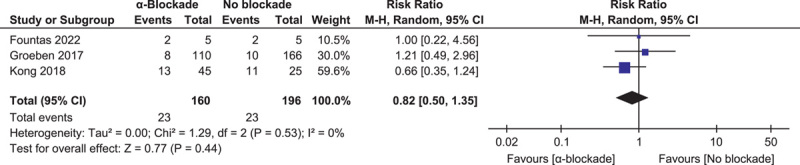
Forest plot of intraoperative hypertensive hemodynamic instability episode comparing α-blockade with no blockade.
Hypotensive hemodynamic instability episode
Intraoperative hypotensive HDI, defined as at least one episode of systolic blood pressure below 80 mmHg or mean arterial pressure below 60 mmHg, was reported in four studies of 523 procedures28,30,34,35. There was also no significant difference between groups: RR=1.07 (0.81, 1.41) (P=0.65, I 2=34%) (Fig. 5).
Figure 5.
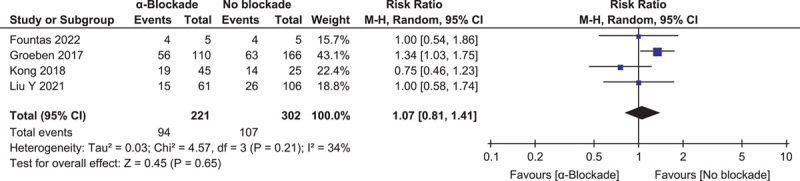
Forest plot of intraoperative hypotensive hemodynamic instability episode comparing α-blockade with no blockade.
Highest heart rate
Peak heart rate was reported in six studies involving 417 procedures22,23,26,27,30,35. Synthesized results showed no significant variation in overall effect: MD=3.58 (−1.42, 8.57) (P=0.16, I 2=27%), as well as in subgroup analysis of two studies focusing on normotensive patients26,30: MD=−0.74 (−7.20, 5.73) (P=0.82, I 2=0%) (Fig. 6).
Figure 6.
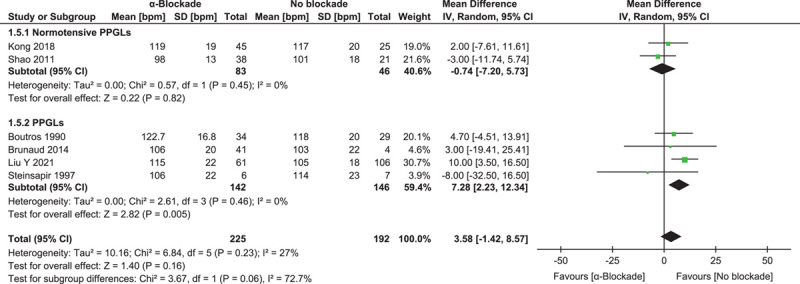
Forest plot of intraoperative highest heart rate comparing α-blockade with no blockade. PPGL, pheochromocytoma and paraganglioma.
Postoperative outcomes
ICU admission
Five studies investigated postoperative ICU admission, involving 854 procedures22,32,33,35,36. The meta-analytic result showed a similar admission rate between groups: RR=1.29 (0.54, 3.09) (P=0.56, I 2=66%) (Fig. 7A).
Figure 7.
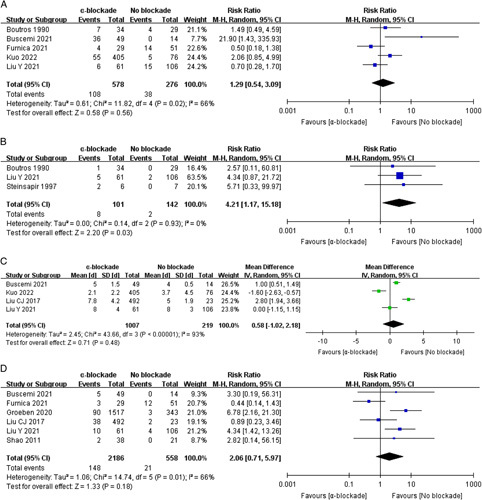
Forest plot of major postoperative events comparing α-blockade with no blockade. ICU admission (A), vasopressor usage (B), length of stay (C), and overall cardiovascular morbidity (D).
Vasopressor usage
Prolonged postoperative hypotension that necessitated vasopressor usage was noted in three studies with 243 procedures22,23,35. Patients with α-blockade pretreatment had a significantly higher risk of postoperative hypertension than those without: RR=4.21 (1.17, 15.18) (P=0.03, I 2=0%) (Fig. 7B).
Length of stay
The pooled result of four studies29,32,35,36 demonstrated a similar postoperative length of stay between groups: MD=0.58 (−1.02, 2.18) days (P=0.48, I 2=93%). There was a high risk of heterogeneity, partly due to the discrepant discharging criteria among different institutions (Fig. 7C).
Overall cardiovascular complications
Six studies incorporating 2744 procedures26,29,31–33,35 investigated the incidence of overall cardiovascular complications, which was higher in the α-blockade pretreatment group, but the difference was not statistically significant: RR=2.06 (0.71, 5.97) (P=0.18, I 2=66%) (Fig. 7D).
Mortality
No short-term mortality was noted in all studies, but two. Boutros et al.22 reported one patient died of intracranial metastatic tumor, Liu et al.35 reported two deaths due to hemorrhagic shock caused by massive intraoperative bleeding and aspiration of regurgitation, respectively. None of the deaths was inferred to be associated with preoperative treatment.
Sensitivity analysis
Sensitivity analysis was performed for pooled outcomes with high risk of statistical heterogeneity identified by the I 2 test. For the comparison of minimum systolic blood pressure, the exclusion of the study by Liu et al.29 that only include open surgeries resulted in a similar overall effect estimate but a significantly reduced statistical heterogeneity: MD=0.21 (−2.76, 3.18) mmHg (P=0.89, I 2=0%).
The analyses showed no remarkable changes in the effect estimate with the sequential exclusion of each study from the analysis (Fig. 8). Besides, the exclusion of studies with serious overall risk of bias yielded similar pooled estimates of intraoperative minimum systolic blood pressure: MD=−8.49 (−23.12, 6.15) (P=0.26, I 2=92%) and postoperative length of stay: MD=0.76 (−1.28, 2.79) days (P=0.47, I 2=95%).
Figure 8.
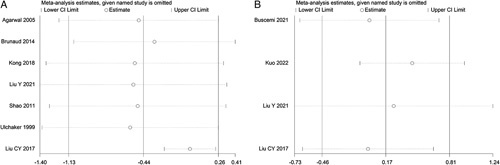
Sensitivity analysis of effect estimates. Minimum systolic blood pressure (A) and length of stay (B).
Discussion
This systematic review and meta-analysis updated the previous study by Schimmack et al 37. They found no beneficial efficacy for α-blockade pretreatment in surgery for pheochromocytoma versus no blockade. For the scarcity of eligible studies, their conclusion was based upon only two outcomes of intraoperative maximum blood pressure and highest heart rate. Considering a comprehensive assessment of the bi-directionality and intensity of hemodynamic fluctuations. We incorporated more parameters to evaluate its effect on hypertensive and hypotensive episodes. Besides, data on major perioperative events and cardiovascular morbidity were initially investigated to ulteriorly reveal patients’ clinical outcomes. Our result demonstrated no beneficial efficacy of α-blockade on stabilizing intraoperative hemodynamics for PPGL resections. Although α-blockade pretreatment was associated with more prolonged hypotension and vasopressor usage than no blockade, perioperative cardiovascular morbidity remained comparable with no blockade.
Nonselective phenoxybenzamine is the first choice among α-blockers used for PPGL patients, which blocks α1 and α2-adrenergic receptors irreversibly to achieve a long-lasting effect38. However, phenoxybenzamine-related complications became the initiating element for abstaining α-blockade pretreatments, such as orthostatic hypotension, reflex tachycardia, and dizziness22,39. Besides, high cost and limited availability in certain regions also hampered its use15. Kuo et al.36 went through a nationwide database in the USA and found a significant shift-away trend from phenoxybenzamine to selective α-blockers and calcium channel blockers in the past decade, likely due to reduced costs, similar length of stay, and ICU admission. Our meta-analysis also demonstrated a higher risk of prolonged hypotension episodes necessitating vasopressor usage associated with preoperative α-blockade. This might be attributed to α1-adrenoceptor irreversibly blocked by phenoxybenzamine that remained irresponsive to normal catecholamines stimulation. Selective α-blockades such as doxazosin, prazosin, and terazosin may be alternatives, however, a recent meta-analysis revealed the inferiority of selective α-blockades in preventing intraoperative blood pressure fluctuations compared with phenoxybenzamine, despite the comparable risk of perioperative morbidity40.
Although tumor size and urine metanephrine levels were found to be associated with intraoperative blood pressure fluctuations, risk factors that precisely predict HDI are still undetermined14,41. The reported incidence of intraoperative HDI remained as high as 40–70% in PPGL patients despite adequate α-blockade pretreatment11–13,42. It was inferred that intraoperative hemodynamic fluctuations were mainly caused by the sudden release and retreat of catecholamines due to tumor manipulation and removal, hardly to be blocked by any preoperative medication43. Moreover, intraoperative HDI rarely leads to major postoperative events. The overall cardiovascular morbidity has fallen to 3–5%, and perioperative mortality was even close to zero in contemporary series5. In contrast, vigorous monitoring and application of various fast-acting vasoactive agents allow anesthesiologists to take control of hemodynamic fluctuations promptly13,44. It is rational to argue that, in most cases, intraoperative HDI episodes should no longer be considered extremely dangerous or even catastrophic events as they once were.
With the development of diagnostic imaging, more pheochromocytomas are diagnosed as incidentalomas rather than symptomatic. Normotensive pheochromocytomas account for up to 40% of incidental adrenal tumors detected on imaging45 and 23–55% of total pheochromocytoma patients46. Compared with hypertensive PPGL, normotensive tumors demonstrated a quite distinct catecholamines secretion pattern with a significantly higher proportion of dopamine, rather than biologically negative43, calcium channel blockers might be an optimal pretreatment regimen in those patients for minimized risk of hypotension and reflex tachycardia47. Subgroup analysis regarding normotensive PPGLs in this meta-analysis yielded a consistent result with general PPGL participants, indicating that prudent omission of α-blockade pretreatment is safe and feasible for those patients.
The patterns of PPGL perioperative management shifted vastly in the past few decades. Multidisciplinary involvements, especially efforts of anesthetic interventions, achieved a great reduction of overall surgical risk and mortality. In contrast, however, more confounding factors were introduced to studies focusing on a single preoperative intervention like α-blockade. For instance, the application of various quick-acting vasoactive agents allows anesthesiologists to correct HDI promptly, which might to some extent compensate for the potential risk of inadequate preoperative preparation. Moreover, patients with hormone-functioning PPGL usually manifest refractory hypertension and cardiovascular comorbidities. Other antihypertensives and vasoactive drugs (calcium channel blockers, β-blockers, etc.) may be indicated as per specific preoperative preparation criteria, regardless of whether they were α-blockaded or not, which may also interfere with the actual efficacy of α-blockade.
There are certain limitations in our study. First, the absence of data from RCTs resulted in a reduced level of evidence regarding our findings, despite the various factors discussed thoroughly previously that impede high-quality RCTs on this topic28,37. Besides, the exact range or dividing values of blood pressure to define intraoperative HDI remain undetermined. We complied with the predefined thresholds to evaluate the overall incidence of intraoperative HDI but might as well neglect its variability.
Therefore, the result of this meta-analysis should not be interpreted as an assertion that α-blockade can be safely omitted without considerations of patient status and institutional expertise, but as a critical appraisal against the adherence to a dogmatic preparation paradigm, which is cumbersome, expensive, and without evident benefits.
Conclusions
This systematic review and meta-analysis showed no beneficial effects of α-blockade pretreatment on intraoperative hemodynamics as well as perioperative morbidity and mortality over no blockade, both in hypertensive and normotensive PPGL patients. However, multicenter RCTs are still warranted for further verification.
Ethical approval
No ethical approval was given since it is not applicable for a meta-analysis.
Sources of funding
This work was supported by grants from the Chongqing Municipal Health Commission (2020jstg018).
Author contribution
D.W.: supervision, project administration, and funding acquisition. J.W.: conceptualization, methodology, and writing – original draft preparation. Q.L.: data curation and formal analysis. S.J.: data curation and software. J.Z.: writing – review and editing. J.H.: validation. Y.L.: visualization.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO database.
Unique Identifying number or registration ID: CRD42022370263.
Hyperlink to your specific registration (must be publicly accessible and will be checked):https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022370263
Guarantor
Delin Wang.
Data availability statement
Data sharing is not applicable to this study as no original data were created or analyzed in this study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 11 April 2023
Contributor Information
Jue Wang, Email: 18096306759@163.com.
Qingyuan Liu, Email: qyliuxxt@outlook.com.
Shihao Jiang, Email: 2019010063@stu.cqmu.edu.cn.
Jindong Zhang, Email: 1328124070@qq.com.
Jinke He, Email: hejk-2009@qq.com.
Yunfan Li, Email: 1010647347@qq.com.
Delin Wang, Email: wandelin@hospital.cqmu.edu.cn.
References
- 1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol 2015;173:757–764. [DOI] [PubMed] [Google Scholar]
- 2. Prejbisz A, Lenders JWM, Eisenhofer G, et al. Cardiovascular manifestations of phaeochromocytoma. J Hypertens 2011;29:2049–2060. [DOI] [PubMed] [Google Scholar]
- 3. Prys-Roberts C. Phaeochromocytoma – recent progress in its management. Br J Anaesth 2000;85:44–57. [DOI] [PubMed] [Google Scholar]
- 4. Lenders JWM, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metabol 2014;99:1915–1942. [DOI] [PubMed] [Google Scholar]
- 5. Lenders JWM, Kerstens MN, Amar L, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens 2020;38:1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yip L, Duh QY, Wachtel H, et al. American Association of Endocrine Surgeons Guidelines for Adrenalectomy: Executive Summary. JAMA Surg 2022;157:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen H, Sippel RS, O’Dorisio MS, et al. , The North American Neuroendocrine Tumor Society Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors. Pancreas 2010;39:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel D, Phay JE, Yen TWF, et al. Update on pheochromocytoma and paraganglioma from the SSO Endocrine and Head and Neck Disease Site Working Group, part 2 of 2: perioperative management and outcomes of pheochromocytoma and paraganglioma. Ann Surg Oncol 2020;27:1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castinetti F, de Freminville JB, Guerin C, et al. Controversies about the systematic preoperative pharmacological treatment before pheochromocytoma or paraganglioma surgery. Eur J Endocrinol 2022;168:D17–D24. [DOI] [PubMed] [Google Scholar]
- 10. Livingstone M, Duttchen K, Thompson J, et al. Hemodynamic stability during pheochromocytoma resection: lessons learned over the last two decades. Ann Surg Oncol 2015;22:4175–4180. [DOI] [PubMed] [Google Scholar]
- 11. van den Heede K, Paspala A, Chander N, et al. To block, or not to block … is it still the question? Effectiveness of alpha- and beta-blockade in phaeochromocytoma surgery: an institutional analysis. Ann R Coll Surg Engl 2022;104:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tariel F, Dourmap C, Prudhomme T, et al. Adrenalectomy for pheochromocytoma: complications and predictive factors of intraoperative hemodynamic instability. Am Surg 2022. 10.1177/00031348221135774. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Ma L, Shen L, Zhang X, et al. Predictors of hemodynamic instability in patients with pheochromocytoma and paraganglioma. J Surg Oncol 2020;122:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JH, Lee HC, Jin Kim S, et al. Perioperative hemodynamic instability in pheochromocytoma and sympathetic paraganglioma patients. Sci Rep 2021;11:18574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson LH, Makay Ö, Brunaud L, et al. Adrenalectomy for incidental and symptomatic phaeochromocytoma: retrospective multicentre study based on the Eurocrine® database. Br J Surg 2021;108:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buisset C, Guerin C, Cungi PJ, et al. Pheochromocytoma surgery without systematic preoperative pharmacological preparation: insights from a referral tertiary center experience. Surg Endosc 2021;35:728–735. [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- 21. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boutros AR, Bravo EL, Zanettin G, et al. Perioperative management of 63 patients with pheochromocytoma. Cleve Clin J Med 1990;57:613–617. [DOI] [PubMed] [Google Scholar]
- 23. Steinsapir J, Carr AA, Prisant LM, et al. Metyrosine and pheochromocytoma. Arch Intern Med 1997;157:901–906. [PubMed] [Google Scholar]
- 24. Ulchaker JC, Goldfarb DA, Bravo EL, et al. Successful outcomes in pheochromocytoma surgery in the modern era. J Urol 1999;161:764–767. [PubMed] [Google Scholar]
- 25. Agarwal A, Gupta S, Mishra AK, et al. Normotensive pheochromocytoma: Institutional experience. World J Surg 2005;29:1185–1188. [DOI] [PubMed] [Google Scholar]
- 26. Shao Y, Chen R, Shen ZJ, et al. Preoperative alpha blockade for normotensive pheochromocytoma: Is it necessary. J Hypertens 2011;29:2429–2432. [DOI] [PubMed] [Google Scholar]
- 27. Brunaud L, Boutami M, Nguyen-Thi PL, et al. Both preoperative alpha and calcium channel blockade impact intraoperative hemodynamic stability similarly in the management of pheochromocytoma. Surgery 2014;156:1410–1418. [DOI] [PubMed] [Google Scholar]
- 28. Groeben H, Nottebaum BJ, Alesina PF, et al. Perioperative α-receptor blockade in phaeochromocytoma surgery: an observational case series. Br J Anaesth 2017;118:182–189. [DOI] [PubMed] [Google Scholar]
- 29. Liu C, Lv Q, Chen X, et al. Preoperative selective vs nonselective α-blockade in PPGL patients undergoing adrenalectomy. Endocr Connect 2017;6:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong H, Li N, Li X-Y, et al. The role of pre-operative α-blockade in patients with normotensive phaeochromocytoma or paraganglioma. Eur J Anaesthesiol 2018;35:898–899. [DOI] [PubMed] [Google Scholar]
- 31. Groeben H, Walz MK, Nottebaum BJ, et al. International multicentre review of perioperative management and outcome for catecholamine-producing tumours. Br J Surg 2020;107:e170–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buscemi S, G, et al. Perioperative management of pheochromocytoma: From a dogmatic to a tailored approach. J Clin Med 2021;10:3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furnica RM, Dusoruth MM, Persu A, et al. Influence of secretory phenotype and preoperative preparation on surgical outcome in pheochromocytoma. Endocr Connect 2021;10:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fountas A, Kanti G, Glycofridi S, et al. Pre- and peri-operative characteristics, complications and outcomes of patients with biochemically silent pheochromocytomas; a case series. Endocrine 2022;78:570–579. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Jin X, Gao J, et al. Preoperative alpha-blocker therapy in patients with missed preoperative diagnosis of extra-adrenal retroperitoneal paraganglioma undergoing resection: a retrospective study of 167 cases at a single center. Neuroendocrinology 2022;112:457–466. [DOI] [PubMed] [Google Scholar]
- 36. Kuo EJ, Chen L, Wright JD, et al. Phenoxybenzamine is no longer the standard agent used for alpha blockade before adrenalectomy for pheochromocytoma: a national study of 552 patients. Surgery 2023;173:19–25. [DOI] [PubMed] [Google Scholar]
- 37. Schimmack S, Kaiser J, Probst P, et al. Meta-analysis of α-blockade versus no blockade before adrenalectomy for phaeochromocytoma. Br J Surg 2020;107:e102–e108. [DOI] [PubMed] [Google Scholar]
- 38. Naranjo J, Dodd S, Martin YN. Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth 2017;31:1427–1439. [DOI] [PubMed] [Google Scholar]
- 39. Newell KA, Prinz RA, Brooks MH, et al. Plasma catecholamine changes during excision of pheochromocytoma. Surgery 1988;104:1064–1073. [PubMed] [Google Scholar]
- 40. Zawadzka K, Wieckowski K, Malczak P, et al. Selective vs non-selective alpha-blockade prior to adrenalectomy for pheochromocytoma: Systematic review and meta-analysis. Eur J Endocrinol 2021;184:751–760. [DOI] [PubMed] [Google Scholar]
- 41. Urabe F, Kimura S, Iwatani K, et al. Risk factors for perioperative hemodynamic instability in pheochromocytoma: a systematic review and meta-analysis. J Clin Med 2021;10:4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Araujo-Castro M, García Centero R, López-García MC, et al. , Surgical outcomes in the pheochromocytoma surgery. Results from the PHEO-RISK STUDY. Endocrine 2021;74:676–684. [DOI] [PubMed] [Google Scholar]
- 43. Lentschener C, Gaujoux S, Tesniere A, et al. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal-time for a reappraisal? Eur J Endocrinol 2011;165:365–373. [DOI] [PubMed] [Google Scholar]
- 44. Lentschener C, Gaujoux S, Thillois JM, et al. Increased arterial pressure is not predictive of haemodynamic instability in patients undergoing adrenalectomy for phaeochromocytoma. Acta Anaesthesiol Scand 2009;53:522–527. [DOI] [PubMed] [Google Scholar]
- 45. Isaacs M, Lee P. Preoperative alpha-blockade in phaeochromocytoma and paraganglioma: is it always necessary? Clin Endocrinol (Oxf) 2017;86:309–314. [DOI] [PubMed] [Google Scholar]
- 46. Araujo-Castro M, García Sanz I, Mínguez Ojeda C, et al. Differences in intraoperative and surgical outcomes between normotensive pheochromocytomas and sympathetic paragangliomas (PPGLs) and hypertensive PPGLs: results from the PHEO-RISK STUDY. J Endocrinol Invest 2022;46:805–814. [DOI] [PubMed] [Google Scholar]
- 47. Lafont M, Fagour C, Haissaguerre M, et al. Per-operative hemodynamic instability in normotensive patients with incidentally discovered pheochromocytomas. J Clin Endocrinol Metab 2015;100:417–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this study as no original data were created or analyzed in this study.


